Abstract
Background
The specificity of breast MRI is only moderate. The unsatisfactory specificity of breast MRI has prompted evaluation of high signal intensity (SI) on T2-weighted imaging (T2WI). The purpose of the study was to investigate the prevalence of prepectoral edema determined using high SI on T2WI with fat-suppression 3 T MRI and to correlate its presence with prognostic factors of breast cancer.
Methods
The retrospective study comprised 589 consecutive histopathologically confirmed lesions, 460 malignant and 129 benign, identified by 3 T MRI. Presence of prepectoral edema was evaluated on T2WI with fat suppression, and its diagnostic value for malignancies and correlation with clinicopathological findings in histopathologically confirmed breast cancer were assessed.
Results
Prepectoral edema was present in 54 of the 460 breast cancers (9 % of the total 589) and none of the 129 benign lesions. Its sensitivity and specificity were 12 and 100 %, respectively. The positive predictive value was 100 %. Young age (p = 0.01), large tumor size (p < 0.0001), high histological grade (p < 0.0001), invasive ductal carcinoma (p < 0.0001), high lymphovascular invasion degree (p < 0.0001), high axillary lymph node positivity (p < 0.0001), high inflammatory breast cancer rate (p < 0.0001), high neoadjuvant chemotherapy rate (p < 0.0001), and chemoresistant breast cancers (p < 0.0001) were significantly associated with prepectoral edema. There was no association of the morphological lesion type on MRI and dynamic enhancement imaging pattern with the presence of prepectoral edema.
Conclusion
Prepectoral edema has low prevalence but is specific for breast cancer and correlated with prognostic factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, 3.0-T breast MRI has become more widely used in routine clinical settings [1–6]. The signal-to-noise ratios of the 3.0-T MRI unit can result in higher spatial resolution and faster imaging times for breast MRI. Nevertheless, even with the use of 3 T MRI, it is often not possible to differentiate malignant from benign lesions on the basis of kinetic and morphological descriptors alone. The specificity of 3 T breast MRI is only moderate, ranging from 72 to 94 % [1–4]. Recently, the unsatisfactory specificity of breast MRI has prompted evaluation of high signal intensity (SI) on T2-weighted imaging (T2WI) [6–14].
Edema, defined as high SI on T2WI, is a useful breast MRI finding. Prepectoral edema can be particularly valuable because it is a very important MRI finding for inflammatory breast cancer (IBC) [14–17]. Several reports have indicated that prepectoral edema is a specific MRI finding of IBC [14–17], although prepectoral edema is also observed for some locally advanced breast cancers and acute mastitis [15, 16]. Moreover, a recent study reported that the presence of prepectoral edema on T2WI is an important finding that should suggest the diagnosis of occult IBC before pathological examination [18]. No reported studies have addressed its prevalence and predictive value. Therefore, prepectoral edema has not yet been found useful as a diagnostic characteristic on breast MRI.
The purpose of this study was to investigate the prevalence of prepectoral edema determined using high SI on T2WI with fat-suppression 3 T breast MRI and correlate its presence with prognostic factors of breast cancer.
Materials and methods
Patients and lesions
We recorded extensive information from breast MRI examinations in our database according to the BI-RADS MRI lexicon [19]. We also recorded the presence of prepectoral edema as high SI on T2WI. From March 2010 through October 2011, 915 breast MRI at 3-T studies were conducted because of either clinically suspicious findings or mammographically and/or ultrasonographically abnormal findings. The criteria for selection included histopathologically confirmed lesions. On the basis of this criterion, a computer search of the database at our institute found 589 consecutive histopathologically confirmed lesions identified by 3 T breast MRI in 582 patients (6 patients had bilateral breast cancer and 1 patient had bilateral benign lesions; mean patient age was 54 years, range 26–87 years). Histopathological analysis of 589 lesions revealed 460 malignant and 129 benign lesions. The 460 malignant lesions included 342 invasive ductal carcinomas, 17 invasive lobular carcinomas, 78 ductal carcinoma in situ (DCIS), nine mucinous carcinomas, six tubular carcinomas, three medullary carcinomas, two metaplastic carcinomas, one apocrine carcinoma, one invasive micropapillary carcinoma, and one adenoid cystic carcinoma. The 129 benign lesions included 54 fibrocystic changes (mainly ductal hyperplasia without atypia), 45 fibroadenomas, 10 intraductal papillomas, four multiple micropapillomas, three adenomas, three complex sclerosing lesions, two benign phyllodes tumors, two hamartomas, two cases of mastitis, two cases of atypical lobular hyperplasia, one xanthogranuloma, and one granular cell tumor. The histopathological diagnoses of the 589 lesions were established by core biopsy (n = 56), vacuum-assisted biopsy (n = 19), or surgery (n = 514). All diagnostic intervention was performed after MRI examination to avoid confounding features, for example inflammation and hemorrhage. The protocols for this retrospective study were approved by the institutional review board. All patients provided written informed consent for review of their medical records, files, and diagnostic images.
