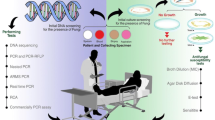
Abstract
Purpose of Review
The incidence of invasive aspergillosis has increased substantially over the past few decades, accompanied by a change in susceptibility patterns of Aspergillus fumigatus with increasing resistance observed against triazole antifungals, including voriconazole and isavuconazole, the most commonly used antifungal agents for the disease. Culture-based methods for determining triazole resistance are still the gold standard but are time consuming and lack sensitivity. We sought to provide an update on non-culture-based methods for detecting resistance patterns to Aspergillus.
Recent Findings
New molecular-based approaches for detecting triazole resistance to Aspergillus, real-time polymerase chain reaction (PCR) to detect mutations to the Cyp51A protein, have been developed which are able to detect most triazole-resistant A. fumigatus strains in patients with invasive aspergillosis.
Summary
Over the last few years, a number of non-culture-based methods for molecular detection of Aspergillus triazole resistance have been developed that may overcome some of the limitations of culture. These molecular methods are therefore of high epidemiological and clinical relevance, mainly in immunocompromised patients with hematological malignancies, where culture has particularly limited sensitivity. These assays are now able to detect most triazole-resistant Aspergillus fumigatus strains. Given that resistance rates vary, clinical utility for these assays still depends on regional resistance patterns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections caused by Aspergillus fumigatus (A. fumigatus) affect patients with immune impairment, including those with hematologic malignancies and those with pre-existing lung conditions or liver cirrhosis, and are associated with devastating mortality rates [1••, 2••, 3]. Early and reliable diagnosis of invasive aspergillosis (IA) and subsequent rapid initiation of appropriate antifungal therapy has been shown to improve survival significantly [4, 5•]. However, IA progresses rapidly and is difficult to diagnose at early stages [6, 7•], and once diagnosed, emergence of triazole resistance complicates selection of appropriate antifungal treatment [8, 9, 10••].
Optimally, diagnostic tests for IA would therefore detect not only the presence or evidence of invasive growth of Aspergillus spp. but also triazole resistance. While most cases of IA are now diagnosed by fungal biomarkers, including galactomannan and 1,3-β-d-glucan [4, 11,12,13], these fail to provide information on antifungal susceptibility of the causative pathogen. Culture-based approaches have the potential to both detect the causative fungal species and detect resistance. However, culture-based approaches are limited by low sensitivities—in particular during early phases of infection—and long turnaround time [11]. Importantly, triazole resistance in A. fumigatus is caused by point mutations in certain genes, such as the cyp51A gene, which encodes the triazole target enzyme required for the biosynthesis of ergosterol [14]. These point mutations may be detected via molecular methods, overcoming the limitations of low sensitivity and long turnaround time that affect culture-based methods and—potentially—improving clinical management and survival in patients infected with triazole resistant A. fumigatus. Several polymerase chain reaction (PCR) assays have been developed that can detect triazole resistance mechanisms [15,16,17,18,19,20], and two are commercially available, AsperGenius® (PathoNostics, Maastricht, the Netherlands) and MycoGenie® (Adamtech, Pessac, France). In this review, we will discuss established and new methods for detection of A. fumigatus resistance in clinical samples.
Culture-Based Methods for Detection of Resistance in Aspergillus fumigatus
Susceptibility patterns of A. fumigatus to the most commonly used antifungal agents have changed over recent years, some of which may also be attributed to increased environmental use of azole fungicides [21] given their relatively low cost and effectiveness against a broad range of fungi [22]. For instance, in the European Union, half of all cereals and grapevine are treated with fungicides, including triazoles [23], and it is estimated that if azoles were no longer used in Europe, a significant fall in wheat production would result with an economic loss in the billions of Euros [22]. Thus, in vitro susceptibility testing for initial antifungal selection and monitoring while on antifungal therapy has become increasingly important.
In addition, antifungal susceptibility testing is important in monitoring background resistance and when comparing the in vitro activity of new antifungal agents to existing agents. Increasing resistance of A. fumigatus against triazoles is being documented, including resistance to isavuconazole, the newest triazole which was recently approved for first line treatment of IA [24]. Isavuconazoe resistance has been emerging also among non-A. fumigatus species [25] and even among wild-type isolates [26],
Collaboration between investigators and the Clinical and Laboratory Standards Institute (CLSI) Subcommittee on Antifungal Susceptibility Testing has generated consensus documents detailing standardized methods for broth- and agar-based antifungal susceptibility testing [27]. A number of culture-based diagnostic tests exist to detect antifungal resistance, and we describe the most commonly used methods here.
