Abstract
Background
Circulating tumor cells (CTCs) are detected in peripheral blood of breast cancer patients, and they may play an important role as a prognostic and predictive marker. We conducted this study to determine the presence of CTCs with the CellSearch System™ and the clinical significance in treatment of metastatic breast cancer (MBC).
Method
Twenty-eight MBC patients were enrolled. These patients were followed by assessing CTCs, imaging studies, and serum tumor markers. Blood samples were collected before starting a new treatment and at the treatment evaluation period (2–3 months after starting chemotherapy). The cutoff for CTC level was 5.
Results
At baseline, 9 of 28 patients (32%) had ≥5 CTCs per 7.5 mL of blood. At the evaluation period, 5 of 23 patients (22%) had ≥5 CTCs. The baseline CTC number did not contribute to determine their overall survival (OS); however, CTCs at the evaluation period were available to predict their OS (p < 0.001). In two cases, both CTCs and tumor markers were available as predictors of treatment efficacy. In two other cases, although alterations of tumor markers might not reflect disease condition, CTC alteration corresponded to their condition. One patient who had multiple skeletal metastasis only, experienced a decrease in her CTCs in spite of tumor marker alteration.
Conclusions
We suggest that monitoring the number of CTCs may be helpful in predicting the efficacy of the treatment and the prognosis. CTCs might be especially useful with patients whose lesions are difficult to assess.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequent type of cancer in women. In Japan, approximately 50,000 women are newly diagnosed with breast cancer each year, and 10,000 patients die of breast cancer every year. Recently, many chemotherapeutic agents, endocrine therapy, and targeted therapy have been introduced to treat metastatic breast cancer patients, but the curability rate is very low. The aim of treatment is to improve the patient’s quality of life, not to cure completely. Therefore, useful prognostic and predictive markers would help to select an appropriate treatment modality to obtain maximum treatment effects and minimize unnecessary side effects.
Circulating tumor cells (CTCs) are detected in peripheral blood of cancer patients, and their existence has been known for a long time [1]. Although the source of CTCs is unknown and the clinical significance is not yet established, it is reported that circulating epithelial cells in breast cancer patients are malignant, that the cells are derived from clones in the primary tumor site which suggests that they may reflect the tumor burden at all stages of tumor progression [2], and that CTCs could be scattered seeds to develop distant metastasis. The presence of CTCs may predict the presence of micrometastasis or of aggressive primary tumors. Some reports have related the existence of CTCs to shorter survival time and progression-free survival [3, 4]. In short, CTCs might be important as a prognostic and predictive marker. Cristofanilli et al. [3] described a cutoff of 5 CTCs per 7.7 mL of blood to distinguish patients with a favorable prognosis.
We conducted a small study to determine the presence of CTCs and the clinical significance in treatment of metastatic breast cancer patients in our institution.
Materials and methods
Patients
A total of 28 metastatic breast cancer patients were enrolled. For all patients, the Eastern Cooperative Oncology Group score of performance status was 0–1. The institutional review board approved this study protocol, and all patients provided written informed consent. These patients were followed by assessing CTCs, imaging studies, and blood chemistry (especially tumor markers). Blood samples were collected before starting a new treatment and at the treatment evaluation period (2–3 months after starting chemotherapy). At the same points, imaging studies including computed tomography (CT), tumor markers, carcinoembryonic antigen (CEA), and carbohydrate antigen 15-3 (CA15-3) were also evaluated.
Sample preparation and sample analysis
Of the several approaches to detecting CTCs, we employed The CellSearch System™ (Immunicon Corp., Huntington Valley, PA, USA). First, blood samples were drawn into 10-mL EDTA Vacutainer tubes (Becton–Dickinson, Franklin Lakes, NJ, USA), to which a cell preservative was subsequently added. Samples were maintained at room temperature and processed within 72 h after collection. The CellSearch System™ includes the CellPrep system, the CellSearch Epithelial Cell Kit, and the CellSpotter Analyzer. The CellPrep system is a semiautomated sample preparation system, and the CellSearch Epithelial Cell Kit contains ferrofluids coated with epithelial cell specific EpCAM (epithelial cell adhesion molecule) antibodies to immunomagnetically enrich epithelial cells. Isolated cells were fluorescently labeled with nucleic acid dye 4′,6-diamidino-2-phenylindole (DAPI) and monoclonal antibodies specific for leukocytes (CD45-allophycocyanin), and epithelial cells (cytokeratin 8,18,19-phycoerythrin), and were then put through the CellSpotter Analyzer (Veridex LLC, Warren, NJ, USA). The CellSpotter Analyzer reveals images of candidate CTCs in a sample. To qualify as a CTC, a cell must be round or oval with a nucleus (as determined by positive DAPI staining) contained within the cytoplasm (negative CD45-allophycocyanin staining, positive cytokeratin 8,18,19-phycoerythrin staining). As a characteristic of this system, nonviable cells are removed in counting the CTCs, thereby reducing false positive cells. Finally, results are always expressed as the number of cells per 7.5 mL of whole blood. To ensure reproducibility, these processes were performed two times by two different technical experts.