MRI technique
Breast MRI examinations were performed using a 3 T MRI unit (Achieva 3.0 T TX; Philips Medical Systems, Best, The Netherlands). Commercially available dedicated 16-element SENSE-compatible breast surface coils, multiple transmit parallel radiofrequency technology, and sophisticated image-based shimming software were used with the 3 T MRI unit. Our imaging protocol includes a localizing sequence followed by bilateral fast spin-echo T2WI (TR/TE: 6072/90 ms, echo train length = 15, matrix = 400 × 295) with fat suppression by spectral attenuated inversion recovery. Other settings were field of view = 330 mm, section thickness = 3 mm, and interslice gap = 0.6 mm. This examination was followed by combined dynamic contrast-enhanced bilateral axial breast imaging for high spatial and temporal resolution and unilateral sagittal breast imaging for in-plane and through-plane high spatial resolution with an active fat-suppression protocol. The dynamic contrast-enhanced breast MRI protocol consisted of six parts [5, 6]. An enhanced T1-weighted high-resolution isotropic volume examination (eThrive) 3D gradient echo sequence with active fat suppression was performed before and after contrast material injection. The image settings were: TR/TE/FA, 3.4 ms/1.8 ms/10°; FOV, 33 × 33 cm; matrix, 400 × 320; section thickness, 1.8 mm interpolated to 0.9 mm; and acquisition time, 56 s. The temporal resolution was 60 s per dynamic acquisition. The dynamic study in the axial plane was performed before, then 60, 120, and 300 s after, initiation of intravenous injection of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma) at a rate of 2 mL/s, followed by 20-mL saline flush at a rate of 2 mL/s. In addition, contrast-enhanced unilateral sagittal breast imaging studies of the breast on the diseased side and the contralateral breast with an eThrive 3D gradient echo imaging studies with active fat suppression, for in-plane and through-plane high spatial resolution, were performed 180 and 360 s after initiation of the intravenous injection. The image settings were: TR/TE/FA, 3.9 ms/1.9 ms/10°; FOV, 20 × 20 cm; matrix, 256 × 230; section thickness, 1.0 mm interpolated to 0.5 mm; and acquisition time, 119 s. The temporal resolution was 60 s.
MRI interpretation
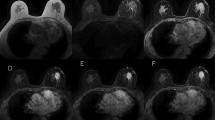
We recorded extensive information from breast MRI examinations in our database. Results were based on data extracted from this clinical database and included detailed imaging characteristics of lesions according to the BI-RADS MRI lexicon [19], presence of prepectoral edema as high SI on T2WI [14–17], and histopathological results. On T2WI, the presence or absence of prepectoral edema, defined as SI similar to that of water, in the area in front of the pectoral muscle, was assessed (Figs. 1, 2) [14–17]. Visual analysis of the time course of enhancement in the three dynamic phases in the axial plane was performed, and findings were categorized according to the BI-RADS MRI lexicon [19]. All breast MRI examinations were prospectively interpreted by an experienced radiologist with 12 years of breast MRI experience, who was informed about the patients’ clinical course and imaging history, including mammograms and ultrasound examinations of the breasts.