Broth and Agar Dilution Methods
Dilution methods using broth and agar are considered the reference standard methods for determining the minimum inhibitory concentration (MIC) of antibiotic and antifungal agents. During antifungal testing with broth microdilution, varying concentrations of antifungal agents are placed in microdilution plate wells filled with broth culture media containing serial dilutions of antifungal agents. Following incubation, the plates are removed and examined for fungal growth, indicated by the presence of cloudy broth. The MIC to the antifungal agent is defined as the lowest concentration, in milligrams per liter, of the agent that inhibits growth of the fungus. In agar dilution, varying concentrations of antifungal agents are combined with melted agar to produce plates with varying serial dilutions of the antifungal agents being tested. A. fumigatus is added to spots on the plate, and following incubation, the plates are examined to determine if growth has occurred at the inoculated spots. The lowest concentration of antifungals that prevent A. fumigatus growth is considered to be the MIC of the antifungal agent. Although these methods are labor-intensive and expensive, they are the most frequently used method to determine the efficacy of new antibiotic or antifungal agents.
Etest® Method
Etest® (Biomérieux, Marcy-l’Étoile, France) is a commercially available, pre-formed test that consists of a predefined gradient of antibiotic concentrations on a plastic strip. After placement of the test strip and incubation of A. fumigatus on an agar plate for 24 to 48 h, an ellipse of growth inhibition occurs that can be used to determine the minimum inhibitory concentration (MIC) of the antifungal agents being tested. This method can be used to determine in vitro activity of a variety of antifungal agents including amphotericin B, flucytosine, caspofungin, and a number of triazoles including voriconazole and posaconazole. While not approved for use by the U.S. Food and Drug Administration (FDA) in the detection of antifungal resistance by filamentous fungi, including A. fumigatus, it is still a commonly used test and results have been shown to be general agreement with the broth dilution reference method for determining susceptibility to amphotericin B [28, 29], itraconazole [29], posaconazole [28, 30, 31], isavuconazole [32, 33], and voriconazole [34], although correlation between broth dilution and caspofungin has proven to not be as robust [35].
Sensititre® Method
Sensititre® (TREK Diagnostic Systems, West Sussex, UK) is a commercially available colorimetric microdilution test that is used for susceptibility testing of yeast and fungi including Candida species, Cryptococcus species, and Aspergillus species. Similar to the broth microdilution method, individual plates are dosed with varying concentrations of antifungal agents as well as a colorimetric indicator. Unlike the broth dilution method, growth or lack of fungal growth is determined by color change rather than presence of turbidity in the plates. Following incubation for 24 h, MIC results are read at the first well that demonstrates a color change of red (indicating growth), purple (inhibition of growth), and blue (no growth). Thus, the lowest MIC is interpreted as the plate with the lowest concentration of antifungal agent that still retains a blue color. This method can be used to determine antifungal activity of amphotericin B, flucytosine, a number of echinocandin agents, and a number of triazoles including voriconazole and posaconazole. Compared to the Etest® method and broth dilution, the Sensititre® method has similar efficacy in determining susceptibility to amphotericin B and itraconazole against Aspergillus spp. in one study [29] but was inferior to the Etest® in another [36, 37].
MALDI-TOF MS Method
Newer approaches for identifying pathogens and performing antimicrobial and antifungal testing, such as matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), are being explored. By analyzing the composite correlation index (CCI), or similarities between spectra generated by microorganisms treated with different concentrations of drug, the minimal profile change concentration (MPCC) can be determined [38], which is the lowest drug concentration where the spectra are more similar to the maximum drug concentration that the null drug concentration. The MPCC has been shown to correlate well with the MIC determined by broth dilution in drug susceptibility testing of Candida albicans [39]. MALDI-TOF MS has shown good agreement with the CLSI reference method in the detection of resistance patterns to caspofungin with a number of Candida and Aspergillus spp. including A. fumigatus [40]. In a proof-of-concept study, MALDI-TOF MS was shown to accurately detect strains of A. fumigatus with reduced voriconazole susceptibility, although this method did not prove to be more rapid or simpler compared to testing with PCR and the Sensititre® microdilution panel [41]. Still, this method needs improved standardization, expanded availability of databases, and databases need to be continuously updated to include reference spectra of unusual pathogens [42, 43].
Molecular Approaches for Detection of Resistance of Aspergillus fumigatus
Incidence of IA caused by triazole-resistant A. fumigatus (ARAf) is increasing, particularly in patients with underlying hematological malignancies. As the diagnosis of IA is rarely based on positive culture in this group of patients, molecular detection of resistance mutations directly from clinical samples is crucial. Furthermore, the high mortality rates associated with ARAf underline the necessity of using non-culture-based assays for the detection of both Aspergillus spp. and triazole resistance directly from clinical samples [44,45,46,47,48, 49••].
