Abstract
Methanol (MeOH) extract of the aerial parts of Dendropanax morbifera (Araliaceae) has demonstrated a significant dose-dependent inhibitory effect on the RANKL-induced differentiation of bone marrow-derived macrophages to osteoclasts. Bioassay-guided fractionation of the extract resulted in the isolation of a novel diacetylene carboxylic acid (1), together with a known diacetylenic compounds (2) as phytochemicals to strongly inhibit the osteoclast differentiation. The chemical structure of 1 was determined by spectroscopic analyses as (9Z,16S)-16-O-acetyl-9,17-octadecadiene-12,14-diynoic acid, that is acetyl derivative of 2. Two diacetylenic components of D. morbifera, 1 and 2 exhibited a dose-dependent inhibitory effect on the RANKL-induced formation of tartrate-resistant acid phosphatase-positive multinucleated cells with IC50 values of 2.4 and 3.1 μM, respectively. Seven other known components (3–9) were also isolated from the extract: dendropanoxide (3), friedelin (4), epifriedelanol (5), α-amyrin (6), β-amyrin (7), β-sitosterol (8), and stigmasterol (9). The significant anti-osteoclastogenic activities of 3, 4, 5, and 7 were first reported in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendropanax morbifera (Araliaceae) is a subtropical broad-leaved evergreen tree endemic to Korea. The tree is widely distributed in the southern part of the Korean peninsula and Jeju island (Han et al. 1998) and produces a resinous sap that can be used as a varnish. In addition, the leaves and stems of this plant have been used in folk medicine for the treatment of headache, skin diseases, and infection (Park et al. 2004). Recently, biologists and pharmacologists have paid much attention to this plant because it has been reported to have many beneficial effects on human health, such as anti-cancer, anti-oxidant, anti-complement, and anti-diabetic activities (Chung et al. 2011; Moon 2011; Hyun et al. 2013; Kim et al. 2015). In particular, some diacetylenic components such as (3S,8S)-falcarindiol, (3S)-diyene, and (9Z,16S)-16-hydroxy-9,17-octadecadiene-12,14-diynoic acid of Dendropanax genus have been reported to be active components responsible for the anti-complement and anti-inflammatory activities (Park et al. 2004; Chung et al. 2011; Chien et al. 2014).
An osteoclast is a type of bone cell that breaks down bone tissue. It is a large multinucleated cell generated originally from the monocytes/macrophages of hematopoietic stem cells. Two essential cytokines, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), are known to be involved in osteoclastogenesis, which induces the differentiation of bone marrow-derived macrophages (BMMs) into tartrate-resistant acid phosphatase-positive multinucleated cells (TRAP+-MNCs) (Suda et al. 1999; Boyle et al. 2003). Therefore, a pharmaceutical trial to discover potent substances capable of inhibiting osteoclast differentiation, particularly from natural resources, could be a promising therapeutic strategy for preventing and/or treating bone disorders and related bone fractures (Del Fattore et al. 2008).
During the investigation to identify traditional Korean herbal medicines with anti-osteoclastogenic activity, the methanol extract of aerial parts of D. morbifera was found to potently inhibit the RANKL-induced differentiation of BMMs into TRAP+-MNCs (Fig. 1). Therefore, we conducted extensive phytochemical studies of the extract of D. morbifera, which finally resulted in the isolation of two diacetylene carboxylic acids, (9Z,16S)-16-O-acetyl-9,17-octadecadiene-12,14-diynoic acid (1) and (9Z,16S)-16-hydroxy-9,17-octadecadiene-12,14-diynoic acid (2), as active components responsible for the inhibitory effect on the RANKL-induced differentiation of BMMs into TRAP+-MNCs (Fig. 2).
Anti-osteoclastogenic activity of the D. morbifera MeOH extract (a) and EtOAC fractions, E1–E4 (b). BMMs (1 × 104 cells/well) were cultured with M-CSF (30 ng/mL) and RANKL (10 ng/mL) in the presence of 0.1% DMSO (vehicle control), D. morbifera MeOH extract, Bonviva Plus or EtOAC fractions for 4 days. Multinucleated cells were fixed with 4% formalin, permeabilized with 0.1% triton X-100 in PBS, and stained with TRAP solutions. The stained cells were photographed under a light microscope and the TRAP activity measured at 405 nm. *p < 0.05; **p < 0.01; ***p < 0.001
Materials and methods
Plant material
Dendropanax morbifera was cultivated in a greenhouse at Hadong Kyungnam (Korea) and the aerial parts collected in July 2015 and identified by Dr. Jo Chang Soo. A voucher specimen (KR1507) was deposited at the herbarium of the Korea Research Institute of Chemical Technology.