Study analysis
The objective of this study was to evaluate the prediction of response to therapy with the CTCs and progression-free survival (PFS) and overall survival (OS) as patient’s prognosis. PFS was defined as the period between the date when the treatment was started and tumor progression or stopping treatment because of severe adverse events and a patient’s unfavorable condition, and OS was the period until death. Statistical analysis was performed using SPSS 17.0 (Chicago, IL, USA). Survival distributions were estimated with the Kaplan–Meier method, and the log-rank statistic was used to test for difference groups. All p values were two-sided, and p < 0.05 was considered significant. Tumor response was evaluated after 2 or 3 months for measurable or evaluable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST).
Results
Patient characteristics
The average age of the 28 metastatic breast cancer patients enrolled was 54.5 years (range 37–73). Fifty-four percent of the patients were positive for estrogen receptors (ER) and/or progesterone receptors (PgR). Twenty-nine percent of the tumors were HER2/neu overexpressed or amplified (immunohistochemistry (IHC) 3+ or fluorescence in situ hybridization (FISH)+) (Table 1). In this study, 82% of patients were starting a regimen containing vinorelbine: either vinorelbine monotherapy for HER2/neu negative patients, or vinorelbine and trastuzumab combination therapy for HER2/neu positive patients. Almost all patients (82%) had been heavily pretreated as the 3rd line treatment or more (Table 1). Except for lymph nodes, lung was the major metastatic site, and 43% of patients had three or more metastatic sites (Table 1). Before evaluation, six patients were withdrawn. Two of them changed hospitals, three died, and one had unexpected severe cardiac symptoms and stopped her treatment. Therefore, their CTC samples could not be collected.
Number of CTCs and clinical efficacy
The number of patients who had at least one CTC was 15 (53.6%) out of 28. The range of CTC count was 0–223. In this study, five or more CTCs were regarded as positive in accordance with a previous report [3]. At baseline, nine of 28 patients (32.1%) had five or more CTCs per 7.5 mL of blood. Five of 22 patients (22.7%) had five or more CTCs at the evaluation period (Table 2).
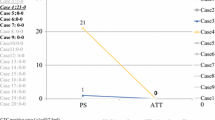
According to the clinical efficacy determined with the radiodiagnostic procedure at the evaluation period, only one patient (3.6%) had complete response (CR), five patients (17.9%) had partial response (PR), seven patients (25.0%) had stable disease (SD), 14 (50%) patients had progressive disease (PD), and one patient (3.6%) was not evaluated. Unfortunately, CTCs were not detected in the CR patient. Although with the PD case, the positive rate of CTCs increased between baseline and the treatment evaluation period, with the PR and SD cases, this rate decreased (Table 2). Figure 1 shows that, especially for patients whose baseline CTCs number was above the cutoff, with one PR patient the CTCs disappeared, two of three SD patients’ CTCs dropped below the cutoff, and one PD patient’s CTCs level increased. In contrast, one PD patient had CTCs below the cutoff at first, but the number of CTCs had increased at the evaluation period.
Alterations of the number of CTCs between baseline and evaluation period. Of the patients whose baseline CTCs number was above the cutoff, one PR patient’s CTCs disappeared, two of three SD patients’ CTCs dropped below the cutoff, and one PD patient’s CTCs level increased. In contrast, one PD patient had CTCs below the cutoff at first, but the number of CTCs had increased at the evaluation period
Among all positive CTC patients at baseline (n = 9), except for 2 patients whose CTCs were not collected at the evaluation period, CTCs of all ER and/or PgR positive patients (n = 4) did not decrease below the cutoff, and three of these four patients’ disease progressed. By contrast, CTCs of all ER and PgR negative patients (n = 3) decreased below the cutoff at the evaluation period, and their disease did not become PD.
