Abstract
Complement cascade plays an important role in the field of transfusion medicine. The study aimed to detect the complement levels of different blood components and different blood types to explore the risk of transfusion of stored blood. The samples including red blood cells (n = 110), fresh frozen plasma (n = 120), and platelet concentrates (n = 104) from healthy blood donors in our center were collected. Complement components (C3, C4, C3b, C3d, and CH50) were assayed to evaluate the activation of complement. The complement levels of various blood components at different storage times were observed. The differences in complement levels of four blood types in various blood components were compared. The complement levels of red blood cells in storage were low, with no significant changes (P > 0.05). C3b and C3d levels in platelets began to significantly increase after storage for 3 days (P < 0.05). The fresh frozen plasma during storage had higher complement levels, and the concentrations of C3 and C4 decreased and C3b and C3d increased at month 4 (P < 0.05). The differences in complement levels of four blood types in various blood components did not significantly change (P > 0.05), but the C3b and C3d levels of AB fresh frozen plasma remained stable during storage, which different from other blood types. The transfusion of red blood cells was relatively safe in terms of complement activation. The activation of complement proteins occurred during the storage of platelet and plasma, except group AB plasma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are three pathways for complement activation cascade, of which C3 participates in three pathways and C4 participates in common classical pathways. C3b produced during the activation of C3 can trigger the alternative pathway and eventually cleavage into C3dg, C3c and C3d. The complement cascade plays an important role in the transfusion medicine [1, 2]. In addition, complement is also involved in the development of various diseases. Studies have shown that incomplete activation of complement is involved in acute hemolysis by C3b opsonization and mononuclear macrophage-induced phagocytosis of red blood cells (RBCs) in the liver and spleen [3]. The classical complement pathway and terminal pathway are involved to some extent in the immune pathogenesis of autoimmune hemolytic anemia (AIHA). Studies on the etiology and pathogenesis of AIHA and complement destruction on RBCs have expanded [4, 5]. Besides, complement hyperactivation is also involved in the development of various clinical phenotypes of thrombotic microangiopathy [6,7,8].
Long-term storage of blood products is an important step in meeting the needs of clinical. The activation of complement system factor and pathway may occur during preservation of the blood products. Once the activated complement component is introduced into patients, the complement activation cascade can be accelerated through the amplification loop. It is especially serious for patients with complement activation caused by diseases such as malignant tumors. Multiple retrospective and observational studies have shown that the transfusion of older RBCs may increase the adverse clinical outcome of recipients [9, 10]. During storage, changes in the membrane of RBCs may also lead to complement activation [11]. Moreover, long-term storage of plasma can alters some complement components and the levels of complement activation products [12].
Therefore, it is of great clinical significance to determine the level of complement in the blood components and to grasp the changes in its storage period. C3, C3b, C3d, and C4 are the main components involved in the complement activation pathway. Besides, a study have showed that the CH50 can be kept as a routine laboratory procedure to supplement C3 and C4 [13]. This study examined the C3, C3b, C3d, and C4 contents and serum total complement activity (CH50) of the blood components including RBCs, fresh frozen plasma (FFP), and platelet concentrates at different storage time points. Meanwhile, we compared the differences in the complement of the four blood types in each blood component. We aimed to explore the changes in complement levels of each blood component during the storage period, thereby investigating the possible risks of the transfusion of stored blood.
Materials and Methods
The basic study started in January 2018 and ended in December 2018. All blood samples were collected from healthy blood donors at the Clinical Blood Transfusion Center of the Chinese PLA General Hospital. Blood products were collected and stored following the AABB Technical Manual [14]. The study has been approved by the Ethics Committee of the Chinese PLA General Hospital.
Blood Products
Fresh Whole Blood
The acid citrate deoxtrose (ACD)-B blood collection bag purchased from Niagle Biotechnology (Sichuan, China) was used to collect 400 mL the whole blood from healthy blood donors. The collected blood samples from the whole blood included 21 group O, 14 group A, 14 group B and 11 group AB. The complement components were tested immediately after samples retention.
RBCs
RBCs were removed from the whole blood with CPDA-1 as an additive solution (Niagle Biotechnology, Sichuan, China) which had a storage period of 35 days. All units were stored at 4 °C under standard blood banking conditions. A total of 110 RBCs samples including 30 group O, 30 group A, 30 group B and 20 group AB were kept. Approximately 20 mL of blood was collected from each RBC using a new transfer bag under sterile conditions. 2–3 mL of blood was collected from the bags into 5-mL polypropylene tubes on days 1, 7, 14, 21, 28, and 35 for the detection of the complement components.