General instrumental procedures
NMR spectra were obtained using a Bruker AM 500 spectrometer using TMS as an internal standard for 1H NMR, 13C NMR, DEPT, HMQC, and HMBC. High-resolution electrospray ionization (HRESIMS) and electron impact (EI) mass spectra were obtained using a Q-Tof micro LC–MS/MS instrument (Waters, Milford, MA, USA) and Varian CP3800-1200L mass spectrometry (Agilent, Lexington, MA, USA), respectively. Preparative-HPLC was performed on a Futecs P-4000 system with a Shim-pack prep-ODS(H) kit column (5 µm, 20 mm × 25 cm). Isolation and purification were carried out using a medium-pressure liquid chromatography (MPLC) system (BUCHI pump Module C-601, silica gel 60, 230–400 mesh, Merck Millipore Sigma, Burlington, MA, USA).
Extraction and isolation
The dried aerial parts (5 kg) of D. morbifera were soaked twice in 50 L of methanol (MeOH) at room temperature for 7 days. The concentrated MeOH extract (950 g) was suspended in 10 L of distilled water and then extracted sequentially with equal volumes of ethylacetate (EtOAc) and n-butanol (n-BuOH), which resulted in 258 g of the EtOAc soluble fraction and 159 g of the n-BuOH soluble fraction. The EtOAc fraction (220 g) was subjected to silica gel column (Ø = 15.0 × 60 cm) chromatography with 2 L of MeOH in dichloromethane solution (0, 2, 10, and 50%) in a stepwise gradient manner to obtain four fractions: E1–E4.
E3 (34 g) was subjected to silica gel column chromatography and eluted with EtOAc in n-hexane solution (10–50%) in a stepwise gradient manner to obtain five sub-fractions: E31–E35. E34 was further purified by prep-HPLC (Shim-pack, 70% MeOH) to yield 201.0 mg of 1 and 112.0 mg of 2 (Fig. 2). E32 was purified by silica gel column chromatography and eluted with 10% EtOAc in n-hexane solution to yield 8 and 9.
E2 (20 g) was rechromatographed on silica gel and eluted with EtOAc in n-hexane solution (2–50%) in a stepwise gradient manner to obtain five sub-fractions: E21–E25. E21 was further purified through silica gel column chromatography and eluted with 3% MeOH in MC solution to yield 10.0 mg of 3 and 14.5 mg of 4. E22 was dissolved in EtOAc and allowed to crystallize at room temperature to yield 120.0 mg of 5. The remaining filtrate of E22 was fractionated into five sub-fractions (E221–E225) using silica gel column chromatography and eluted with EtOAc in n-hexane in a stepwise gradient manner. (3–50%). E224 was crystallized in EtOAc at room temperature to yield a mixture (490.7 mg) of 6 and 7.
(9Z,16S)-16-O-acetyl-9,17-octadecadiene-12,14-diynoic acid (1): Viscous liquid; \([\alpha ]_{D}^{20}\)-43.6 (c 0.50, CH3OH); 1H NMR (CDCl3, 500 MHz); 13C NMR (CDCl3, 125 MHz) (Table 1); HRESIMS m/z 330.1825 (calculated for C20H26O4, 330.1831).