Real-time PCR testing has been developed to detect triazole resistance to A. fumigatus. The Cyp51A protein, a central enzyme with lanosterol-14α-demethylase activity in the ergosterol biosynthesis pathway, is a target for triazole antifungals with at least 13 mutations at six amino acid positions causing a resistance phenotype to triazoles [20], including itraconazole, posaconazole [50], voriconazole, and isavuconazole. Molecular PCR-based methods for detecting Cyp51A alterations directly from clinical samples have to be very sensitive and specific to ensure the amplification of small amounts of Aspergillus DNA and to avoid cross-reactivity with human DNA. It is difficult to amplify the whole Cyp51A gene from primary clinical samples due to the very small amount of intact fungal molecules in these samples. In this scenario, nested PCR assays using two consecutive PCRs amplifying the same gene region have shown the highest sensitivities. In addition to in-house triazole resistance, such as ARAf PCR assays detecting the frequent mutation combinations TR34/L98H, TR46/Y121F/T289A, and M220 in the A. fumigatus Cyp51A gene by subsequent DNA sequence analysis [44, 45], some real-time PCR systems without DNA sequence analysis are commercially available. For the Cyp51A amino acid substitutions G54 and M220 and other known and unknown mutations, several in-house PCR assays plus DNA sequencing from clinical A. fumigatus isolates have been established [20, 51, 52]. To detect potential mutations in the PCR products analyzed by DNA sequence analysis, the sequence of the products has to be compared to the sequence of the A. fumigatus Cyp51A wild-type sequence using the NCBI alignment service Align Sequence Nucleotide Blast (http://www.ncbi.nlm.nih.gov/) or e.a. the FunResDB-A [53].
An overview of commercially available DNA sequence analysis independent real-time PCR systems for detection of Cyp51A mutations was published by Rath and Steinmann in 2018 [54••]. The diagnostic performance for the detection of Aspergillus DNA of five commercial tests was described, including the MycAssay Aspergillus® system, Microgen Bioproducts Ltd. (Camberley, UK), the AsperGenius® assay from PathoNostics (Maastricht, the Netherlands), the MycoGenie® assay from AdamTech (Pessac, France), and he SeptiFast® system, which is a multiplex-real-time PCR assay for the detection of bacterial and fungal pathogens and last the RenDX Fungiplex® assay from Renishaw Diagnostics Ltd. (Glasgow, UK).
In addition to the detection of Aspergillus DNA, the AsperGenius® and MycoGenie® real-time PCR systems are also able to analyze Cyp51A mutations. The AsperGenius® detects the TR34/L98H and Y121F/T289A mutations by melting curve analysis, the MycoGenie® can identify the TR34/L98H combination. The AsperGenius® assay was evaluated in bronchoalveolar lavage (BAL) samples with sensitivity and specificity values of 84% and 80%, respectively. Eight samples revealed Cyp51A triazole resistance mutations [18, 48]. Schauwvlieghe et al. also analyzed BAL samples from patients with positive galactomannan in BAL and suspected invasive Aspergillus infection using the AsperGenius® system detecting a sensitivity of 79%. Triazole resistance mutations were found in the form of TR34/L98H in eight and T289A/Y121F in three patients [55]. This test was also evaluated in 124 serum samples of 49 hemato-oncological patients (14 proven/probable cases, 33 control patients) and 211 plasma samples from 10 patients with proven or probable IA, two possible cases, and 27 controls. The described sensitivity was 78.6% and the specificity 100% for the first group and 80% and 77.8% for the second group of patients, respectively [56, 57]. The detection of resistance markers in BAL fluid using the AsperGenius® method was also associated with an increased probability of treatment-failure to triazoles [48]. The AsperGenius® assay had a good diagnostic performance in BAL and serum samples. An advantage of this of the assay is the time saving aspect by detecting triazole resistance mutations without DNA sequence analysis.
The MycoGenie® PCR system is recommended for the testing of biopsy, respiratory, and serum samples. This assay has very high sensitivity by detecting the 28S rRNA gene of A. fumigatus with a LOD of 1 copy/μl and the major triazole resistance mutation combination TR34/L98H with a LOD of 6 copies/μl. The system was evaluated in 2017 by Dannoui et al. in 88 respiratory samples and 69 serum samples of patients with proven or probable IA, revealing a sensitivity of 92.2% for respiratory samples and of 100% for serum samples [58]. A further study was published by Denis et al. comparing the MycoGenie® assay to an A. fumigatus Bio-Evolution® system (Bio-Evolution®, Bry-sur-Marne, France) using the rRNA gene ITS1 region for Aspergillus DNA detection. BAL samples (n = 73) of hematological and non-hematological patients were investigated. Patients were classified as proven, probable, and noIA groups for data evaluation. Both assays showed 100% specificity for the detection of Aspergillus DNA, and the triazole resistance associated Cyp51A alterations TR34/L98H was not detected.