FFP
FFP were removed from the whole blood and stored at − 40 °C under standard blood banking conditions. A total of 120 plasma samples including 30 group O, 30 group A, 30 group B and 30 group AB were selected. Approximately 20 mL of blood was collected from each plasma using a new transfer bag which finally was divided into 7 bags under sterile conditions. The plasma simples were thawed in a 37 °C water bath before complement determination. The complement components were tested on days 1, 30, 60, 90, 120, 150, and 180.
Platelet Concentrates
Platelet from healthy blood donors were collected using an Amicus blood cell separator (Fresenius, Bad Homburg vor der Höhe, Germany). The platelets were stored in a platelet oscillator at 22 ± 2 °C. A total of 104 platelet samples including 30 group O, 30 group A, 28 group B and 16 group AB were kept. The complement were detected on days 0, 1, 3, and 5.
Complement Assays
The complement biomarkers C3 and C4 (Siemens, Munich, Germany), C3b and C3d (XF Biotech, Shanghai, China), and CH50 (Wako, Tokyo, Japan) were measured by commercial enzyme-linked immunosorbent assay kits. Briefly, all assays were performed according to the manufacturers’ instructions. The samples were tested for supernatant obtained after 3000 rpm/5 min centrifugation. All tests were in accordance with the laboratory operating standards. Two levels of quality controls were included and passed in each assay to ensure quality performance during the entire study.
Statistical Analysis
Data were analyzed with SPSS 22.0 (IBM, Armonk, NY) and presented as the mean and standard deviation (SD) or median with interquartile range. Comparisons among groups with four blood types and different storage times were performed using one-way analysis of variance or Wilcoxon rank-sum test. Pairwise comparison analysis was performed by the t-test and Wilcoxon test. A P value less than 0.05 was considered statistically significant. Graphpad prism 5 was used to create the artwork.
Results
Complement Activation During Blood Component Storage
As shown in Fig. 1, no significant changes in the levels of complement C3, C4, C3b, and C3d during the 35 days storage period of RBCs (P > 0.05). The CH50 value was gradually increased during the 35 days storage period, with values at week 3, 4 and 5 of storage significantly higher than that on day 0 (P < 0.01).
The changes in levels of complement activation product C3 (a), C4 (b), CH50 (c), C3b (d) and C3d (f) during storage of RBC for 5 weeks. The line chart shows mean and SD. Significant differences CH50 between 3w and 0d (P < 0.01), 4w and 0d (P < 0.001) and 5w and 0d (P < 0.001) were observed. There were no differences in C3, C4, C3b, and C3d during the 5 weeks of storage. The normal level of complement: C3: 90–180 mg/dL, C4: 10–40 mg/dL, C3b: 27–158 µg/mL, C3d: 16–105 µg/mL, CH50: 23–46 U/ml. **P < 0.01, ***P < 0.001
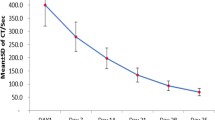
FFP demonstrated a large difference in complement changes within 6 months of preservation (Fig. 2). The complement levels of various blood donors showed large individual differences. The complement C3 and C4 began to significantly increase after 2 months of storage (P < 0.001) and was subsequently reduced to the same level as that on day 0 after 4 months of storage, but was significantly increased again after 5 months of storage (P < 0.001). Nevertheless, they were always lower than the normal human complement level (C3: 90–180 mg/dL, C4: 10–40 mg/dL). Since complements C3b and C3d were products of C3, they decreased with the increase of C3 and increased with the decrease of C3.
The changes in levels of complement activation product C3 (a), C4 (b), CH50 (c), C3b (d) and C3d (f) during storage of plasma for 6 months. The line chart shows median with interquartile range. Significant differences CH50 between 3m and 0d (P < 0.01), 4m and 0d (P < 0.001) and 5m and 0d (P < 0.001) were observed. There were significant differences in C3, C4, CH50, C3b, and C3d during the 6 months of storage. The normal level of complement: C3: 90–180 mg/dL, C4: 10–40 mg/dL, C3b: 27–158 µg/mL, C3d: 16–105 µg/mL, CH50: 23–46 U/ml. **P < 0.01, ***P < 0.001
The complement levels in platelet were relatively stable during the 5 days storage (Fig. 5 in electronic supplementary material). No significant changes in complement C3 and C4 levels were found during the storage period (P > 0.05), and their levels were lower than the normal level. The complement C3b and C3d levels began to significantly increase after 3 days of storage (P < 0.05), but were still lower than the normal level.