Cell culture and osteoclast differentiation
All cell culture materials were purchased from HyClone (Logan, UT, USA). Bone marrow-derived cells (BMCs) were obtained from 5-week-old male ICR mice (Damool Science, Daejeon, Korea) by flushing the femurs and tibias with α-MEM supplemented with antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin). BMCs were cultured in α-MEM supplemented with 10% fetal bovine serum (FBS) with 30 ng/mL of mouse recombinant M-CSF (R&D Systems, Minneapolis, MN, USA) for 1 day. After 1 day, non-adherent BMCs were plated on a Petri dish and cultured for 3 days in the presence of M-CSF (30 ng/mL). After non-adherent cells were washed out, adherent cells were used as BMMs. For the induction of osteoclastogenesis, BMMs were cultured in the presence of 10 ng/mL of mouse recombinant RANKL (R&D Systems) and 30 ng/mL of M-CSF for 4 days to differentiate them into mature TRAP+-MNCs. All experimental procedures for BMC cultures and osteoclast differentiation were performed in strict accordance with the recommendations in the Standard Protocol for Animal Study of Korea Research Institute of Chemical Technology (KRICT; No. 2012-7D-02-01). The protocol (ID No. 7D-M1) was approved by the Institutional Animal Care and Use Committee of KRICT.
TRAP staining and activity assay
For the induction of osteoclastogenesis, isolated BMMs (1 × 104 cells/well) were cultured with M-CSF (30 ng/mL) and RANKL (10 ng/mL) in the presence of 0.1% DMSO (vehicle control) or the test sample for 4 days. Extracts and chemicals were reconstructed to 30 mg/mL and 30 mM DMSO stock solution, and diluted to 1:1000 with the culture medium. Then, cells were fixed with 3.7% formalin for 5 min and permeabilized with 0.1% Triton X-100 for 10 min before staining with TRAP solution (Sigma-Aldrich, St. Louis, MO, USA) for 10 min to visualize TRAP+-MNCs. To measure TRAP activity, cells were fixed with 3.7% formalin for 5 min, permeabilized with 0.1% Triton X-100 for 10 min, and treated with TRAP buffer (100 mM sodium citrate pH 5.0, 50 mM sodium tartrate) containing 3 mM p-nitrophenyl phosphate (Sigma-Aldrich) at 37 °C for 5 min. Reaction mixtures were transferred into new plates containing an equal volume of 0.1 N NaOH and optical density (OD) measured at 405 nm. TRAP activity was performed in triplicate, and commercially available Bonviva Plus® (ibandronate sodium) was used as a reference drug.
Cytotoxicity assay
BMMs were plated in a 96-well plate in triplicate at a density of 1 × 104 cells/well for 24 h. The cells were then treated with isolated constituents 1 and 2 for 3 days. Compounds 1 and 2 were reconstructed to 30 mM DMSO stock solution, and diluted to 1:1,000 with the culture medium. Therefore, 0.1% DMSO was used as the vehicle control. The supernatants of cultured media were transferred to a new 96-well plate. Lactate dehydrogenase (LDH) activity was measured in triplicate using the CytoTox96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Absorbance was measured using the Hidex sense beta plus microplate reader (Hidex, Turku, Finland) at 490 nm.
Statistical analysis
All quantitative values are presented as the mean ± SD. Statistical differences were analyzed using Student’s t test. P < 0.05 was considered significant.
Results
Anti-osteoclastogenic activity of the MeOH extract of D. morbifera
The MeOH extract obtained from the aerial parts of D. morbifera dose-dependently inhibited the formation of TRAP+-MNCs, with the concentration required for a 50% inhibitory effect (IC50) on TRAP activity being 3.49 µg/mL (Fig. 1a). The reference drug, Bonviva Plus®, also dose-dependently inhibited the formation of TRAP+-MNCs, with an IC50 of 6.37 µg/mL.
Identification of phytochemicals in D. morbifera
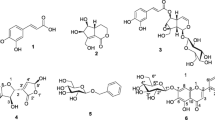
Next, extensive phytochemical investigation of D. morbifera extract was conducted to identify the active ingredients responsible for its anti-osteoclastogenic property. E3 (IC50 on TRAP activity; 5.84 µg/mL) exhibited the most potent anti-osteoclastogenic activity, and E4 (7.76 µg/mL) and E2 (9.46 µg/mL) also demonstrated anti-osteoclastogenic activities (Fig. 1b). In this study, E3 and E2 were further subjected to repeated column chromatography, which finally led to the isolation of two active components, 1 and 2, as well as other constituents (3–9) (Fig. 2).
