Abstract
Clinical reference intervals among Indian population are poorly defined. Therefore, there is an urgent need to establish local clinical laboratory reference intervals for healthy Indian population. The present study aimed to identify the 95 % reference interval for hematological and biochemical parameters in apparently healthy Indian population. We undertook a multicentric cross-sectional study conducted at Apollo Hospitals Educational and Research Foundation across India. Of which 10,665 reference individuals identified as healthy by physicians. The 95 % of the reference distribution was estimated using 2.5th and 97.5th percentile reference limits. The 95 % reference intervals for hemoglobin (Males: 12.3–17 g/dL; Females: 9.9–14.3 g/dL), platelet count (Males: 1.3–3.8; Females: 1.3–4.2 Lakhs/µL), erythrocyte sedimentation rate (Males: 2–22; Females: 4–55 mm/h), serum uric acid in males: 3.5–8.2 mg/dL, gamma glutamyl transferase (Males: 13–61 U/L), fasting blood glucose (Males: 78–110 mg/dL), total cholesterol (Males: 115–254 mg/dL), low density lipoprotein (Males: 60–176 mg/dL) and triglycerides (Males: 55–267 mg/dL, Females: 52–207 mg/dL) were different from currently used reference values. Additionally need for gender based partitioning were observed for triglycerides and gamma glutamyl transferase. The observed findings are of clinical significance and it needs to be validated with additional community based studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reference intervals (RI); generally refer to quantitative data accompanied with upper and lower limits. Population based RI play an important role in screening patients, follow-up, routine clinical care, and clinical management of patients as well as in clinical trials. Definitions and guidelines for each step are elaborated by the Expert Panel on Theory of Reference Value (EPRTV) [1–6] and subsequently in 2000, National Committee for Clinical Laboratory Standards (NCCLS) [7] and also Clinical and Laboratory Standards Institute (CLSI 2008) [8] published guidelines for determining reference intervals for quantitative clinical laboratory tests. Most of the diagnostic laboratories in India are following the reference intervals from the literatures, package inserts, and textbooks or adopted from Western cohorts, this reference population is completely different from Indian ethnic population (viz., dietary habits, lifestyle, ethnicity, socio economic status and environmental factors). Several studies reported significant changes in reference intervals as compared to standard reference ranges [9–12]. A recent cross-sectional study from Africa has also found that significant changes in hematological markers (such as hemoglobin, platelet count and total white blood count) and biochemical parameters like alanine transaminases and creatinine values compared with currently used reference ranges. Detailed survey of literature revealed that many population based studies have been carried out in developed countries especially from Caucasians, while limited studies are available on RI in resource limited settings as well as in Indian ethnic origin. In addition, these studies have been carried out with small sample size in a specific region with limited laboratory analytes. Therefore, it becomes indispensable to establish region-specific reference intervals for our settings. Hence the present study aimed to establish the 95 % reference intervals for biochemical and hematological parameters from a healthy Indian population.
Materials and Methods
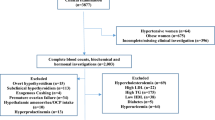
Setting and Participants
This multicentric cross-sectional study was conducted at Apollo Hospitals Educational and Research Foundation (AHERF), (non-profit organization, providing quality health care education, training and also involved in research activities in the field of biomedical, clinical, basic and translational research since the year 2006.) between August 2004 and March 2008 in four different centers from Ahmedabad, Chennai, Hyderabad and New Delhi. The reference population comprised of participants who underwent Apollo Master Health Checkup (MHC), for a preventive health package offered by Apollo group of hospitals. The reference individuals were identified and included as apparently healthy from this population attending the centre MHC package. All the participants were identified and included based on stringent inclusion and exclusion criteria. Participants who met the following criteria were finally included in the reference group (Adults: age between 20 and 70 years, BMI of 18–25 kg/m2; Participants deemed healthy by their respective physicians.) On the other hand participants with any of the following were excluded from the reference group; Participants with known pathologic states, diabetes mellitus, chronic renal insufficiency, hypertension, ischemic heart disease, anemia, thyroid disorders, liver diseases with biochemical and hematological abnormalities, weight loss, fever, chest pain, giddiness, polyarthralgia and loss of appetite, intake of pharmacologically active substances, usage of drugs, multiple and regular vitamins, oral contraceptive pills, smokers, alcohol consumption more than two drinks per week (60 mL of alcohol), past illness of typhoid, tuberculosis, malaria, dengue within 6 months of the study date and jaundice or major surgery, pregnancy, lactating women and blood pressure (BP) ≥140/90 mm of Hg. A total of 76,000 participants were screened and reviewed at four centers of which 10,665 study participants who met the inclusion criteria were included. Informed consents were obtained from all participants before initiation of the study. A detailed family history and medical examinations was performed at screening stage for all the participants. Physicians at each center thoroughly screened the information from health care records to identify persons fulfilling the selection criteria. The study protocol along with informed consents were reviewed and approved by ethics committee at Apollo Hospitals, Chennai.
