Abstract
In the current study, the protective effect of montelukast (ML) on cisplatin induced reproductive toxicity in rats was investigated. Twenty-eight rats were equally divided into four groups; first group was kept as control. In the second group, ML was orally administered at the dose of 10 mg/kg/day for 10 days. In the third group, CP was intraperitoneally administered at the dose of 7 mg/kg a single injection, and in fourth group, CP and ML were given together at the same doses. Although CP induced oxidative stress via significant increase in the formation of TBARS, it caused a significant decline in the levels of GSH, CAT, GPx, and SOD in rats. In contrast, ML prevents these effects of CP through cause an increase in GSH, CAT, GPx, and SOD levels and a decrease in formation of TBARS. In addition, sperm motility and serum testosterone levels significantly decrease and histopathological damage increases with CP treatment. However, the effects of CP on sperm motility, serum testosterone level, oxidative and histopathological changes are eliminated by ML treatment. In conclusion, the current study demonstrated that the reproductive toxicity caused by CP may be prevented by ML treatment. Thus, it was judged that co-administration of ML with CP may be useful to attenuate the negative effects of CP on male reproductive system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin (CP), a chemotherapeutic agent, is successfully used for the treatment of many types of solid tumors such as ovarian, cervical, bladder, breast, and lung [1, 2]. Also, CP is widely used for the treatment of testicular cancer, and it has a >90% cure rate. The success of CP for the treatment of testicular cancer is limited by its undesirable side effects on reproductive system [2, 3]. The reproductive toxicity as well as hepatotoxicity and nephrotoxicity induced by CP is generally ascribed to oxidative stress, characterized by an increase in lipid peroxidation and a decrease in antioxidant enzymes. The testicular oxidative damage related with CP is well documented in the many in vivo experimental studies [4, 5]. It has been reported that both short- and long-term administration of CP cause a significant increase in lipid peroxidation and a significant decrease in antioxidant enzymes in testis tissue of rats [3, 6]. Ahmed et al. [2] indicated that a significant increase in lipid peroxidation and the superoxide anion levels in testicular tissue of rats occurred with low dose (2 mg/kg) CP treatment that was associated with a significant reduction in the activity of the antioxidant enzymes. In addition, it has been demonstrated in numerous studies that CP treatment leads to a significant decrease in reproductive organ weights (testis, epididymis, prostate), sperm quality [3, 7], and inhibition of testosterone secretion from Leydig cells as well as damages the testicular tissue [5, 8].
Montelukast (ML), a new anti-inflammatory agent, is a selective reversible cycteinyl leukotriene D4 (LTD4) receptor antagonist, and it directly interferes with leukotriene production and their receptors [9, 10]. Leukotriens increase in many illness and inflammatory conditions such as asthma, peptic ulcer, and ischemia/reperfusion (I/R) [11]. In this context, ML used oral treatments of asthma in adults and children [12]. On the other hand, because of their anti-inflammatory property through binding LTD4 receptor, ML provides a significant positive effect for preventing oxidative damage in some tissues such as testis and liver [13, 14]. A previous study [14] determined that ML prevents oxidative damage in testis tissue caused by I/R injury in rats through release of reactive oxygen species and an increase in glutathione (GSH) levels.
Experimental studies [2, 3, 5, 6, 15] show that administration of antioxidant compound such as lycopene, royal jelly, curcumin, melatonin, vit-C, and amifostine may protect reproductive system against CP. However, there is no study about whether the treatment of ML can prevent CP toxicity or not. In the present study, we aim to determine the protective effects of ML against reproductive toxicity of CP via examination of sperm characteristics, serum testosterone levels, histopathological, and oxidative changes in testis tissue.
Materials and methods
Chemicals
CP (10 mg/10 ml, Code 1876A) was obtained from Faulding Pharmaceuticals Plc (Warwickshire, UK). ML as Onceair® was provided by Abdi İbrahim drug company (İstanbul, Turkey). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) and were of analytical grade or of the highest grade available.