40-year-old woman with 87 mm high-grade invasive ductal carcinoma and marked lymphovascular invasion with six positive axillary lymph nodes. a Axial contrast-enhanced MR image in the late dynamic phase shows segmental enhancement in the middle region of the left breast. Skin thickening and skin pathological enhancement are not visualized. b Axial T2-weighted image with fat suppression shows prepectoral edema defined as high signal intensity in the area in front of the left pectoral muscle. Faint subcutaneous edema also is visualized. c Axial contrast-enhanced MR image in the late dynamic phase shows some left axillary lymphadenopathy. d Photomicrograph of a histological specimen shows intraductal component (asterisk) and marked lymphovascular invasion (arrows) of the tumor cells (H&E stain; ×20)
60-year-old woman with 18 mm intermediate-grade invasive ductal carcinoma and marked lymphovascular invasion with one positive axillary lymph node. a Axial contrast-enhanced MR image in the early dynamic phase shows a spiculated enhancing mass in the upper region of the left breast. Skin thickening and skin pathological enhancement are not visualized. b Axial T2-weighted image with fat suppression shows prepectoral edema defined as high signal intensity in the area in front of the left pectoral muscle (arrows). No subcutaneous edema is visualized. c Axial contrast-enhanced MR image in the early dynamic phase shows symmetrically normal axillary lymph nodes (arrows). US axillary examination findings were normal. d Photomicrograph of a histological specimen shows marked lymphovascular invasion (arrows) of the tumor cells (H&E stain; ×10)
Axillary ultrasound examination
Axillary lymph node ultrasonography (US) using 4–14 MHz linear transducer was performed with one of the three US units (LOGIQ 9; GE Medical Systems, Milwaukee, WI, USA; Aplio XG SSA-790A, Toshiba, Tokyo, Japan; or ProSound F75, Hitachi-Aloka, Tokyo, Japan). US was performed by one of four US technologists with 8–10 years of experience in breast US. US results were considered positive if cortical thickening (including loss of hilum) and/or nonhilar blood flow to the cortex were observed. Cortical thickening, either diffuse or focal, was defined as a maximum cortical thickness equal to or greater than the width of the fatty hilum [20]. US findings were interpreted by an experienced radiologist with 20 years of breast US experience and one of four US technologists who did the axillary lymph node US examination.
Pathological examination
Samples for histopathological examination were prepared by making serial 5-mm slices of breast-conserving surgical specimens and 5–10-mm slices of mastectomy specimens. All surgical specimens were sliced perpendicular to an imaginary line from the nipple to the axilla. Histological diagnoses were made by one pathologist with 17 years of experience in breast histology. Hematoxylin and eosin staining was used to assess lymphovascular invasion at the periphery of the tumor as a standard pathological evaluation. Special immunostaining markers were not used to identify endothelial cells as part of the routine pathological evaluation. The degree of lymphovascular invasion was subjectively divided into four grades:
-
ly 0 (no lymphovascular invasion);
-
ly 1 (minimal lymphovascular invasion);
-
ly 2 (moderate lymphovascular invasion); and
-
ly 3 (marked lymphovascular invasion).
For breast cancers resected after neoadjuvant chemotherapy (NAC), the histological therapeutic effects were determined according to the criteria established by the Committee for Production of Histopathological Criteria for Assessment of Therapeutic Response of the Japanese Breast Cancer Society [21]. The histological therapeutic effect was classified into one of four categories:
-
Grade 0, no response or almost no change in cancer cells after treatment;
-
Grade 1, slight response defined as mild changes in cancer cells, irrespective of extent, or marked changes in fewer than one-third of cancer cells, or marked changes in one-third or more but fewer than two-thirds of cancer cells;
-
Grade 2, marked response defined as marked changes in two-thirds or more of tumor cells with apparent remaining cancer cells or marked changes approaching a complete response with only a few remaining cancer cells; and
-
Grade 3, complete response defined as necrosis and/or disappearance of all tumor cells and/or replacement of cancer cells by granulation and/or fibrosis [21].
Breast cancer was divided into two types, according to histological therapeutic effect:
-
chemosensitive breast cancer was defined when the histological therapeutic effect was evaluated as Grade 2 or 3; and
-
chemoresistant breast cancer was defined when the histological therapeutic effect was evaluated as Grade 0 or 1.
Statistical analysis
For analysis of dichotomous variables in different groups, we used the Chi-squared and Fisher exact tests. The Student t test was used to analyze the significance of age and tumor size. The SPSS 11.0 statistical software package (SPSS, Chicago, IL, USA) was used to perform all analyses, and statistical significance was assumed for values of p < 0.05.
Results
Prevalence and predictive value
Prepectoral edema was present in 54 (9 % of total 589 lesions) of the 460 lesions with breast cancer, but in none of the 129 benign lesions. All 54 breast cancers were invasive ductal carcinomas. The resulting sensitivity and specificity of prepectoral edema were 12 and 100 %, respectively. The positive and negative predictive values were 100 and 24 %, respectively.