Compound 1 was obtained as a viscous liquid. The molecular formula was determined to be C20H26O4 at m/z 330.1825 (calc. 330.1831) [M+Na]+ by HRESIMS. The 1H NMR spectra of 1 exhibited a similar pattern to that of 2. The 1H NMR spectrum of 2 showed signals for five olefinic protons at δH 5.95 (ddd, J = 17.0, 10.2, 5.4 Hz), δH 5.52 (m), δH 5.48 (d, J = 17.0 Hz), δH 5.40 (m) and δH 5.25 (d, J = 10.2 Hz), a methine signals at δH 4.93 (d, J = 5.4 Hz), and fourteen aliphatic protons at δH 3.04 (d, J = 7.0 Hz, 2H), δH 2.36 (t, J = 7.5, 7.4 Hz, 2H), δH 2.05 (dd, J = 7.2, 6.7 Hz, 2H), δH 1.65 (m, 2H) and δH 1.37 (m, 8H). When the 1H NMR spectrum of 1 was compared with that of 2, an acetoxy signal newly observed at δH 2.12 (s, 3H) in 1. By comparing the 13C spectrum of 1 with that of 2, additional acetoxy signals at δC 169.6 and δC 20.9 were observed for 1. In the HMBC spectrum, the typical correlation between H-16 (δH 5.92) and C-19 (δC 169.6) indicated that the acetoxy group was attached at C-16, and that the hydroxyl group at the C-16 position of 2 was replaced with an acetoxy group to become 1. The absolute stereochemistry at C-16 of 2 was reported to be an S-configuration, a compound from D. morbifera (Park et al. 2004). In this study, 1 and 2 had a negative optical rotation value, \([\alpha ]_{D}^{20}\)-43.6 (c 0.50, CH3OH) and \([\alpha ]_{D}^{20}\)-15.9 (c 0.25, CH3OH), suggesting the absolute stereochemistry at C-16 of 1 to be S-configuration. To the best of our knowledge, 1 had never been reported in this species, or even from other natural plant sources. However, 1 appears to be an artefact derived from 2 rather than a genuine novel constituent of the species, as 1 is easily obtained by acetylation of 2 with pyridine and acetic anhydride at room temperature. The chemical structure and stereochemistry of 2 was determined to be (9Z,16S)-16-hydroxy-9,17-octadecadiene-12,14-diynoic acid in a direct comparison to spectroscopic data in the literature (Park et al. 2004). Thus, the chemical structure of 1 was established to be (9Z,16S)-16-O-acetyl-9,17-octadecadiene-12,14-diynoic acid (Fig. 2). Both compounds 1 and 2 were somewhat unstable, even with refrigeration, and there was some decomposition into insoluble matter in MeOH at room temperature within a month.
The other isolated components (3–9) were identified as dendropanoxide (3) (Motoo et al. 2005), friedelin (4) (Trinh et al. 2007), epifriedelanol (5) (Trinh et al. 2007), α-amyrin (6) (Liliana et al. 2012), β-amyrin (7) (Liliana et al. 2012), β-sitosterol (8) (Virgilio et al. 2015), and stigmasterol (9) (Virgilio et al. 2015) by comparing their spectroscopic data to the literature (Fig. 2).
Anti-osteoclastogenic activity of phytochemicals in D. morbifera
Since Bonviva Plus® is a mixture of vitamin D3 (or cholecalciferol; 240 mg) and ibandronate sodium (168 mg), the effects of all isolated components (1–9) on the RANKL-induced formation of TRAP+-MNCs were evaluated at 30 µM compared to those of vitamin D3 and ibandronate sodium (Fig. 3). Both of vitamin D3 and ibandronate sodium used as the positive controls strongly inhibited the TRAP activity, and also two diacetylenic fatty acids (1 and 2) strongly inhibited the TRAP activity. Compounds 3, 4, 5, and 7 also significantly inhibited the TRAP activity, but in the following dose-dependent experiments, the anti-osteoclastogenic activities of 1 and 2 were confirmed by TRAP staining and measuring its activity. Both 1 and 2 dose-dependently inhibited the RANKL-induced formation of TRAP+-MNCs (Fig. 4a) and TRAP activity (Fig. 4b). The IC50 values of 1 and 2 for TRAP activity were calculated to be 2.41 and 3.11 μM, respectively (Fig. 4b). They did not exhibit a distinct cytotoxic effect on BMMs below 30 μM, suggesting that their anti-osteoclastogenic activity was not due to their potential to inhibit the survival of BMMs.