Animals and treatment
A total of 28 healthy adult male Spraque-Dawley rats (between 2 and 3 months old and 250–300 g in weight) obtained from Experimental Animal Institute, Malatya, Turkey, for this experiment. Animals were housed in sterilized polypropylene rat cages, in 12-h light–dark cycle, at an ambient temperature of 21°C. Diet and water for them were given ad libitum. Experiments were performed based on animal ethics guidelines of Institutional Animals Ethics Committee.
Rats were randomly divided into 4 equal groups (n = 7 in each group). CP was intraperitoneally (ip.) administered at the dose of 7 mg/kg single injection. The previous studies [5, 7] indicate that single injection of CP caused testicular damage in rats at the dose of 7 mg/kg (i.p.). ML, suspended in tap water, was given at the doses of 10 mg/kg/day for ten consecutive days. This dose was guided by previous studies [9, 14]. Group 1 (control) served as negative control and was given isotonic saline (i.p.) and tap water (orally) as vehicles. In group 2 (CP group), rats were treated with a single injection of CP and then with tap water for 10 days. Rats in the group 3 (ML group) were treated with ML for 10 days without CP. In the group 4, rats were treated with CP and ML (CP + ML group) together. Tissue and blood samples were collected on day 10 of CP treatment. The animals were killed under ether anesthesia, and testis tissues were immediately removed and dissected over ice-cold glass. Blood samples were collected from the left ventricle with an injector under anesthesia. Sera were obtained after whole blood centrifugation (3,000×g, 20 min, at 4°C). Tissue and serum samples were stored at −45°C in a deepfreeze until analysis.
Biochemical assay
The homogenization of tissues was carried out in teflonglass homogenizer with 150 mM KCl (pH 7.4) to obtain 1:10 (w/v) dilution of the whole homogenate. The homogenates were centrifuged at 18,000×g (4°C) for 30 min to determine thiobarbituric acid–reactive substances (TBARS), reduced glutathione (GSH) levels, and catalase (CAT) activities and at 25,000×g for 50 min to determine glutathione peroxidase (GPx) and CuZn-superoxide dismutase (SOD) activities.
The levels of homogenized tissue TBARS, as an index of lipid peroxidation, were determined by thiobarbituric acid reaction using the method of Yagi [16]. The product was evaluated spectrophotometrically at 532 nm, and results are expressed as nmol/g tissue. The GSH content of the testis homogenate was measured at 412 nm using the method of Sedlak and Lindsay [17]. The GSH level was expressed as nmol/ml. SOD activity was measured by the inhibition of nitroblue tetrazolium (NBT) reduction due to O2 − generated by the xanthine/xanthine oxidase system [18]. One unit of SOD activity was defined as the amount of protein causing 50% inhibition of the NBT reduction rate. The product was evaluated spectrophotometrically at 560 nm. Results are expressed as IU/mg protein. CAT activity of tissues was determined according to the method of Aebi [19]. The enzymatic decomposition of H2O2 was followed directly by the decrease in absorbance at 240 nm. The difference in absorbance per unit time was used as a measure of CAT activity. The enzyme activities are given in k/mg protein. GPx activity was measured by the method of Paglia and Valentina [20]. In the presence of glutathione reductase and NADPH, the oxidized glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP. The decrease in absorbance at 340 nm was measured. GPx activity is expressed as IU/mg protein. Tissue protein content was determined according to the method developed by Lowry et al. [21] using bovine serum albumin as standard.
Evaluation of sperm parameters
The epididymal sperm concentration was determined with a hemocytometer using a modified method briefly described by [22]. The right epididymis was finely minced by anatomical scissors in 1 ml of isotonic saline in a Petri dish and allowed to incubate at room temperature. Then, the supernatant fluid containing all epididymal sperm cells was counted with the help of a light microscope at 200× magnifications. The percentage of forward progressive sperm motility was evaluated using a light microscope with heated (37°C) stage as described by [23]. The percentage of forward progressive sperm motility was evaluated visually at 400× magnification. Motility estimates were performed from three different fields on each sample. The mean of the three successive estimations was used as the final motility score. To determine the percentage of morphological abnormal spermatozoa, the slides stained with eosin–nigrosin were prepared. The slides were then viewed under a light microscope at 400× magnification. A total of 300 sperm cells was examined on each slide (2,100 cells in each group), and the head, tail, and total abnormality rates of spermatozoa were expressed as a percentage [22, 23].