Clinicopathological findings
Table 1 shows the clinicopathological data for breast cancers with and without prepectoral edema. The mean age of the patients with breast cancers with prepectoral edema was significantly lower than that of the patients with breast cancers without prepectoral edema (p = 0.01). The mean tumor size of breast cancers with prepectoral edema was significantly larger than that of breast cancers without prepectoral edema (p < 0.0001). Breast cancers with prepectoral edema were more likely to be histologically high-grade tumors (43 %: 23/54 vs. 20 %: 81/406, respectively; p < 0.0001) than those without prepectoral edema. Among the pathological subtypes, invasive ductal carcinoma (p < 0.0001) was significantly associated with breast cancers with prepectoral edema. Breast cancers with prepectoral edema were more likely to have marked/moderate lymphovascular invasion (48 %: 26/54 vs. 7 %: 29/406, respectively; p < 0.0001) and axillary lymph node positivity (87 %: 47/54 vs. 23 %: 94/406, respectively; p < 0.0001) than those without prepectoral edema. Of the 47 axillary lymph node-positive cases, 15 (32 %) gave negative US results. Breast cancers with prepectoral edema were more likely to be IBC (p < 0.0001) and to have had NAC (p < 0.0001). Of the 7 marked lymphovascular invasion cases with prepectoral edema, 5 were IBC and 2 were occult IBC. Of the 2 marked lymphovascular invasion cases without prepectoral edema, both had localized marked lymphovascular invasion.
Kinetic and morphological MRI descriptors
There was no association of morphological lesion type on MRI and dynamic enhancement imaging pattern with breast cancers with or without prepectoral edema (Tables 2, 3).
NAC effect
Of the 460 breast cancers, 90 (20 %) had NAC. The histological therapeutic effects showed chemosensitive and chemoresistant breast cancers in 40 % (36/90) and 60 % (54/90; Table 4) of the lesions, respectively. Chemoresistant breast cancers were significantly associated with prepectoral edema (p < 0.0001). Most of the breast cancers with prepectoral edema were chemoresistant (86 %). For breast cancers without prepectoral edema cases, 24 lesions were chemoresistant (44 %) and 31 were chemosensitive (56 %).
Discussion
Edema, defined as high SI on T2WI, is a very important MRI finding, especially for IBC and occult IBC [14–18]. Our study also showed that prepectoral edema was significantly associated with IBC. Previous studies have reported that prepectoral edema was closely related to a dilated lymphovascular system filled with cancer cells in the retromammary area [16, 17]. Although the primary lymphatic drainage of the breast is to the axillary lymph nodes, when it is involved with cancer cells, drainage to the internal mammary nodes and interpectoral nodes can be the main lymphatic drainage route [22]. After involvement with cancer cells, prepectoral edema appears [14, 18]. Lymphovascular invasion has been reported to have prognostic significance because its presence is most likely responsible for lymph node metastasis that is the most important prognostic variable for patients with breast cancer [23]. Lymphovascular invasion is very useful for predicting the pathological response and outcome of patients who receive NAC [24, 25]. In addition, lymphovascular invasion is a risk estimation factor for sentinel lymph node metastasis, but it can be evaluated only after surgery [26]. Our study showed that a high degree of lymphovascular invasion was significantly associated with breast cancers with prepectoral edema. Five out of 26 marked/moderate lymphovascular invasion cases were IBC. Because the remaining 21 marked/moderate lymphovascular invasion cases were not IBC, they could not be precisely estimated as having a marked/moderate degree of lymphovascular invasion before surgery by clinical examination, because IBC is a clinical diagnosis characterized by the presence of such symptoms and signs as erythema, tenderness, edema, pain, increased cutaneous temperature, and breast swelling [18]. However, approximately 10 % of patients do not have clinical signs of IBC, and diagnosis can be made only on the basis of pathologically proved dermal lymphovascular invasion. These cases are called occult IBC [18]. Actually, 5 out of 21 marked/moderate lymphovascular invasion cases were occult IBC in this study. Therefore, our study revealed that some marked/moderate lymphovascular invasion cases cannot show clinical signs of IBC. In addition, our study also showed that the presence of prepectoral edema determined from the T2WI MRI characteristics can indicate marked/moderate lymphovascular invasion cases without clinical signs of IBC. The T2WI MRI findings can yield data that could assist in both pretreatment planning and prognosis for clinically occult marked/moderate lymphovascular invasion cases before surgery.