Effects of phytochemicals isolated from D. morbifera MeOH extract on osteoclast differentiation. BMMs (1 × 104 cells/well) were cultured with M-CSF (30 ng/mL) and RANKL (10 ng/mL) in the presence of 0.1% DMSO (vehicle control) or 30 μM of other isolated phytochemicals (1–9) for 4 days. The TRAP activity of osteoclasts was measured at 405 nm. *p < 0.05; **p < 0.01; ***p < 0.001
Anti-osteoclastogenic activity of compounds 1 and 2. BMMs (1 × 104 cells/well) were cultured with M-CSF (30 ng/mL) and RANKL (10 ng/mL) in the presence of 0.1% DMSO (vehicle control) or the indicated compounds for 4 days. Multinucleated cells were fixed, permeabilized, and stained with TRAP solutions. The stained cells were photographed under a light microscope (a) and the TRAP activity of osteoclasts measured at 405 nm (b; black bar). The cytotoxicity of the compounds was also evaluated (b; white bar). *p < 0.05; ***p < 0.001
Discussion
In this study, we found anti-osteoclastogenic activity of the MeOH extract of aerial parts of D. morbifera with an IC50 for TRAP activity comparable to that of Bonviva Plus®. Bonviva Plus® is the most widely used medicine in clinics; it contains ibandronate sodium monohydrate and concentrated cholecalciferol at a ratio of 1:1. The anti-osteoclastogenic activity of ibandronate has been well reported in vitro (Zhang et al. 2013).
Further bioassay-guided fractionation of the extract resulted in the isolation of a new diacetylene carboxylic acid (1) and related congener (2) as active principles responsible for the inhibitory effect on osteoclast differentiation. Compounds 1 and 2 exhibited potent inhibitory effects on the RANKL-induced differentiation of BMMs into TRAP+-MNCs in a dose-dependent manner. Compounds 1 and 2 are diacetylene carboxylic acids, generally called plant polyacetylenes, and consist of at least two triple carbon–carbon bonds, which are frequently produced by higher plants of the Apiaceae and Araliaceae families. More than 1100 different acetylenes and biogenetically related substances have been identified in plants. The most common polyacetylenes are falcarinol-type aliphatic C17-polyacetylenes, which are produced from C18-polyacetylene carboxylic acid by decarboxylation (Christensen and Brandt 2006; Dawid et al. 2015) and have been reported to have a variety of health-promoting metabolic effects as well as potential anti-cancer, anti-fungal, and anti-inflammatory properties.
Compounds 3, 4, 5, and 7 also significantly inhibited TRAP activity at 30 μM. Compound 3 exhibits several biological activities, including antiplasmodial activity (Chung et al. 2009), antidiabetic activity (Moon 2011), and autophagy-inducing activity (Lee et al. 2013). Compound 4 also exhibits several biological activities, including anti-oxidant, anti-inflammatory, analgesic, and antipyretic activities (Antonisamy et al. 2011; Sunil et al. 2013). Several biological activities of compounds 5 and 7, such as anti-cancer activity and anti-inflammatory activity (Holanda Pinto et al. 2008), have also been reported. However, this is the first report of their anti-osteoclastogenic activity, though their anti-osteoclastic potential was relatively less than that of compounds 1 and 2.
In summary, D. morbifera exhibited anti-osteoclastogenic activity, and its potential could be due to several anti-osteoclastogenic phytochemicals. Compound 1, a novel phytochemical, showed anti-osteoclastogenic activity, as well as that of known compounds 2, 3, 4, 5, and 7, were first reported in this study, suggesting that the anti-osteoclastogenic activity of D. morbifera and its phytochemicals could serve as novel natural resources for treating osteoclast-related disorders, such as osteoporosis, rheumatoid arthritis, periodontal disease, and cancer bone metastasis.