Histological examination
For histopathological examination, testicle samples were taken and fixed in 10% neutral-buffered formalin, processed routinely, and stained with hematoxylin–eosin (H&E). The testicular sections were analyzed for qualitative changes and classified into two groups, normal and abnormal tubules. The normal tubules were the ones showing normal cell associations without any qualitative changes. The abnormal tubules were ones with a decline germinal epithelium, degeneration, desquamation, and disorganization of germinal cells. In each testicular section, abnormal and normal tubules were counting in 5 randomly selected microscopic fields, and abnormal tubules percentages were calculated. In addition, about 25 horizontally sectioned seminiferous tubules per rat were measured to determine the mean tubule diameters by ocular micrometer and thickness of germinal layers from the basal membrane to luminal surface.
Hormonal analysis
Serum testosterone level was determined by enzyme-linked immunosorbent assay (ELISA) using anti-rat ELISA commercial kits from Cayman Chemical Company (Ann Arbor, MI, USA) according to the manufacturer’s instructions. The plates were read at 405 nm using the CA-2000 ELISA microplate reader and washer (CIOM Medical Co., Ltd. in China). Testosterone quantities in the samples were calculated from standard curves of testosterone using a linear regression method.
Statistical analysis
All values were presented as mean ± SEM. Significant differences (P value) are given in Table 1. A computer program SPSS 11.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. For the comparison of biochemical and spermatological parameters, statistical analyses were performed using one-way ANOVA and post hoc Duncan significant difference test. Histological results were compared with Kruskal–Wallis variance analysis and chi square.
Results
Biochemical evaluation
The levels of SOD, GPx, CAT, GSH, and TBARS for rat testis are given in Table 1. The results showed that CP treatment caused a significant increase in TBARS levels compared with control and other experimental group. Moreover, GSH, CAT, SOD, and GPx levels were significantly decreased in CP group according to control. On the other hand, ML treatment together with CP caused a significant decrease in elevated TBARS levels and a significant increase in only SOD levels in comparison with the CP alone group. But CAT, GPx, and GSH levels did not significantly change in CP + ML group compared with CP group.
Organ weights and sperm parameters
The effects of CP and ML on the testis, epididymis, seminal vesicles, prostate weight, epididymal sperm concentration, sperm motility, and abnormal sperm rate are presented in Table 2. The results indicate that there was no significant change in organ (testis epididymis, seminal vesicles, prostate) weights, abnormal sperm rate, and sperm concentration with CP or ML treatment compared with control group. However, CP causes a significant (P < 0.05) decrease in sperm motility compared with control and other groups. On the other hand, sperm motility was significantly increased in ML group according to CP groups. In addition, ML treatment together with CP significantly increases low sperm motility caused by CP.
Histological evaluation
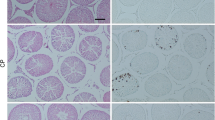
The testicular structure in the control and ML-treated groups was normal without any pathological changes in the cell associations in the seminiferous tubules or the intertubular area (Fig. 1a). The effects of CP and ML on tubular diameter, epithelial germinal layer thickness, and abnormal tubules percentage for each group are presented in Table 3. CP affected the testicular structure, and 24.75% tubules were abnormal. The major findings on testis of CP were germ cell degeneration, epithelial sloughing, vacuoles, thinning of germinal cell layers, and tubular shrinkage (Fig. 1b, c). In these abnormal tubules, generally maturation arrests were found. In addition, a various degree edema was seen in the interstitial spaces. Compared with CP alone group, the ratio of abnormal tubules in the CP + ML group was decreased, 12.82% versus 24.75%. In CP + ML group, tubular degeneration and atrophy, epithelial sloughing were also seen (Fig. 1d). Histopathological changes observed in CP + ML group were milder than the CP alone group. However, ML was unable to recover the structural affects induced by CP to the control group level.