Specimen Collection and Handling Techniques
Specimens were collected from all the reference individuals between 7.30 and 9.30 am in the morning after an overnight fast. All participants were advised to take rest at least for 15 min before blood collection. Venous blood was drawn and collected in a plain vacutainer red topped tubes or serum separator tubes (SST, Becton–Dickinson, Franklin Lakes, NJ, USA) gold topped gel tubes for laboratory investigations. Biochemical parameters such as liver function tests, urea, creatinine, uric acid were analyzed from SST tubes by standard methods. In plain tubes, protein profile and lipid profile were investigated. Additionally, SST or sodium fluoride/Na2 EDTA tubes were used for blood glucose analysis. Whole blood was drawn in a 3 mL vacutainer tubes containing K2 EDTA 5.4 mg (Becton–Dickinson, Franklin lakes, NJ, USA) tubes were transported immediately to the laboratory and analysis was completed within 3 h.
Laboratory Investigations
All laboratory investigations were carried out in accordance with standard operating procedures (SOPs) followed by good laboratory practices (GLP). Prior testing two levels of internal quality controls (IQC) from Bio-Rad (Bio-Rad Laboratories Inc. USA) were used and IQC results were interpreted in agreement with Westgard multirule algorithm and plotted as levy Jennings. As a part of quality control programme, the testing laboratories have been accredited by NABL in accordance with international standard ISO 15189. All four centers participated in regular EQAS programme conducted by national (AIIMS, New Delhi) and international (RCPA, Australia, Randox, UK) laboratories for hematological parameters. Fully Automated clinical chemistry analyzers were used in all the four centers viz., Hitachi 911/912 (Roche, USA) automated analyzer from was used at Chennai, Delhi and Ahmedabad centers. Dade Behring Dimension RXL (now Siemens) fully automated high throughput clinical chemistry analyzer was used at the Hyderabad center. Instruments were regularly calibrated and specimens were cross checked to rule any analytical variations due to instrument, reagents and other factors in all four centres. Reagents, calibrators, methodologies and quality controls were unchanged during the entire period of the study for both hematological and biochemical investigations.
Hematological investigations were performed on SysmexKX-21 Fully automated hematology analyzer, (Sysmex Corporation, Kobe, Japan) at Chennai, Delhi, Ahmedabad centers and ABX Pentra 120 (Biomerieux) was used in Hyderabad. The performance of Pentra 60 was compared with other automated hematology analysers [13]. In addition, all centers have participated in external quality program (EQAS) conducted by national and international agencies. The following analytes were investigated: haemoglobin (HGB), mean corpuscular haemoglobin volume (MCV), mean corpuscular haemoglobin concentration (MCHC), ESR, RBC, WBC, neutrophils, eosinophils, lymphocytes, basophils, monocytes, platelet count, blood glucose, 2 h post glucose, blood urea, creatinine, uric acid, total cholesterol (Tc), HDL-C, LDL-C, TGL, total bilirubin, total protein, albumin, AST and GGT.