Evaluation of the A. fumigatus Bio-Evolution® data revealed a sensitivity of 81% and of the MycoGenie® data of 71%. Apart from promising approaches, the sensitivity and clinical feasibility of the MycoGenie® system have to be further evaluated in different clinical settings.
A further real-time PCR system for the detection of Aspergillus DNA and the Cyp51A triazole resistance alterations TR34 and TR46 is the Fungiplex Aspergillus Azole-R test system (Bruker Daltonik GmbH, Bremen, Germany). The assay is validated for testing of serum, plasma, and BAL fluid samples with different detection limits in different samples. The two Cyp51A alterations are detected by using different DNA detection channels of the real-time PCR instruments. In serum, the limit of detection was 50, in plasma 75, and in BAL 25 genome equivalents/500 μl sample for TR34 and in serum 50, in plasma 75, and in BAL 50 Aspergillus genome equivalents/500 μl sample for TR46. The assay is not yet validated in a clinical setting.
Triazole resistance in A. fumigatus is not only a problem in immunocompromised patients but is also an emerging concern for treating chronically infected and/or colonized patients. A study of Guegan et al. had the aim to evaluate the performance of PCR assays to detect Aspergillus fungi together with triazole resistance in sputum samples from cystic fibrosis (CF) patients. The study compared the diagnostic performance of the MycoGenie® assay with the AsperGenius® system and two in-house assays plus DNA sequencing by investigating 119 sputum samples from 87 CF patients. PCR results were also compared to mycological culture. The overall rate of Aspergillus detection with the four qPCR assays ranged from 47.9 to 57.1%; the detection rate for positive cultures with A. fumigatus was 42/119 (35.3%). Five out of 41 isolated strains were triazole resistant, whereby three revealed Cyp51A mutations and only one isolates the TR34/L98H mutation combination. The authors concluded that “Cyp51A targeting was only moderately effective for triazole resistance monitoring” [59].
To perform some comparable analyses between six in-house ARAf PCR assays plus DNA sequence analysis [14, 44, 45] and a commercial realtime PCR assay, Postina et al. investigated in parallel the commercially available AsperGenius® system in detecting the Cyp51A alterations TR34/L98H and Y121F/T289A directly from 52 clinical samples (15 biopsies, 22 bronchoalveolar lavage (BAL), 15 cerebrospinal fluid (CSF) samples) and ARAf isolates (n = 3) of immunocompromised patients. Both methods were compared concerning amplification and detection of Aspergillus DNA and Cyp51A alterations. The rate of positive ARAf PCR results plus successful sequencing using the ARAf PCR assays was 61% in biopsies, 29% in CSF, 67% in BAL samples, and 100% in isolates. In comparison, the amount of positive PCRs using the AsperGenius® assays was 47% in biopsies, 42% in CSF, 59% in BAL samples, and 100% in isolates. Altogether, 17 Cyp51A alterations were detected using the ARAf PCRs plus subsequent DNA sequencing and therefrom 10 alterations also by the AsperGenius® system. The comparative evaluation of the data revealed that the conventional PCR assays were more sensitive in detecting ARAf in BAL and biopsy samples, whereby differences were not significant. The advantage of the AsperGenius® system was the time saving aspect [14].
Future Perspectives
In the future, knowledge about the epidemiology of Aspergillus susceptibility patterns will represent a cornerstone for guiding the appropriate selection of antifungal prophylaxis and treatment. Given that more areas may be burdened with high rates of environmental triazole resistance, triazoles may not be universally recommended as primary antifungal treatment, but instead, treatment choice may depend on local epidemiology of ARAf. While triazole resistance is considered an emerging threat for patients infected by A. fumigatus [60] leaving very limited treatment options for those patients, triazole resistance in Aspergillus terreus [61] may be even more threatening, because Aspergillus terreus is non-susceptible to amphotericin B.
To enable early diagnosis and treatment, more rapid and sensitive methods to diagnose A. fumigatus and ARAf need to be developed. Optimally, these methods would be applied as point-of-care tests in blood specimens and detect a broader spectrum of resistance markers including markers for resistance in individuals on prolonged triazole therapy [62].
Conclusion
In summary, non-culture-based molecular detection of Aspergillus triazole resistance is of high epidemiological and clinical relevance, mainly in immunocompromised patients with hematological malignancies, where culture has limited sensitivity. Molecular assays are now able to detect most triazole-resistant A. fumigatus strains in patients with IA. Given that resistance rates vary, clinical utility for these assays still depends on regional resistance patterns.