a Photomicrograph of a control group showing normal histological appearance of testis. Hematoxylin and eosin X320. b Photomicrograph of a testicular section of a rat treated with cisplatin alone group. Many tubules show degeneration, thinning of germinal cell layers (arrowheads), vacuoles of epithelial cells (arrows). Hematoxylin and eosin X320. c High magnification of a testicular section of a rat treated with cisplatin alone group; Note that the tubular degeneration and shrinkage (asterisks), thinning of germinal cell layers (arrowhead) and maturation arrest. Hematoxylin and eosin X640. d A testicular section of a rat treated with CP + ML group showing tubular atrophy (arrow) and germ cell degeneration and epithelial sloughing (asterisks). Hematoxylin and eosin X320
The tubular diameter and germinal layer thickness of control and ML-treated rats did not show any significant differences. CP decreased the tubular diameter and germinal layer thickness, and the differences in the data between control and CP alone were significant (P < 0.001). ML decreased the morphometric affects of CP; however, there was also a significant differences (P < 0.05) (Table 3).
Hormonal results
Serum testosterone levels are presented in Fig. 2. It was determined that serum testosterone levels with CP treatment were significantly decreased (P < 0.01) compared with control and other groups. ML treatment did not significantly change serum testosterone levels compared with control. However, ML treatment reversed the effects of CP on serum testosterone levels, and this value was near to that in the control group.
Discussion
Although CP has amazing anticancer properties, the therapeutic use of it limited due to its severe toxic effects on some organs and system such as kidney, heart, liver, and reproductive system [24–26]. In this context, many studies examined that how toxic effects of CP prevented when clinically used. In the present study, it was shown that CP treatment induced reproductive toxicity via increased lipid peroxidation, decreased antioxidant status, changed sperm characteristics, and serum testosterone level in rats. Also, we first determined that ML can reverse these toxic effects of CP on the reproductive system when used together with CP.
Oxidative and histological changes
Oxidative stress is a condition of an imbalance between the free radicals (such as TBARS) and antioxidant defense system (activity of SOD, CAT, GPx, and GSH levels). TBARS generated with peroxidation by reactive oxygen species of fatty acids is regarded as an indicator of lipid peroxidation and leads to irreversible cell damage [27]. Testis is the major target organs for oxidative stress because of its high content of polyunsaturated membrane lipids [28]. Our results showed that CP significantly induced lipid peroxidation in rat testis tissue via increased TBARS levels. Similarly, previous studies [5, 26] as well as our study indicate that CP treatment caused significant lipid peroxidation in rat testis tissue and these studies confirmed our findings. On the other hand, ML treatment could prevent lipid peroxidation through reduced TBARS levels in testis tissue. As far as we are aware there was no study about how ML affects testis tissue against lipid peroxidation. However, Ozturk et al. [14] determined that ML treatment reduced TBARS levels, elevated with I/R, in testis tissue of rats. Besides, it was observed that ML prevents lipid peroxidation in liver and blood caused by carbon tetrachloride [29]. These results are in agreement with our findings. Enzymatic (SOD, CAT, GPx) and nonenzymatic (GSH) antioxidants protect cells against lipid peroxidation damage and scavenge free radicals [30]. In the present study, the results indicate that SOD, CAT, GPx, and GSH levels are decreased with CP treatment in testis tissue. It is well known that CP leads to a significant reduced antioxidant status in humans and animals [2, 3]. On the other hand, ML given together CP generally reversed toxic effects and numerically increased the SOD, CAT, GPx, and GSH levels. Similarly previous studies [11, 12] showed that ML has significant antioxidant property, and these results are paralleled with our findings. However, CAT, GSH, and GPx levels did not significantly changed in CP + ML group compared with CP group. This situation may be explained with sensitivity of the rats and duration of drug treatment. In the histological evaluation, it was determined that ML treatment did not histopathologically change in testis tissue compared with control. On the other hand, CP histopathologically caused significant alterations in testis tissue structure such as germ cell degeneration, epithelial sloughing, vacuoles, thinning of germinal cell layers, and tubular shrinkage. In addition, our findings indicate that histopathological damages decreased when ML combined with CP. Many previous studies [1, 3] are in agreement with our findings, and they determined CP treatment increased histopathological damage in testis tissue. It was considered that the histopathological effects may attribute to the imbalance between oxidant and antioxidant statuses in testis tissue induced by CP. Moreover, this situation may contribute to male infertility by reducing sperm function. For this reason, a decrease in elevated oxidative stress in testis tissue with ML is very important in terms of infertility.