Statistical Analysis
All statistical analysis was performed using SPSS software package version 11.0. The calculations for reference interval was carried out in accordance with IFCC, NCCLS and CLSI approved guidelines [7, 8]. To identify the 95 % reference interval, the central 95 % of the reference distribution was estimated using reference limits at 0.025 fractile (2.5th percentile) for the lower reference limit and 0.975 fractile (97.5th percentile) as the upper reference limit. All data was expressed as median 25th–75th percentile, since the analytes did not follow a Gaussian (normal) distribution (as tested by one sample Kolmogorov–Sminorv test).
Results
Of the 10,665 healthy subjects studied, 7,478 (70.1 %) were males and 3,187 (29.9 %) were females. The age ranged from 20 to 70 years. The mean BMI was 24.34 ± 3.1 kg/m2 and diet history was available for 9,859 subjects, of these 4,921 (49.9 %) were vegetarians and 4,938 (50.08 %) were non-vegetarians. The identified 95 % reference intervals for hematological parameters are presented in Table 1. The currently used reference ranges are given for comparison. The reference intervals from our study showed differences from the currently used reference intervals for hemoglobin, platelet count, ESR, eosinophil count, serum uric acid, total bilirubin and GGTP. As currently decision limits and recommended values are used for blood glucose and lipid profile, differences were also observed for these parameters.
ESR values were almost 3-fold higher in females than males (4–55 mm/h (F) vs. 2–22 mm/h (M)) and the ULN was elevated almost 2-fold when compared with reporting ranges. The median and the average co-efficient of variation (CV) of all biochemical and hematological parameters are shown in Tables 2 and 3.
Statistical differences between the means for the two genders were observed for all analytes except for MCV. However the magnitude of the difference was small. Larger differences were observed for the following parameters for which separate reference intervals for the two genders must be considered in clinical decision making: Hemoglobin, PCV, ESR, serum creatinine, uric acid, HDL, triglycerides and GGTP (Tables 1, 4). Center-based analysis revealed that regional differences between the centers were appreciated among the population studied. We noted that high ESR, eosinophil and low hemoglobin and platelet counts were observed in Chennai. In Ahmedabad center, persons had elevated total cholesterol, LDL and HDL and uric acid levels. Similarly, high total protein, albumin and ALT levels were seen in Delhi centre and high levels of 2 h post glucose, ALT and GGTP were noted in Hyderabad centre.
Established reference intervals with 90 % CI for blood chemistries are shown in Table 4. In lipid profile, the 95 % reference interval for our population was significantly higher than the currently recommended ideal values (Table 4). Some of the biochemical parameters like urea and bilirubin differed from the current reference ranges. We also calculated the reference intervals for persons who were below and above 40 years of age (Tables 5, 6).We identified that some of the parameters like ESR, Tc, HDL, LDL-c and TGL were higher in persons below 40 years age group, while hemoglobin and platelets were lower in persons above 40 years age group (Table 5). Region and gender wise reference intervals are shown in Tables 7 and 8.
Discussion
Currently, developing countries like India are facing serious public health challenges like obesity, cardiovascular disease, diabetes and infectious diseases. In this arena, clinical laboratory plays a major role for early diagnosis of life threatening disease and also provide valuable information about health of an individual. The present study aimed at establishing region-specific reference intervals for hematological and biochemical markers among apparently healthy Indian population. In our study, we found that established reference intervals of certain biochemical and hematological parameters were altered from current reporting ranges. It highlights the importance of establishing and incorporating the region-specific reference intervals in the laboratory reporting system in our ethnic population. It was also observed that the 2.5 % lower reference limit was lower for hemoglobin, mean cell volume, platelet count and 2 h post glucose and the 97.5 % upper reference interval was higher for ESR, fasting glucose, total cholesterol, low density lipoprotein, triglycerides, total cholesterol, serum uric acid, total bilirubin, alkaline phosphatase and gamma glutamyltransferase.
Surprisingly, platelets were low in males than females and also the upper limit of normal was marginally lower than current reporting ranges. The reason for low platelet count is unknown. But, genetic environmental and dietary factors have been speculated from earlier studies [14, 15]. The erythrocyte sedimentation rate which is an indirect marker for inflammation and uric acid levels were found to be higher in our apparently healthy population. It is assumed that diet rich in purines as observed in animal proteins increases serum uric acid levels. Gender differences were observed for the following parameters such as haemoglobin, PCV, ESR, serum creatinine, uric acid, HDL, triglycerides and GGTP as in the current clinical practice gender based partitioning is not routinely employed on a large scale in our setting.