As a general rule, the field of view is always limited when breast coils are used for axillary evaluation, which can lead to an incomplete evaluation of axillary lymph nodes. Therefore, use of breast MRI with a breast coil for evaluation of axillary lymph nodes remains controversial. However, our study showed that high axillary lymph node positivity was significantly associated with breast cancers with prepectoral edema based on T2WI MRI characteristics. Our study revealed that 15 out of 47 (32 %) axillary lymph node-positive cases were not diagnosed on US examination and all 15 cases were not IBC. Therefore, the 15 cases can be predicted as axillary lymph node-positive on the basis of the presence of prepectoral edema. Thus, breast MRI without complete bilateral assessment of axillary nodes using only breast coils can predict axillary lymph node positivity on the basis of the presence of prepectoral edema determined from T2WI MRI characteristics. Moreover, current conventional anatomical modalities, for example MRI, computed tomography, and US, rely on insensitive size and morphological criteria, and thus, lack the desired accuracy for characterizing lymph nodes. This is mainly because metastases can be present in non-enlarged lymph nodes and not all enlarged nodes are malignant. However, our approach using T2WI MRI characteristics may be a functional and physiological assessment of axillary lymph nodes that can be a new promising functional technique for differentiating metastatic from inflammatory lymph nodes on breast MRI.
Further, our study showed that other clinicopathological findings, for example young age, large tumor size, high histological grade, invasive ductal carcinoma, and high neoadjuvant chemotherapy rate were significantly associated with breast cancers with prepectoral edema. Younger age has been generally accepted as an independent adverse prognostic indicator of survival in breast cancer [27, 28]. Warwick et al. [29] reported that tumor size and histological grade at the time of diagnosis have a lasting effect on subsequent survival. Thus, young age, large tumor size, and high histological grade are prognostic factors used in routine practice. Therefore, breast MRI with T2WI can predict those prognostic factors on the basis of the presence of prepectoral edema. Our study also showed that non-invasive ductal carcinomas were not associated with breast cancers with prepectoral edema. Thus, no association with prepectoral edema in invasive lobular carcinoma and DCIS might be explained by a lower incidence of involved axillary lymph nodes [30]. In addition, our results showed that prepectoral edema can be used to estimate the response of neoadjuvant chemotherapy.
As far as we are aware there have been no published reports addressing the prevalence and predictive value of prepectoral edema on breast MRI. Our study showed that prepectoral edema had a prevalence of 9 %, sensitivity of 12 %, and specificity of 100 %. The positive predictive value was 100 %. Prepectoral edema has limited sensitivity, but very high specificity and positive predictive value.
Chemoresistance was significantly associated with breast cancers with prepectoral edema. Most breast cancers with prepectoral edema were chemoresistant (86 %). Some reports have indicated that breast MRI is the most effective technique for assessing residual disease and monitoring tumor response [31]. However, the main concern is the delay between the MRI findings and treatment response of NAC. Uematsu et al. [32] reported that early MRI prediction of therapeutic efficacy before NAC can assist in both pre-treatment planning and prognosis, and is an effective non-invasive imaging technique. The presence of prepectoral edema based on the baseline T2WI MRI characteristics before NAC also might predict patients who will respond.
There was no association of morphological lesion type on MRI and dynamic enhancement imaging pattern with breast cancers with or without prepectoral edema. Therefore, the presence of prepectoral edema can be an independent breast MRI predictor of malignancy.