Hormonal evaluation
This study showed that serum testosterone level has significantly decreased with CP treatment in rats. However, ML treatment did not change serum testosterone levels compared with the control group. On the other hand, ML treatment reversed toxic effect of CP on testosterone levels and increased it when given together with CP. It was showed that CP treatment significantly decreases the testosterone levels as well as our study [5, 7]. As far as we are aware there was no study about the effects of ML treatment on serum testosterone levels. We and other researchers [5, 7] thought that this effect of CP may occur through interference with LH receptor expression, impairment of the cholesterol mobilization to mitochondrial cytochrome P450scc, or reduction of the activity of this enzyme, thus interfering with the first steps in testosterone production.
Organ weight and spermatological evaluation
Although CP treatment caused significant decrease in sperm motility, it had no significant effect on the reproductive organ weights (testis, seminal vesicles, prostate), epididymal sperm concentration, and abnormal sperm rate in the present study. Similarly, Türk et al. [31] indicated that sperm motility in rats treated with CP was found to decrease at the dose of 7 mg/kg in rats and this result is in agreement with our study. However, in the same study, they claimed that CP could significantly decrease organ weights, sperm concentration, and increase abnormal sperm rate in contrast to our study. It is thought that this difference about organ weight, sperm concentration, and abnormal sperm rate may be due to sensitivity of the rats and duration of drug treatment. Additionally, in this study, we showed that ML significantly increased sperm motility and prevented toxic effect of CP. Previously, spermatological effects of ML did not examine and our results constitute the first data in this field. “In this context, the decrease in sperm motility after CP administration may be explained by the increased free-radical production, decreased antioxidant enzymes, and ATP depletion that is mainly necessary for the flagellum movement of the sperm”. For that, when CP toxicity occurred, ML treatment can be beneficial due to its antioxidant properties and may be positively affect infertility caused by CP.
Conclusion
In the present study, (1) we confirmed the toxic effects of CP at the single dose of 7 mg/kg on testicular (increased oxidative stress and histological changes in tissue), spermatological (decreased sperm motility), and hormonal (decreased serum testosterone levels) damage in rats. Also, (2) it is shown that ML treatment in combination with CP at the dose of 10 mg/kg/day for ten consecutive days generally reversed toxicity of CP about the reproductive system. The beneficial effects of ML against CP-induced reproductive damage may be due to its antioxidant and anti-inflammatory properties. Therefore, according to our results, we claim that ML, a specific LTD4 receptors antagonist, may attenuate the CP-induced reproductive toxicity.