Given the importance, gender based studies should be carried out to investigate the reason for the biological fluctuation and also to identify the people at risk of developing the disease. On the other hand, there were no differences appreciated in the parameters like lymphocytes, monocytes and basophils. These analytes are correlated well with the reporting values. Therefore, it is clear that establishment of local reference intervals carries utmost significance in the laboratory reporting system and in safety monitoring of clinical trial participants.
Many studies reported that Indians are more susceptible to metabolic complications especially to diabetes, cardiovascular disease, dyslipidemia and other lipid abnormalities. The present study demonstrated that the levels of total cholesterol, triglycerides and serum uric acids were higher than current reporting ranges. This could be attributed to changes in life style, socio-economic status, age, sedentary habits, consumption of fried foods, lack of exercise and genetic factors.
Recent studies from different geographical locations of India have established reference intervals for certain biochemical parameters and also identified significant changes in liver markers and lipid levels in healthy Indian population [16–21]. Furthermore, we have documented that the renal and cell integration markers like creatinine, AST and GGT were lower in females than males as supported by previous studies done elsewhere [22, 23]. Subsequently, we found lower levels of haemoglobin and platelets in subjects above 40 years age group and higher levels of lipids & lipoproteins in below 40 years as supported by a previous study [24]. As age increases RI may differ from younger adults that emphasize the importance of establishing age-specific reference values and their significance in our clinical milieu prior to developing laboratory reporting intervals for our ethnic population various factors should be considered (viz. pre-analytical, analytical and post-analytical factors) that would affect the outcome of results and it was recently reported by a CALIPER substudy among healthy children [25]. International federation of clinical chemists recommends that each clinical laboratory should establish its own reference interval for local ethnic population and not to adopt the manufacturer references ranges. Moreover, clinical laboratory values should be validated every 5 years to ensure laboratory reporting system adhering to the guidelines published elsewhere for clinical laboratory.
There are few limitations in this study that could be cited such as data on smoking, socioeconomic status, genetic, exercise and history of alcohol were not available for all reference population. However, strengths of the present study are larger sample size, study participants from different region and standard laboratory techniques.
In conclusion, gender based partitioning is required for hemoglobin, packed cell volume, ESR, high density lipoprotein, triglycerides, creatinine, uric acid, and GGTP. However many community based studies are warranted to validate the region-specific laboratory reference intervals for our ethnic population.
References
Solberg HE. International Federation of Clinical Chemistry, expert panel on theory of reference values: approved recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. Clin Chim Acta. 1987;165:111–8.
PetitClerc C, Solberg HE. International Federation of Clinical Chemistry, expert panel on theory of reference values: approved recommendation (1987) on the theory of reference values. Part 2. Selection of individuals for the production of reference values. J Clin Chem Clin Biochem. 1987;25:639–44.
Solberg HE, PetitClerc C. International Federation of Clinical Chemistry (IFCC), expert panel on theory of reference values: approved recommendation (1988) on the theory of reference values. Part 3. Preparation of individuals and collection of specimens for the production of reference values. J Clin Chem Clin Biochem. 1988;26:593–8.
Solberg HE, Stamm D. International Federation of Clinical Chemistry (IFCC) recommendation: the theory of reference values. Part 4. Control of analytical variation in the production, transfer and application of reference values. J Automat Chem. 1991;13:231–4.
Solberg HE. International Federation of Clinical Chemistry, expert panel on theory of reference values: approved recommendation: on the theory of reference values. Part 5. Statistical treatment of collected reference values: determination of reference limits. Clin Chim Acta. 1987;170:S13–32.
Dybkaer R, Solberg HE. International Federation of Clinical Chemistry (IFCC), expert panel on theory of reference values: approved recommendation (1987) on the theory of reference values. Part 6. Presentation of observed values related to reference values. Clin Chim Acta. 1987;170:S33–42.