Our study had some limitations such as selection and sampling biases. First, there were only two mastitis cases, for which prepectoral edema may be observed [16]. Further studies are needed to analyze the relationship between prepectoral edema and a benign inflammatory breast disease such as acute mastitis. Thus, further validation using larger study populations and prospective studies is warranted. Second, the MR images were only reviewed by one experienced radiologist. Third, the study used only visual assessment of kinetic analysis. A recent study showed diagnostic accuracy of visual assessment, manual region of interest (ROI) assessment, and computer-aided diagnosis (CAD) for kinetic analysis was not based on statistically significant differences [33]. In addition, one advantage of visual assessment versus manual ROI assessment is excellent intra and interobserver reproducibility [34]. ROI assessment is operator and technique-dependent [35, 36]. However, CAD for kinetic analysis may have diagnostic benefit in the evaluation of breast lesions without mass effect [37]. Further validation in prospective studies is thus warranted. Fourth, lymphovascular invasion was assessed by hematoxylin and eosin staining, and the extent of lymphovascular invasion was assessed subjectively. It is ideal to use immunostaining with D2-40 antibodies to confirm that lymphovascular invasion identified by hematoxylin and eosin staining truly involves tumor lymphatic emboli [23]; however, this special immunostaining marker is not used in routine pathological evaluation. The effect of D2-40 antibody immunostaining should be further validated in the future.
In conclusion, prepectoral edema as high SI on T2WI with fat-suppression 3 T breast MRI has low prevalence, but its presence was highly specific for breast cancer. Evaluation of the presence of prepectoral edema is a simple and readily available noninvasive technique for estimation of prognosis for patients with breast cancer. Prepectoral edema is especially indicative of extensive lymphovascular invasion and high axillary lymph node positivity. The presence of prepectoral edema based on the baseline T2WI MRI characteristics before NAC also might predict patients who will respond to treatment.
References
Elsamaloty H, Elzawawi MS, Mohammad S, Herial N. Increasing accuracy of detection of breast cancer with 3-T MRI. Am J Roentgenol. 2009;192:1142–8.
Schmitz AC, Peters NH, Veldhuis WB, et al. Contrast-enhanced 3.0-T breast MRI for characterization of breast lesions: increased specificity by using vascular maps. Eur Radiol. 2008;18:355–64.
Pinker K, Grabner G, Bogner W, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Invest Radiol. 2009;44:553–8.
Pinker-Domenig K, Bogner W, Gruber S, et al. High resolution MRI of the breast at 3 T: which BI-RADS® descriptors are most strongly associated with the diagnosis of breast cancer? Eur Radiol. 2012;22:322–30.
Uematsu T, Kasami M, Yuen S, Igarashi T, Nasu H. Comparison of 3- and 1.5-T Dynamic Breast MRI for Visualization of Spiculated Masses Previously Identified Using Mammography. Am J Roentgenol. 2012;198:W611–7.
Uematsu T, Kasami M. High-spatial-resolution 3-T breast MRI of nonmasslike enhancement lesions: an analysis of their features as significant predictors of malignancy. Am J Roentgenol. 2012;198:1223–30.
Baltzer PA, Dietzel M, Kaiser WA. Nonmass lesions in magnetic resonance imaging of the breast: additional T2-weighted images improve diagnostic accuracy. J Comput Assist Tomogr. 2011;35:361–6.
Baltzer PA, Yang F, Dietzel M, et al. Sensitivity and specificity of unilateral edema on T2w-TSE sequences in MR-mammography considering 974 histologically verified lesions. Breast J. 2010;16:233–9.
Yuen S, Uematsu T, Kasami M, et al. Breast carcinomas with strong high-signal intensity on T2-weighted MR images: pathological characteristics and differential diagnosis. J Magn Reson Imaging. 2007;25:502–10.
Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009;250:638–47.
Kawashima H, Kobayashi-Yoshida M, Matsui O, Zen Y, Suzuki M, Inokuchi M. Peripheral hyperintense pattern on T2-weighted magnetic resonance imaging (MRI) in breast carcinoma: correlation with early peripheral enhancement on dynamic MRI and histopathologic findings. J Magn Reson Imaging. 2010;32:1117–23.
Malich A, Fischer DR, Wurdinger S, et al. Potential MRI interpretation model: differentiation of benign from malignant breast masses. Am J Roentgenol. 2005;185:964–70.
Dietzel M, Baltzer PA, Vag T, et al. Application of breast MRI for prediction of lymph node metastases—systematic approach using 17 individual descriptors and a dedicated decision tree. Acta Radiol. 2010;51:885–94.
Uematsu T. MRI findings of inflammatory breast cancer, locally advanced breast cancer, and acute mastitis: T2-weighted images can increase the specificity of inflammatory breast cancer. Breast Cancer. 2012;19:289–94.
Renz DM, Baltzer PA, Böttcher J, et al. Inflammatory breast carcinoma in magnetic resonance imaging: a comparison with locally advanced breast cancer. Acad Radiol. 2008;15:209–21.