Comparison of Complement Activation in Different Blood Groups
As shown in Table 1, significant differences in complement C3 and C4 levels were observed in the four different blood types in fresh whole blood (P < 0.05). The C3 in the group A was significantly lower than the other groups (P = 0.01), whereas the C4 in group AB was significantly higher than the other groups (P = 0.019). However, all complement were within the normal levels.
This study showed significant differences in complement C4, C3d and CH50 among the four different blood types in RBCs (Fig. 3). The complement C4 was relatively high in group O and low in the group A (P < 0.01), while the complement C3d were relatively high in the group B in the 35 days storage (P < 0.01). The CH50 in group AB was significantly higher than the other blood types in preservation from day 0 to week 5 (P < 0.01).
The comparison of four blood groups in levels of complement activation product C3 (a), C4 (b), CH50 (c), C3b (d) and C3d (f) during storage of RBCs for 5 weeks. The line chart shows mean only. There were significant differences in C4, CH50, and C3d among the four blood groups at different storage times. The normal level of complement: C3: 90–180 mg/dL, C4: 10–40 mg/dL, C3b: 27–158 µg/mL, C3d: 16–105 µg/mL, CH50: 23–46 U/ml. *P < 0.05, **P < 0.01, ***P < 0.001
The plasma complement levels of different blood types varied greatly at different storage time points (Fig. 4). The C3 was significantly lower in the group AB on month 3 and higher on month 4 than the other blood types (P < 0.01). The complement C4 of the group AB was significantly higher than the group O and B on month 4 (P < 0.01). Meanwhile, the complement C3b and C3d of the group AB on month 4 was significantly lower than the other blood types (P < 0.01). Importantly, both group A and group B preserved for month 4 had reduced C3 and C4 levels but increased C3b and C3d levels. In contrast, AB-type blood samples had very stable complement levels of C3, C4, C3b and C3d throughout the preservation process.
The comparison of four blood groups in levels of complement activation product C3 (a), C4 (b), CH50 (c), C3b (d) and C3d (f) during storage of plasma for 6 months. The line chart shows mean only. There were significant differences in C3, C4, CH50, C3b and C3d among four blood groups at several different storage times. The normal level of complement: C3: 90–180 mg/dL, C4: 10–40 mg/dL, C3b: 27–158 µg/mL, C3d: 16–105 µg/mL, CH50: 23–46 U/ml. *P < 0.05, **P < 0.01, ***P < 0.001
A small difference in complement levels of the platelet was observed in different groups (Fig. 6 in electronic supplementary material). The C4 in the group B preserved for day 0 and 5 was significantly higher than the other groups (P < 0.01). No significant difference in other complement levels was observed among the different groups (P > 0.05).
Discussion
Deposition of complement components may occur during the storage of blood, further increasing the risk of systemic complications of blood recipients after transfusion.
This study showed a little change in complement levels of RBCs, FFP and apheresis platelet in the storage period, and the overall complement levels were still lower than the normal level. A study of Schleuning et al. [15] showed that C3 and C4 were activated in the whole blood stored in CPDA-1 for 10 days. They suggested that the cause of complement activation may be the degradation of white blood cells which followed by the release of components with potential complement activation properties. In current practice, it is appropriate to store the different components after the separation of whole blood.
RBCs may occur storage lesion during the preservation. A previous study suggested that activation of the complement system occurred during the preservation of RBCs, which may be one of the causes of storage lesion [16]. Another study showed the presence of C3 on the stored RBC membrane and soluble membrane attack complex levels were elevated during the storage of RBCs [17]. However, the levels of C3, C4, C3b, and C3d in the RBCs during the preservation period in this study remained stable. No complement deposition or complement system activation of the stored RBCs were observed. Unfortunately, we did not detect the complement deposition on the RBC membrane. In a recent study, although no complement deposition was found in the stored RBCs, C3 deposition, and IgG binding were observed in the RBCs of some donors after incubating the RBCs with serum [18]. Therefore, differences in some blood donors may lead to complement deposition and cascading of RBCs, which require further validation by tracking patients who receive complement activation of blood components and by measuring the binding of complement and antibody to donor RBCs.