National Committee for Clinical Laboratory Standards (NCCLS). How to define and determine reference intervals in the clinical laboratory; approved guideline, 2nd ed, NCCLS document C28-A2. Wayne, PA: NCCLA.
CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline, 3rd ed, CLSI document C28-A3. Wayne, PA: CLSI; 2008.
Yadav D, Mishra S, Gupta M, Sharma P. Reference intervals of certain liver specific biochemical analytes in Indian population. Indian J Clin Biochem. 2011;26:98–9.
Horn PS, Pesce AJ. Effect of ethnicity on reference intervals. Clin Chem. 2002;48:1802–4.
Dosoo DK, Kayan K, Adu-Gyasi D, Kwara E, Ocran J, Osei-Kwakye K, et al. Haematological and biochemical reference values for healthy adults in the middle belt of Ghana. PLoS ONE. 2012;7:e36308.
Johnson AM, Hyltoft Petersen P, Whicher JT, Carlstrom A, MacLennan S. Reference intervals for plasma proteins: similarities and differences between Caucasian and Asian Indian males in Yorkshire, UK. Clin Chem Lab Med. 2004;42:792–9.
Davis BH, Bigelow NC. Performance evaluation of a hematology blood counter with five-part leukocyte differential capability. Am Clin Lab. 1999;18:8–9.
Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49:664–6.
Azikiwe AN. Platelet count values in healthy Nigeria medical students in Jos. East Afr Med J. 1984;61:482–5.
Sundaram M, Mohanakrishnan J, Murugavel KG, Shankar EM, Solomon S, Srinivas CN, et al. Ethnic variation in certain hematological and biochemical reference intervals in a south Indian healthy adult population. Eur J Intern Med. 2008;19:46–50.
Yadav D, Mishra S, Gupta M, John PJ, Sharma P. Establishment of reference interval for liver specific biochemical parameters in apparently healthy north Indian population. Indian J Clin Biochem. 2013;28:30–7.
Das M, Saikia M. Estimation of reference interval of lipid profile in Assamese population. Indian J Clin Biochem. 2009;24:190–3.
Durgawale P, Patil S, Shukla PS, Sontakke A, Kakade S, Yadav S. Evaluation of reference intervals of serum lipid profile from healthy population in western Maharashtra. Indian J Clin Biochem. 2009;24:30–5.
Kaur V, Verma M, Kaur A, Gupta S, Singh K. To establish the reference intervals of lipid profile in Punjab. Indian J Clin Biochem. 2012;27:290–5.
Malati T, Mahesh MRU. To Reference intervals for serum total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, Lp (a), apolipoprotein A-I, A-II, B, C-II, C-III and E in healthy South Indians from Andhra Pradesh. Indian J Clin Biochem. 2009;24:343–55.
Furruqh S, Anitha D, Venkatesh T. Estimation of reference values in liver function test in health plan individuals of an urban south Indian population. Indian J Clin Biochem. 2004;19:72–9.
Verma M, Khadapkar R, Sahu PS, Das BR. Comparing age-wise reference intervals for serum creatinine concentration in a reality check of the recommended cut-off. Indian J Clin Biochem. 2006;21:90–4.
Baliarsingh S, Sharma N. Serum uric acid level is an indicator of total cholesterol and low density lipoprotein cholesterol in men below 45 years in age but not older males. Clin Lab. 2012;58:545–50.
Pasic MD, Colantonio DA, Chan MK, Venner AA, Brinc D, Adeli K. Influence of fasting and sample collection time on 38 biochemical markers in healthy children: a CALIPER substudy. Clin Biochem. 2012;45:1125–30.
Acknowledgments
We thank Prof. Ranjit Roy Chaudhury for his guidance and support. We also thank AHERF research team who helped for data collection and management. Our special thanks to Dr. Prathap C. Reddy, Ms. Shobana Kamineni and Mr. Sridhar for funding the project.
Conflict of Interest
The authors have declared that no competing interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sairam, S., Domalapalli, S., Muthu, S. et al. Hematological and Biochemical Parameters in Apparently Healthy Indian Population: Defining Reference Intervals. Ind J Clin Biochem 29, 290–297 (2014). https://doi.org/10.1007/s12291-013-0365-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-013-0365-5