Change history
10 July 2019
Funding information was incomplete. The correct information is given below.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Hoenigl M, Gangneux JP, Segal E, Alanio A, Chakrabarti A, Chen SC, et al. Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: new leadership is about working together. Mycoses. 2018;61(11):885–94. https://doi.org/10.1111/myc.12836 Describes global guidelines and optimal collaboration strategies to optimize clinical care in resource-rich and resource-limited settings.
•• Jenks JD, Mehta SR, Taplitz R, Aslam S, Reed SL, Hoenigl M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test in bronchoalveolar lavage. Mycoses. 2019;62(3):230–6. https://doi.org/10.1111/myc.12881 Paper highlights the difficulties of defining, categorizing, and diagnosing Invasive aspergillosis in non-neutropenic hosts.
Cornely OA, Lass-Florl C, Lagrou K, Arsic-Arsenijevic V, Hoenigl M. Improving outcome of fungal diseases - guiding experts and patients towards excellence. Mycoses. 2017. https://doi.org/10.1111/myc.12628.
Heldt S, Prattes J, Eigl S, Spiess B, Flick H, Rabensteiner J, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect. 2018;77(3):235–41. https://doi.org/10.1016/j.jinf.2018.05.001.
• Hoenigl M, Orasch T, Faserl K, Prattes J, Loeffler J, Springer J, et al. Triacetylfusarinine C: a urine biomarker for diagnosis of invasive aspergillosis. J Infect. 2019;78(2):150–7. https://doi.org/10.1016/j.jinf.2018.09.006 Important paper, as point-of-care diagnostics play a major role in critically ill patients.
Jenks JD, Mehta SR, Taplitz R, Law N, Reed SL, Hoenigl M. Bronchoalveolar lavage Aspergillus galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test for diagnosis of invasive pulmonary aspergillosis in patients with hematological malignancies. The Journal of infection. 2019;78(3):249–59. https://doi.org/10.1016/j.jinf.2018.10.014.
• Jenks JD, Salzer HJF, Hoenigl M. Improving the rates of Aspergillus detection: an update on current diagnostic strategies. Expert Rev Anti-Infect Ther. 2019;17(1):39–50. https://doi.org/10.1080/14787210.2018.1558054 Diagnosis of fungal infections is a major issue in the treatment of immunocompromised patients. This review provides an important overview on the current status of diagnostic strategies.
Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: which drug and when? Med Mycol. 2019;57(Supplement_2):S168–s78. https://doi.org/10.1093/mmy/myy052.
Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62(3):362–8. https://doi.org/10.1093/cid/civ885.
•• Schauwvlieghe A, de Jonge N, van Dijk K, Verweij PE, Bruggemann RJ, Biemond BJ, et al. The diagnosis and treatment of invasive aspergillosis in Dutch haematology units facing a rapidly increasing prevalence of azole-resistance. A nationwide survey and rationale for the DB-MSG 002 study protocol. Mycoses. 2018;61(9):656–64. https://doi.org/10.1111/myc.12788 This paper describes the increasing incidence of azole resistance in the Netherlands, an increasing problem globally.
Eigl S, Hoenigl M, Spiess B, Heldt S, Prattes J, Neumeister P, et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017;55(5):528–34. https://doi.org/10.1093/mmy/myw102.
Hoenigl M. Fungal translocation: a driving force behind the occurrence of non-AIDS events? Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz215.
Hoenigl M, Eigl S, Heldt S, Duettmann W, Thornton C, Prattes J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses. 2018;61(1):40–3. https://doi.org/10.1111/myc.12704.
Postina P, Skladny J, Boch T, Cornely OA, Hamprecht A, Rath PM, et al. Comparison of two molecular assays for detection and characterization of Aspergillus fumigatus triazole resistance and Cyp51A mutations in clinical isolates and primary clinical samples of immunocompromised patients. Front Microbiol. 2018;9:555. https://doi.org/10.3389/fmicb.2018.00555.
White PL, Perry MD, Moody A, Follett SA, Morgan G, Barnes RA. Evaluation of analytical and preliminary clinical performance of Myconostica MycAssay Aspergillus when testing serum specimens for diagnosis of invasive aspergillosis. J Clin Microbiol. 2011;49(6):2169–74. https://doi.org/10.1128/jcm.00101-11.
White PL, Hibbitts SJ, Perry MD, Green J, Stirling E, Woodford L, et al. Evaluation of a commercially developed semiautomated PCR-surface-enhanced Raman scattering assay for diagnosis of invasive fungal disease. J Clin Microbiol. 2014;52(10):3536–43. https://doi.org/10.1128/jcm.01135-14.