Currently, plasma exchange is an important therapeutic method for autoimmune disease. We must consider the possibility of complement activation in stored plasma, which may also be a cause of adverse reactions to transfusion and increase the risk of disease complications. A study by Hyllner et al. [19] showed a phenomenon of complement activation in stored plasma and many small molecule cleavage fragments produced by complement activation were extensively involved in the immune regulation and inflammatory response in the body. In this study, the levels of C3 and C4 reduced and those of C3b and C3d increased in stored plasma. Although these levels were not much different from normal levels, complement activation in plasma occurred during storage. In addition, this study has shown significant individual differences in the complement levels of different donors, but the changes in each complement during storage are highly consistent. One study suggested that genetic factors and environmental factors may affect blood properties of individual donors. For example, RBCs of some blood donors are more susceptible to storage damage than those from other donors [20]. However, activation of the complement system on the one hand will down-regulate the immune system to improve the disease, on the other hand, it will up-regulate the inflammatory response and aggravate the disease condition. Therefore, further research is needed to evaluate the effects of transfusion of stored plasma with complement activation on patients, especially in plasma exchange patients who received large amounts of stored plasma.
Platelet transfusion is a very important treatment in the clinic. Compared to the other blood products, the incidence of adverse reactions caused by platelet-related transfusion is the highest [21]. Studies have shown that factors involved in the adverse effects of platelet transfusion are biological response modifiers including cytokines, platelet microparticles, soluble CD40 ligands, anti-leukocyte antibodies, and mitochondrial DNA [22,23,24]. However, activation of the complement system may also be one of the contributing factors. In this study, the C3b and C3d levels of platelet after 3 days of storage began to increase. Although the complement components were different, our results were basically consistent with the findings of Chen et al. [25].Therefore, further studies on whether the activation of complement activation in platelet poses a potential risk to the inflammatory response of the patients are necessary.
Although some researchers believe that there are many differences in the complement levels and complement activation of blood components of different blood types, no relevant research has been reported to date. In this study, the complement levels of fresh whole blood of four different blood types were first determined, and showed that complement C3 levels of group A was significantly lower than those of the other groups (P < 0.01). However, complement C4 levels of the group AB was significantly higher than those of the other groups (P < 0.01), even though these levels were all in the normal range. In addition, this study compared the complement activation of different blood components in different blood types. The changes of C3b and C3d of the RBCs and the platelet in four different blood types were basically the same during the blood storage period, whereas the changes in plasma complement composition in the four blood types showed differences. The C3b and C3d levels of the plasma of group AB remained stable at different storage time points, while complement activation was observed in the other three different blood types after 4 months of storage. Therefore, there was no difference between the transfusion risk of RBCs and platelet of different blood types. However, for the FFP, transfusion of group AB plasma may have fewer adverse events caused by complement activation compared with the other three blood types, which requires clinical data for further validation.
In addition, this study determined CH50 as an auxiliary indicator of changes in complement composition. However, significant differences among the three components were observed and some of the specimens even showed zero. We found that a small change of detection could led to significant changes in CH50. Moreover, the environmental factors and systematic errors in the measurement may lead to greater coefficient of variation, suggesting that CH50 should probably not be included as a reference for testing the complement level stability of the three blood components.
In conclusion, the current practice of separating plasma and RBC from whole blood is appropriate, and the transfusion of RBCs is relatively safe in terms of complement activation. Moreover, this study demonstrated the activation of complement proteins during the storage of platelet and plasma, except group AB plasma. Due to the large individual differences in complement levels, further research may be needed in patients receiving blood components with complement activation.