References
Lirdi LC, Stumpp T, Sasso-Cerri E, Miraglia M (2008) Amifostine protective effect on cisplatin-treated rat testis. Anat Rec (Hoboken) 291:797–808
Ahmed EA, Omar HM, Elghaffar SKH, Ragb SM, Nasser AY (2011) The antioxidant activity of vitamin C, DPPD and l-cysteine against cisplatin-induced testicular oxidative damage in rats. Food Chem Toxicol 49:1115–1121
Ilbey YO, Ozbek E, Cekmen M, Simsek A, Otunctemur A, Somay A (2009) Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum Reprod 24:1717–1725
Amin A, Hamza AA (2006) Effects of roselle and ginger on cisplatin-induced reproductive toxicity in rats. Asian J Androl 8:607–612
Silici S, Ekmekcioglu O, Eraslan G, Demirtas A (2009) Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology 74:545–551
Fung C, Vaughn DJ (2011) Complications associated with chemotherapy in testicular cancer management. Nat Rev Urol 8:213–222
Ateşşahin A, Karahan I, Türk G, Gür S, Yilmaz S, Ceribaşi AO (2006) Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol 21:42–47
Al Vawda (1994) Effect of testosterone on cisplatin-induced testicular damage. Arch Androl 32:53–57
Muthuraman A, Sood S (2010) Antisecretory, antioxidative and antiapoptotic effects of montelukast on pyloric ligation and water immersion stress induced peptic ulcer in rat. Prostaglandins Leukot Essent Fat Acids 83:55–60
Drazen JM, Israel E, O’Byrne PM (1999) Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 340:197–206
Dengiz GO, Odabasoglu F, Halici Z, Cadirci E, Suleyman H (2007) Gastroprotective and antioxidant effects of montelukast on indomethacin-induced gastric ulcer in rats. J Pharmacol Sci 105:94–102
Canbay E, Agachan B, Ozturk T, Giris M, Asoglu O, Balik E, Bugra D (2010) Dual inhibition of wound healing and oxidative process by montelukast in experimental colon anastomoses. Surg Innov 17:248–255
Ozkan E, Yardimci S, Dulundu E, Topaloğlu U, Sehirli O, Ercan F, Velioğlu-Oğünç A, Sener G (2010) Protective potential of montelukast against hepatic ischemia/reperfusion injury in rats. J Surg Res 159:588–594
Ozturk H, Ozturk H, Gideroglu K, Terzi H, Bugdayci G (2010) Montelukast protecs against testes ischemia/reperfusion injury in rats. Can Urol Assos J 4:174–179
Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A (2009) Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril 92:1124–1132
Yagi K (1998) Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol 108:101–106
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sun Y, Oberley LW, Li YA (1988) Simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) In methods of enzymatic analysis. Academic Press, NY, pp 673–677
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Lowry OH, Rosebrough NJ, Farr AL, Randall RI (1951) Protein measurement with folin phenol reagent. J Biol Chem 193(1):265–275
Ciftci O, Aydin M, Ozdemir I, Vardi N (2011a) Quercetin prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced testicular damage in rats. Andrologia. doi:10.1111/j.1439-0272.2010.01126
Ciftci O, Ozdemir İ, Aydin M, Beytur A (2011b) Beneficial effects of chrysin on the reproductive system of adult male rats. Andrologia. doi:10.1111/j.1439-0272.2010.01127
Sawhney P, Giammona CJ, Meistrich ML, Richburg JH (2005) Cisplatin-induced long-term failure of spermatogenesis in adult C57/Bl/6J mice. J Androl 26:136–145
Ciftci O, Ozdemir I, Vardi N, Gurbuz N (2010) Novel platinum-N-heterocyclic carbene complex is more cardiotoxic than cis-platin in rats. Hum Exp Toxicol. doi:10.1177/096032711039006
Ateşşahin A, Sahna E, Türk G, Ceribaşi AO, Yilmaz S, Yüce A, Bulmuş O (2006) Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res 41:21–27
Montjean D, Ménézo Y, Benkhalifa M, Cohen M, Belloc S, Cohen-Bacrie P, de Mouzon J (2010) Malonaldehyde formation and DNA fragmentation: two independent sperm decays linked to reactive oxygen species. Zygote 18:265–268
Chainy GBN, Samantaray S, Samanta L (1997) Testosterone-induced changes in testicular antioxidant system. Andrologia 29:343–349
Cuciureanu M, Căruntu ID, Păduraru O, Stoica B, Jerca L, Crauciuc E, Nechifor M (2009) The protective effect of montelukast sodium on carbon tetrachloride induced hepatopathy in rat. Prostaglandins Other Lipid Mediat 88:82–88
Schiller HJ, Reilly PM, Bulkley GB (1993) Tissue perfusion in critical illnesses. Antioxidant therapy. Crit Care Med 21:92–102
Türk G, Ateşşahin A, Sönmez M, Ceribaşi AO, Yüce A (2008) Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril 89:1474–1481
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beytur, A., Ciftci, O., Oguz, F. et al. Montelukast attenuates side effects of cisplatin including testicular, spermatological, and hormonal damage in male rats. Cancer Chemother Pharmacol 69, 207–213 (2012). https://doi.org/10.1007/s00280-011-1692-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1692-y