Overall survival
For all 28 patients, the median OS was approximately 23.8 months (1.3–44.7), and the median OS of the patients who had positive and negative CTCs at baseline was 12.7 (1.3–44.7) and 29.1 (2.6–44.2) months respectively. The median OS of the patients who had positive and negative CTCs at the evaluation period was 4.7 (3.4–21.4) and 37.9 (16.8–44.7) months respectively. Figure 2 shows Kaplan–Meier curves of OS according to the number of CTCs at baseline and the evaluation period. According to these curves, baseline CTCs did not contribute significantly to determine the prognosis (p = 0.477). On the other hand, the patients with negative CTCs at evaluation had a better prognosis than positive CTCs patients (p < 0.001).
Progression-free survival
For all 28 patients, the median PFS was approximately 5.2 months (0.2–44.2), and the median PFS of the patients who had positive and negative CTCs at baseline was 1.9 (0.3–33.9) and 5.2 (0.2–44.2) months respectively. The median PFS of the patients who had positive and negative CTCs at the evaluation period was 1.9 (1.1–6.8) and 8.3 (0.2–44.2) months respectively. Figure 3 shows Kaplan–Meier curves of PFS according to the number of CTCs at baseline and the evaluation period. According to these curves, the baseline CTC level did not contribute to predict the treatment efficacy (p = 0.542). However, the patients with negative CTCs at evaluation had a better prognosis than positive CTCs patients (p = 0.002).
Kaplan–Meier estimates of probabilities of progression-free survival for those positive and negative CTCs at baseline and evaluation period. Baseline CTC level did not contribute to predict treatment efficacy. Patients with negative CTCs at evaluation had a better prognosis than positive CTCs patients
Alterations of CTCs level between pre- and post-treatment
In addition, although the correlations between the PFS and the difference of CTC numbers between baseline and the evaluation period were inconsistent, some patients whose pre-treatment CTC number was greater than their post-treatment CTCs number had longer a PFS (Fig. 4). In Fig. 5, the patients who had positive CTCs during treatment had a worse PFS and OS than patients whose CTCs level decreased below the cutoff between the pre- and post-treatment period.
Correlation of progression-free survival and the difference of CTC numbers between baseline and evaluation period. Although correlations between PFS and the difference of CTC numbers between baseline and evaluation period were inconsistent, some patients whose pre-treatment CTC number was greater than post-treatment CTCs had longer PFS
Kaplan–Meier estimates of probabilities of progression-free survival and overall survival for those with positive CTCs at both pre- and post-treatment and those positive with CTCs changed to negative at evaluation period. Patients who had positive CTCs during treatment had worse PFS than patients whose CTCs level changed to negative during treatment. Patients who had positive CTCs during treatment had worse OS than patients whose CTCs level decreased below the cutoff during treatment
Examples
Figures 6, 7, and 8 reveal the alterations of CTCs and tumor markers (CEA, CA15-3) of typical patients between baseline and the treatment evaluation period. Figure 6 shows that in case A (PD patient) and case B (PR patient), both CTCs and tumor markers altered according to the patients’ condition. In Fig. 7, although alterations of tumor markers did not reflect the patient’s condition, CTC alteration corresponded to each patient’s condition. With case C, though her metastatic sites shrank, tumor markers did not change. On the other hand, her CTC level decreased; therefore CTC alteration was suitable to evaluate efficacy. In case D, in spite of decreased tumor markers, the CTC level increased. Four months later her metastatic sites progressed.
Case C A patient whose metastatic sites showed a tendency to shrink, tumor markers did not change. On the other hand, her CTC level decreased; therefore CTC alteration was suitable to evaluate efficacy. Case D In spite of decreased tumor markers, the CTC level increased. Four months later, her metastatic sites progressed
In case E who had multiple skeletal metastasis only, both CTCs and tumor markers decreased and the patient had a long SD (SD more than 6 months) with treatment (Fig. 8).
Discussion
CTCs have been detected in the peripheral blood of all major carcinomas, such as prostate, breast, colorectal, and lung. Fehm et al. [2] reported that CTC chromosomal abnormalities resembled those in primary epithelial cancer lesions, indicating that the CTCs were derived from the tumor sites. Furthermore, the number of CTCs in the blood of healthy control and nonmalignant disease is extremely low [5], as confirmed more recently by many studies on the various methods used to detect CTCs. As for biological techniques, immunomagnetic isolation, flow cytometry, immunofluorescent microscopy, reverse transcriptase-polymerase chain reaction (RT-PCR), polymerase chain reaction (PCR), and fluorescence in situ hybridization (FISH) are used to achieve high specificity and accuracy. For example, elevated CTCs were found in 50–75% of metastatic breast cancer patients by using either immunofluorescent approach or RT-PCR methods [6].