Renz DM, Baltzer PA, Böttcher J, et al. Magnetic resonance imaging of inflammatory breast carcinoma and acute mastitis: a comparative study. Eur Radiol. 2008;18:2370–80.
Yasumura K, Ogawa K, Ishikawa H, Takeshita T, Nakagawa Y, Osamura RY. Inflammatory carcinoma of the breast: characteristic findings of MR imaging. Breast Cancer. 1997;4:161–9.
Uematsu T, Kasami M, Watanabe J. Can T2-weighted 3-T breast MRI predict clinically occult inflammatory breast cancer before pathological examination? A single-center experience. Breast Cancer. 2012. [Epub ahead of print].
American College of Radiology. Breast imaging reporting and data system (BI-RADS). 4th ed. Reston: American College of Radiology; 2003.
Abe H, Schmidt RA, Kulkarni K, Sennett CA, Mueller JS, Newstead GM. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-gauge core-needle biopsy—clinical experience in 100 patients. Radiology. 2009;250:41–9.
Kurosumi M, Akashi-Tanaka S, Akiyama F, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer. 2008;15:5–7.
Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004;239:232–7.
Hasebe T, Yamauchi C, Iwasaki M, Ishii G, Wada N, Imoto S. Grading system for lymph vessel tumor emboli for prediction of the outcome of invasive ductal carcinoma of the breast. Hum Pathol. 2008;39:427–36.
Uematsu T, Kasami M, Watanabe J, et al. Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast Cancer. 2011;18:309–13.
Tamura N, Hasebe T, Okada N, et al. Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer Sci. 2009;100:1823–33.
Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25:3670–9.
Xiong QH, Valero V, Kau V, et al. Female patients with breast carcinoma age 30 years and younger have a poor prognosis—the M. D. Anderson Cancer Center experience. Cancer. 2001;92:2523–8.
Yildirim E, Dalgic T, Berberoglu U. Prognostic significance of young age in breast cancer. J Surg Oncol. 2000;74:267–72.
Warwick J, Tabar L, Vitak B, Duffy SW. Time-dependent effects on survival in breast carcinoma: results of 20 years of follow-up from the Swedish Two-County Study. Cancer. 2004;100:1331–6.
Vandorpe T, Smeets A, Van Calster B, et al. Lobular and non-lobular breast cancers differ regarding axillary lymph node metastasis: a cross-sectional study on 4,292 consecutive patients. Breast Cancer Res Treat. 2011;128:429–35.
Weinstein S, Rosen M. Breast MR imaging: current indications and advanced imaging techniques. Radiol Clin N Am. 2010;48:1013–42.
Uematsu T, Kasami M, Yuen S. Neoadjuvant chemotherapy for breast cancer: correlation between the baseline MR imaging findings and responses to therapy. Eur Radiol. 2010;20:2315–22.
Baltzer PA, Freiberg C, Beger S, et al. Clinical MR-mammography: are computer-assisted methods superior to visual or manual measurements for curve type analysis? A systemic approach. Acad Radiol. 2009;16:1070–6.
Kinkel K, Helbich TH, Esserman LJ, et al. Dynamic high-spatial-resolution MR imaging of suspicious breast lesions: diagnostic criteria and interobserver variability. Am J Roentgenol. 2000;175:35–43.
Mussurakis S, Buckley DL, Coady AM, Turnbull LW, Horsman A. Observer variability in the interpretation of contrast enhanced MRI of the breast. Br J Radiol. 1996;69:1009–16.
Mussurakis S, Buckley DL, Horsman A. Dynamic MRI of invasive breast cancer: assessment of three region-of-interest analysis methods. J Comput Assist Tomogr. 1997;21:431–8.
Vag T, Baltzer PA, Dietzel M, et al. Kinetic analysis of lesions without mass effect on breast MRI using manual and computer-assisted methods. Eur Radiol. 2011;21:893–8.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Uematsu, T., Kasami, M. & Watanabe, J. Is evaluation of the presence of prepectoral edema on T2-weighted with fat-suppression 3 T breast MRI a simple and readily available noninvasive technique for estimation of prognosis in patients with breast cancer?. Breast Cancer 21, 684–692 (2014). https://doi.org/10.1007/s12282-013-0440-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-013-0440-z