References
Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT (2015) Complement system part I—molecular mechanisms of activation and regulation. Front Immunol 6:262
Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Foumenina LT (2015) Complement system part II: role in immunity. Front Immunol 6:257
Panch SR, Montemayor-Garcia C, Klein HG (2019) Hemolytic transfusion reactions. N Engl J Med 381(2):150–162
Berentsen S, Sundic T (2015) Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy. Biomed Res Int 2015:363278
Berentsen S, Beiske K, Tjønnfjord GE (2007) Primarychronic cold agglutinin disease: an update on pathogenesis, clinical features and therapy. Hematology 12(5):361–370
Karpman D, Tati R (2013) Complement activation in thrombotic microangiopathy. Hamostaseologie 33:96–104
Westwood JP, Langley K, Heelas E, Machin SJ, Scully M (2014) Complement and cytokine response in acute thrombotic thrombocytopenic purpura. Br J Haematol 164:858–866
Jodele S, Licht C, Goebel J, Dixon BP, Zhang K, Sivakumaran TA, Davies SM, Pluthero FG, Lu L, Laskin BL (2013) Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant–associated thrombotic microangiopathy. Blood 122:2003–2007
Purdy FR, Tweeddale MG, Merrick PM (1997) Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth 44:1256–1261
Sanders J, Patel S, Cooper J, Berryman J, Farrar D, Mythen M, Montgomery HE (2011) Red blood cell storage is associated with length of stay and renal complications after cardiac surgery. Transfusion 51:2286–2294
Flatt JF, Bawazir WM, Bruce LJ (2014) The involvement of cation leaks in the storage lesion of red blood cells. Front Physiol. https://doi.org/10.3389/fphys.2014.00214
AABB (2014) Technical manual, 18th edn. AABB Press, Bethesda
Salkie ML, Kelker DH (1981) An investigation into the relationships between serum levels of C3, C4 and CH50. Vox Sang 41(5–6):282–286
Morgan AR, O’Hagan C, Touchard S, Lovestone S, Morgan BP (2017) Effects of freezer storage time on levels of complement biomarkers. BMC Res Notes 10(1):559
Schleunig M, Utz H, Heim M, Mempel W (1992) Decreased formation of the complement component C4ain leukocyte-depleted stored whole blood. Beitrage zur Infusionstherapie 30:182–185
Kamhieh-Milz J, Bartl B, Sterzer V, Kamhieh-Milz S, Salama A (2014) Storage of RBCs results in an increased susceptibility for complement-mediated degradation. Transfus Med 24:392–399
Hu X, Patel RP, Weinberg JA, Marques MB, Ramos TN, Barnum SR (2014) Membrane attack complex generation increases as a function of time in stored blood. Transfus Med 24:114–116
Thielen AJF, Meulenbroek EM, Baas I, Bruggen R, Zeerleder SS, Wouters D (2018) Complement deposition and IgG binding on stored red blood cells are independent of storage time. Transfus Med Hemother 45:378–384
Hyllner M, Arnestad JP, Bengtson JP, Rydberg L, Bengtsson A (1997) Complement activation during storage of whole blood, red cells, plasma, and buffy coat. Transfusion 37(3):264–268
Tzounakas VL, Georgatzakou HT, Kriebardis AG, Voulgaridou AI, Stamoulis KE, Foudoulaki-Paparizos LE, Antonelou MH, Papassideri IS (2016) Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion 56:1274–1286
Oakley FD, Woods M, Arnold S, Young PP (2015) Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion 55:563–570
Hamzeh-Cognasse H, Damien P, Nguyen KA, Arthaud CA, Eyraud MA, Chavarin P, Absi L, Osselaer JC, Pozzetto B, Cognasse F, Garraud O (2014) Immunereactive soluble OX40 ligand, soluble CD40 ligand, and interleukin-27 are simultaneously oversecreted in platelet components associated with acute transfusion reactions. Transfusion 54:613–625
Maurer-Spurej E, Larsen R, Labrie A, Heaton A, Chipperfield K (2016) Microparticle content of platelet concentrates is predicted by donor microparticles and is altered by production methods and stress. Transfus Apheres Sci 55:35–43
Cognasse F, Aloui C, Anh Nguyen K, Hamzeh-Cognasse H, Fagan J, Arthaud CA, Eyraud MA, Sebban M, Fromont E, Pozzetto B, Laradi S, Garraud O (2016) Platelet components associated with adverse reactions: predictive value of mitochondrial DNA relative to biological response modifiers. Transfusion 56:497–504
Chen J, Losos M, Yang S, Li J, Wu H, Cataland S (2017) Increased complement activation during platelet storage. Transfusion 57(9):2182–2188
Acknowledgements
We thank our experimenters in Department of Blood Transfusion, the First Medical Center of Chinese PLA General Hospital for their dedication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RC, YS, YL, HZ, JS and PD. The first draft of the manuscript was written by XL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Chinese PLA General Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X., Cui, R., Song, Y. et al. Changes in Complement Levels and Activity of Red Blood Cells, Fresh Frozen Plasma, and Platelet Concentrates During Storage. Indian J Hematol Blood Transfus 37, 140–146 (2021). https://doi.org/10.1007/s12288-020-01338-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-020-01338-0