Torelli R, Sanguinetti M, Moody A, Pagano L, Caira M, De Carolis E, et al. Diagnosis of invasive aspergillosis by a commercial real-time PCR assay for Aspergillus DNA in bronchoalveolar lavage fluid samples from high-risk patients compared to a galactomannan enzyme immunoassay. J Clin Microbiol. 2011;49(12):4273–8. https://doi.org/10.1128/jcm.05026-11.
Chong GL, van de Sande WW, Dingemans GJ, Gaajetaan GR, Vonk AG, Hayette MP, et al. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53(3):868–74. https://doi.org/10.1128/jcm.03216-14.
Huttner A, Emonet S, Harbarth S, Renzi G, Kaiser L, Schrenzel J. Polymerase-chain reaction/electrospray ionization-mass spectrometry for the detection of bacteria and fungi in bronchoalveolar lavage fluids: a prospective observational study. Clin Microbiol Infect. 2014;20(12):O1059–66. https://doi.org/10.1111/1469-0691.12749.
Klaassen CH, de Valk HA, Curfs-Breuker IM, Meis JF. Novel mixed-format real-time PCR assay to detect mutations conferring resistance to triazoles in Aspergillus fumigatus and prevalence of multi-triazole resistance among clinical isolates in the Netherlands. J Antimicrob Chemother. 2010;65(5):901–5. https://doi.org/10.1093/jac/dkq041.
Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356(14):1481–3. https://doi.org/10.1056/NEJMc061720.
Price CL, Parker JE, Warrilow AG, Kelly DE, Kelly SL. Azole fungicides - understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci. 2015;71(8):1054–8. https://doi.org/10.1002/ps.4029.
Azevedo MM, Faria-Ramos I, Cruz LC, Pina-Vaz C, Rodrigues AG. Genesis of azole antifungal resistance from agriculture to clinical settings. J Agric Food Chem. 2015;63(34):7463–8. https://doi.org/10.1021/acs.jafc.5b02728.
Pfaller MA, Rhomberg PR, Wiederhold NP, Gibas C, Sanders C, Fan H, et al. In vitro activity of isavuconazole against opportunistic fungal pathogens from two mycology reference laboratories. Antimicrob Agents Chemother. 2018;62(10). https://doi.org/10.1128/aac.01230-18.
Pinto E, Monteiro C, Maia M, Faria MA, Lopes V, Lameiras C, et al. Aspergillus species and antifungals susceptibility in clinical setting in the north of Portugal: cryptic species and emerging azoles resistance in A. fumigatus. Front Microbiol. 2018;9:1656. https://doi.org/10.3389/fmicb.2018.01656.
Buil JB, Bruggemann RJM, Wasmann RE, Zoll J, Meis JF, Melchers WJG, et al. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J Antimicrob Chemother. 2018;73(1):134–42. https://doi.org/10.1093/jac/dkx354.
Institute. CaLS. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi.; Approved standard. In: CLSI document M38. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
Espinel-Ingroff A. Comparison of three commercial assays and a modified disk diffusion assay with two broth microdilution reference assays for testing zygomycetes, Aspergillus spp., Candida spp., and Cryptococcus neoformans with posaconazole and amphotericin B. J Clin Microbiol. 2006;44(10):3616–22. https://doi.org/10.1128/jcm.01187-06.
Martin-Mazuelos E, Peman J, Valverde A, Chaves M, Serrano MC, Canton E. Comparison of the Sensititre YeastOne colorimetric antifungal panel and Etest with the NCCLS M38-A method to determine the activity of amphotericin B and itraconazole against clinical isolates of Aspergillus spp. J Antimicrob Chemother. 2003;52(3):365–70. https://doi.org/10.1093/jac/dkg384.
Pfaller MA, Messer SA, Boyken L, Hollis RJ, Diekema DJ. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference M38-A microdilution methods for determining posaconazole MICs. Diagn Microbiol Infect Dis. 2003;45(4):241–4.
Araujo R, Espinel-Ingroff A. Comparison of assessment of oxygen consumption, Etest, and CLSI M38-A2 broth microdilution methods for evaluation of the susceptibility of Aspergillus fumigatus to posaconazole. Antimicrob Agents Chemother. 2009;53(11):4921–3. https://doi.org/10.1128/aac.00862-09.
Arendrup MC, Verweij P, Nielsen HV. Evaluation of MIC strip isavuconazole test for susceptibility testing of wild-type and non-wild-type Aspergillus fumigatus isolates. Antimicrob Agents Chemother. 2017;61(1). https://doi.org/10.1128/aac.01659-16.
Jenks JD, Salzer HJ, Prattes J, Krause R, Buchheidt D, Hoenigl M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:1033–44. https://doi.org/10.2147/DDDT.S145545.
Espinel-Ingroff A, Rezusta A. E-test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J Clin Microbiol. 2002;40(6):2101–7.