References
Antonisamy P, Duraipandiyan V, Ignacimuthu S (2011) Anti-inflammatory, analgesic and antipyretic effects of friedelin isolated from Azima tetracantha Lam. in mouse and rat models. J Pharm Pharmacol 63:1070–1077
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Chien SC, Tseng YH, Hsu WN, Chu FH, Chang ST, Kuo YH, Wang SY (2014) Anti-inflammatory and anti-oxidative activities of polyacetylene from Dendropanax dentiger. Nat Prod Commun 11:1589–1590
Christensen LP, Brandt K (2006) Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J Pharm Biomed Anal 41:683–693
Chung IM, Kim MY, Park SD, Park WH, Moon HI (2009) In vitro evaluation of the antiplasmodial activity of Dendropanax morbifera against chloroquine-sensitive strains of Plasmodium falciparum. Phytother Res 23:1634–1637
Chung IM, Song HK, Kim SJ, Moon HI (2011) Anticomplement activity of polyacetylenes from leaves of Dendropanax morbifera Leveille. Phytother Res 5:784–786
Dawid C, Dunemann F, Schwab W, Nothnagel T, Hofmann T (2015) Bioactive C17-polyacetylenes in carrots (Daucus carota L.): current knowledge and future perspectives. J Agric Food Chem 63:9211–9222
Del Fattore A, Teti A, Rucci N (2008) Osteoclast receptors and signaling. Arch Biochem Biophys 473:147–160
Han SH, Jung YH, Oh MH, Ko MH, Oh YS, Koh SC, Kim MH, Oh MY (1998) Phytogenetic relationships of the Dendropanax morbifera and D. trifidus based on PCR-RAPD. Korean J Genet 20:173–181
Holanda Pinto SA, Pinto LM, Cunha GM, Chaves MH, Santos FA, Rao VS (2008) Anti-inflammatory effect of α, β-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 16:48–52
Hyun TK, Kim MO, Lee H, Kim Y, Kim E, Kim JS (2013) Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem 141:1947–1955
Kim W, Kim DW, Yoo DY, Jung HY, Kim JW, Kim DW, Choi JH, Moon SM, Yoon YS, Hwang IK (2015) Antioxidant effects of Dendropanax morbifera Léveille extract in the hippocampus of mercury-exposed rats. BMC Complement Altern Med 15:247–253
Lee JW, Kim KS, An HK, Kim CH, Moon HI, Lee YC (2013) Dendropanoxide induces autophagy through ERK1/2 activation in MG-63 human osteosarcoma cells and autophagy inhibition enhances dendropanoxide-induced apoptosis. PLoS ONE 8:e83611
Liliana HV, Javier P, Arturo NO (2012) The pentacyclic triterpenes α, β-amyrins: a review of sources and biological activities. In: Rao J (ed) Phytochemicals—a global perspective of their role in nutrition and health. InTech Europe, Rijeka, pp 487–502
Moon HI (2011) Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum Exp Toxicol 30:870–875
Motoo T, Reiko M, Masakazu S, Yoshinori A (2005) 13C NMR assignment of dammarane triterpenes and dendropanoxide: application of 2D long-range 13C-1H correlation spectra. Magn Reson Chem 26:581–590
Park BY, Min BS, Oh SR, Kim JH, Kim TJ, Kim DH, Bae KH, Lee HK (2004) Isolation and anticomplement activity of compounds from Dendropanax morbifera. J Ethnopharmacol 90:403–408
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differenotiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 3:345–357
Sunil C, Duraipandiyan V, Ignacimuthu S, Al-Dhabi NA (2013) Antioxidant, free radical scavenging and liver protective effects of friedelin isolated from Azima tetracantha Lam. leaves. Food Chem 139:860–865
Trinh TT, Nguyen HC, Tran VS (2007) Triterpenes from Celastrus hindsii Benth. J Chem 45:373–376
Virgilio D, Ebajo J, Chien-Chang S, Consolacion YR (2015) Terpenoids and Sterols from Hoya multiflora Blume. J App Pharm Sci 5:33–39
Zhang W, Yang DL, Wang YX, Wang HW, Zhen ZJ, Zhang YZ, Shen Y (2013) In vitro osteoclast-suppressing effect of sodium ibandronate. Chin Med J (Engl) 126:751–755
Acknowledgements
This work was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2015 (C0277170) and by the Ministry of Science, ICT, and Future Planning of Korea (SI-1606).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kim, E.H., Jo, C.S., Ryu, S.Y. et al. Anti-osteoclastogenic diacetylenic components of Dendropanax morbifera. Arch. Pharm. Res. 41, 506–512 (2018). https://doi.org/10.1007/s12272-018-1033-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1033-3