The CellSearch System™ contains immunomagnetic and immunofluorescent microscopic procedures to identify CTCs with high sensitivity and specificity and has been approved for clinical use the by Food and Drug Administration (FDA). This system is semiautomated to be highly reproducible, enabling the enumeration and characterization of CTCs [5]. It appears to provide reasonable prognostic and predictive utility in metastatic breast cancer. Commonly, clinical features including the time to first recurrence, metastatic sites, number of lesions, and tumor burden are used as prognostic factors, whereas hormone receptor status and HER2 status are usually used as predictive factors. In addition to these tools, Cristofanilli et al. described in their use of a cutoff level of CTC defined as 5 per 7.5 mL of blood [3]. The presence of more than 5 CTCs before initiation of treatment was associated with a short PFS and OS, and predicted a poorer outcome than for patients who have less than 5 CTCs detected. Similarly, the CTC level at first follow-up also predicted the treatment efficacy, PFS, and OS [3, 4]. In short, more than 5 CTCs after the initiation of treatments predicted no therapeutic benefit from the treatments. Furthermore, Hayes et al. [7]. reported that continuous detection of more than 5 CTCs at any time during therapy accurately indicated subsequent treatment failure and mortality. Although the present small study could not demonstrate that patients who initially had 5 or more 5 CTCs were associated with a worse prognosis, these data suggest that the CTC status after the treatment may be a prognostic marker. In addition, CTCs were useful to estimate treatment efficacy as a predictive marker. These results suggest that following up the number of CTCs may contribute to predict the efficacy of the treatment like tumor markers.
A subsequent report showed that CTC enumeration might provide an earlier, more reproducible indication of disease status than imaging examination. In particular, the prognosis of radiologically responding patients (stable disease and partial response) was divided into good and unfavorable prognosis groups according to the number of CTCs (5 or more, less than 5). Similarly, radiologically non-responding patients (progressive disease) were also divided into these two groups depending on the number of CTCs [8]. Furthermore, in this report, obvious radiological disease progression patients with more than 5 CTCs demonstrated a significantly worse prognosis than the patients whose CTCs level was fewer than 5.
Commonly, for monitoring patients with metastatic disease during therapy, some tumor markers can be used in conjunction with diagnostic imaging and physical examination. CA15-3 and CEA are often used in monitoring a patient’s condition. However, during the first few weeks of new treatment, these serous tumor marker levels sometimes rise temporarily, and we experience difficulties in evaluating the therapeutic response [6]. As mentioned above, although alterations of tumor markers sometimes did not reflect disease condition, CTC alteration corresponded to each disease condition [9]. In our study and previous reports, CTCs may be usable as a kind of tumor marker or surrogate marker to avoid unnecessary toxic therapy and help us develop better therapeutic strategies for our patients. After a few cycles or treatment, patients with elevated CTCs would be assigned to either the same treatment until clinical disease progression or switched to the next chemotherapeutic agent(s).
On the other hand, it is often difficult to judge treatment efficacy in the absence of measurable disease, especially with patients who have only skeletal metastasis. Usually, skeletal metastatic sites of breast cancer contain both osteolytic and osteoblastic changes. If treatment has been effective, the cancer nests sometimes reveal osteoplastic change. It is difficult to judge by radiography whether osteoblastic change is due to disease progression or to treatment efficacy. Therefore, in addition to other tumor markers, CTCs might be a useful predictor of treatment efficacy, especially with the patients whose lesions are difficult to assess.
According to the American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer, the measurement of CTCs should not be used to diagnose breast cancer or to influence any treatment decision in patients with breast cancer [10]. Similarly, the use of the recently FDA-cleared test for CTC (CellSearch Assay) in patients with metastatic breast cancer cannot be recommended until further validation confirms the clinical value of this test. Therefore, a prospective trial should be held with metastatic breast cancer patients.
References
Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–9.
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res. 2002;8:2073–84.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Clistofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–30.
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904.
Hayes DF, Smerage J. Is there a role for circulating tumor cells in the management of breast cancer? Clin Cancer Res. 2008;14:3646–50.
Hayes DF, Clistofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24.
Budd GT, Clistofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging- predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9.
Terstappen LWMM, Rao C, Gross S, Weiss AJ. Peripheral blood tumor cell load reflects the clinical activity of the disease in patients with carcinoma of the breast. Int J Oncol. 2000;17:573–8.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
Acknowledgment
We greatly appreciate our laboratory staff, Ms. Shibata and Ms. Minowa, who supported our study by searching for and counting numerous CTC numbers.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tokudome, N., Ito, Y., Takahashi, S. et al. Detection of circulating tumor cells in peripheral blood of heavily treated metastatic breast cancer patients. Breast Cancer 18, 195–202 (2011). https://doi.org/10.1007/s12282-011-0259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-011-0259-4