Fuller J, Schofield A, Jiwa S, Sand C, Jansen B, Rennie R. Caspofungin Etest endpoint for Aspergillus isolates shows poor agreement with the reference minimum effective concentration. J Clin Microbiol. 2010;48(2):479–82. https://doi.org/10.1128/jcm.01677-09.
Espinel-Ingroff A, Turnidge J, Alastruey-Izquierdo A, Botterel F, Canton E, Castro C, et al. Method-dependent epidemiological cutoff values for detection of triazole resistance in Candida and Aspergillus species for the Sensititre YeastOne colorimetric broth and Etest agar diffusion methods. Antimicrob Agents Chemother. 2019;63(1). https://doi.org/10.1128/aac.01651-18.
Guinea J, Verweij PE, Meletiadis J, Mouton JW, Barchiesi F, Arendrup MC. How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates. Clin Microbiol Infect. 2018. https://doi.org/10.1016/j.cmi.2018.09.008.
Arnold RJ, Reilly JP. Fingerprint matching of E. coli strains with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of whole cells using a modified correlation approach. Rapid Commun Mass Spectrom : RCM. 1998;12(10):630–6. https://doi.org/10.1002/(sici)1097-0231(19980529)12:10<630::Aid-rcm206>3.0.Co;2-0.
Marinach C, Alanio A, Palous M, Kwasek S, Fekkar A, Brossas JY, et al. MALDI-TOF MS-based drug susceptibility testing of pathogens: the example of Candida albicans and fluconazole. Proteomics. 2009;9(20):4627–31. https://doi.org/10.1002/pmic.200900152.
De Carolis E, Vella A, Florio AR, Posteraro P, Perlin DS, Sanguinetti M, et al. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry for caspofungin susceptibility testing of Candida and Aspergillus species. J Clin Microbiol. 2012;50(7):2479–83. https://doi.org/10.1128/jcm.00224-12.
Gitman MR, McTaggart L, Spinato J, Poopalarajah R, Lister E, Husain S, et al. Antifungal susceptibility testing of Aspergillus spp. by using a composite correlation index (CCI)-based matrix-assisted laser desorption ionization-time of flight mass spectrometry method appears to not offer benefit over traditional broth microdilution testing. J Clin Microbiol. 2017;55(7):2030–4. https://doi.org/10.1128/jcm.00254-17.
Rizzato C, Lombardi L, Zoppo M, Lupetti A, Tavanti A. Pushing the limits of MALDI-TOF mass spectrometry: beyond fungal species identification. J Fungi (Basel, Switzerland). 2015;1(3):367–83. https://doi.org/10.3390/jof1030367.
Sanguinetti M, Posteraro B. Identification of molds by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2017;55(2):369–79. https://doi.org/10.1128/jcm.01640-16.
Spiess B, Postina P, Reinwald M, Cornely OA, Hamprecht A, Hoenigl M, et al. Incidence of Cyp51 A key mutations in Aspergillus fumigatus-a study on primary clinical samples of immunocompromised patients in the period of 1995-2013. PLoS One. 2014;9(7):e103113. https://doi.org/10.1371/journal.pone.0103113.
Spiess B, Seifarth W, Merker N, Howard SJ, Reinwald M, Dietz A, et al. Development of novel PCR assays to detect azole resistance-mediating mutations of the Aspergillus fumigatus cyp51A gene in primary clinical samples from neutropenic patients. AntimicrobAgents Chemother. 2012;56(7):3905–10.
Perfect JR, Hachem R, Wingard JR. Update on epidemiology of and preventive strategies for invasive fungal infections in cancer patients. Clin Infect Dis. 2014;59(Suppl 5):S352–5. https://doi.org/10.1093/cid/ciu639.
Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70(5):1522–6. https://doi.org/10.1093/jac/dku566.
Chong GM, van der Beek MT, von dem Borne PA, Boelens J, Steel E, Kampinga GA, et al. PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bronchoalveolar lavage: a multicentre validation of the AsperGenius assay(R) in 201 patients with haematological disease suspected for invasive aspergillosis. J Antimicrob Chemother. 2016;71:3528–35. https://doi.org/10.1093/jac/dkw323.
•• Koehler P, Hamprecht A, Bader O, Bekeredjian-Ding I, Buchheidt D, Doelken G, et al. Epidemiology of invasive aspergillosis and azole resistance in patients with acute leukaemia: the SEPIA Study. Int J Antimicrob Agents. 2017;49(2):218–23. https://doi.org/10.1016/j.ijantimicag.2016.10.019 This very important study shows the clinical relevance of azole resistance in hematological patients on triazole therapy with azole-resistant A. fumigatus infections.
Trama JP, Mordechai E, Adelson ME. Detection of Aspergillus fumigatus and a mutation that confers reduced susceptibility to itraconazole and posaconazole by real-time PCR and pyrosequencing. J Clin Microbiol. 2005;43(2):906–8. https://doi.org/10.1128/jcm.43.2.906-908.2005.
van der Linden JW, Snelders E, Arends JP, Daenen SM, Melchers WJ, Verweij PE. Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. JClinMicrobiol. 2010;48(4):1478–80.
Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. ClinInfectDis. 2011;52(9):1123–9.
Weber M, Schaer J, Walther G, Kaerger K, Steinmann J, Rath PM, et al. FunResDB-A web resource for genotypic susceptibility testing of Aspergillus fumigatus. Med Mycol. 2018;56(1):117–20. https://doi.org/10.1093/mmy/myx015.
•• Rath PM, Steinmann J. Overview of commercially available PCR assays for the detection of Aspergillus spp. DNA in patient samples. Front Microbiol. 2018;9:740. https://doi.org/10.3389/fmicb.2018.00740 This paper compares the five most important commercially available molecular Aspergillus DNA and azole-resistant detection systems.
Schauwvlieghe A, Vonk AG, Buddingh EP, Hoek RAS, Dalm VA, Klaassen CHW, et al. Detection of azole-susceptible and azole-resistant Aspergillus coinfection by cyp51A PCR amplicon melting curve analysis. J Antimicrob Chemother. 2017;72(11):3047–50. https://doi.org/10.1093/jac/dkx262.
White PL, Barnes RA, Springer J, Klingspor L, Cuenca-Estrella M, Morton CO, et al. The clinical performance of Aspergillus PCR when testing serum and plasma- a study by the European Aspergillus PCR Initiative. J Clin Microbiol. 2015;53:2832–7. https://doi.org/10.1128/jcm.00905-15.
White PL, Posso RB, Barnes RA. Analytical and clinical evaluation of the PathoNostics AsperGenius assay for detection of invasive aspergillosis and resistance to azole antifungal drugs directly from plasma samples. J Clin Microbiol. 2017;55(8):2356–66. https://doi.org/10.1128/jcm.00411-17.
Dannaoui E, Gabriel F, Gaboyard M, Lagardere G, Audebert L, Quesne G, et al. Molecular diagnosis of invasive aspergillosis and detection of azole resistance by a newly commercialized PCR kit. J Clin Microbiol. 2017;55(11):3210–8. https://doi.org/10.1128/jcm.01032-17.
Guegan H, Chevrier S, Belleguic C, Deneuville E, Robert-Gangneux F, Gangneux JP. Performance of molecular approaches for Aspergillus detection and azole resistance surveillance in cystic fibrosis. Front Microbiol. 2018;9:531. https://doi.org/10.3389/fmicb.2018.00531.
Tsitsopoulou A, Posso R, Vale L, Bebb S, Johnson E, White PL. Determination of the prevalence of triazole resistance in environmental Aspergillus fumigatus strains isolated in South Wales, UK. Front Microbiol. 2018;9:1395. https://doi.org/10.3389/fmicb.2018.01395.
Zoran T, Sartori B, Sappl L, Aigner M, Sanchez-Reus F, Rezusta A, et al. Azole-resistance in Aspergillus terreus and related species: an emerging problem or a rare phenomenon? Front Microbiol. 2018;9:516. https://doi.org/10.3389/fmicb.2018.00516.
Buil JB, Zoll J, Verweij PE, Melchers WJG. Molecular detection of azole-resistant Aspergillus fumigatus in clinical samples. Front Microbiol. 2018;9:515. https://doi.org/10.3389/fmicb.2018.00515.
Funding
This work was supported using funds from the Oesterreichische Nationalbank (Anniversary Fund, project number 15346).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclaimer
The funders had no role in the study design, data collection, analysis, interpretation, decision to publish, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Conflict of Interest
Dieter Buchheidt reports being a consultant to Basilea, Gilead Sciences, and Merck Sharp & Dohme/Merck; receiving research grants from Gilead Sciences and Pfizer; serving on the speakers’ bureau of Astellas, Basilea, Gilead Sciences, Merck Sharp & Dohme/Merck, Pfizer, and TEVA; and receiving travel grants from Astellas, Gilead Sciences, Merck Sharp & Dohme/Merck, and Pfizer. Martin Hoenigl reports untied research funding from Gilead. Jeffrey Jenks and Birgit Spiess declare no conflict of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jenks, J.D., Spiess, B., Buchheidt, D. et al. (New) Methods for Detection of Aspergillus fumigatus Resistance in Clinical Samples. Curr Fungal Infect Rep 13, 129–136 (2019). https://doi.org/10.1007/s12281-019-00342-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-019-00342-w