Abstract
5-FU is an anticancer drug that is widely used to treat cancers, including colorectal cancer (CRC); however, chemoresistance to 5-FU remains an important problem to be resolved. The role of microRNAs (miRs) in chemosensitivity has recently been studied in the development of therapeutic strategies to overcome drug resistance. Here, we focused on miR-96, which has been reported to demonstrate chemosensitivity. We investigated whether 5-FU sensitivity may be modulated by miR-96 in monolayer cells and whether this relationship also applies for drug resistance in 3D tumor spheroids (TSs). When the level of miR-96 increased, the expression of the anti-apoptotic regulator XIAP and p53 stability regulator UBE2N decreased, resulting in increased apoptosis and growth inhibition following 5-FU exposure. Transfection of miR-96 inhibitors resulted in an overexpression of XIAP and UBE2N, yet only minimal change was induced in apoptosis. Nonetheless, luciferase assay failed to show direct interactions between miR-96 and these genes. In TSs, 5-FU resistance corresponded to a significantly lower level of miR-96, however only XIAP, not UBE2N, was up-regulated demonstrating partial agreement with the 2D condition regarding target expression. Overall, these results suggest that miR-96 may modulate 5-FU sensitivity in CRC cells by promoting apoptosis; however, differential expression of target genes in TSs warrants further studies on the 5-FU resistance mechanism under 3D conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoropyrimidine 5-fluorouracil (5-FU) is a widely used drug to treat cancer including colorectal cancer (CRC). Thus resistance to 5-FU is an important problem in cancer chemotherapy (Schmidt et al. 2004). Such resistance can be attributed to changes in the expression of apoptosis-regulating genes; in CRC tumors and cell lines resistant to 5-FU, down-regulation of the pro-apoptotic genes BAX and BID and up-regulation of the anti-apoptotic genes cellular inhibitor of apoptosis 1 (cIAP1) and X-linked inhibitor of apoptosis (XIAP) have been reported compared to wild-type cells (Manoochehri et al. 2014). Thus, sensitivity to 5-FU can be rescued by inhibiting the expression of genes causing enhanced apoptosis signaling (Karasawa et al. 2009). Recovery of sensitivity to drug-induced apoptosis may serve as an important strategy for 5-FU-containing chemotherapy regimens (Zhang et al. 2008).
MicroRNAs (miRs) are single-stranded 19–25 nucleotide short non-coding RNAs that affect various processes during tumor development via the post-transcriptional control of target mRNAs (Croce 2009). Not only do miRs have roles in tumorigenesis, but some miRs have also been reported for their role as a modulator of chemotherapeutic efficacy. Recently, gemcitabine resistance has been shown to be induced indirectly by the miR-200 family, which promotes epithelial-mesenchymal transition in pancreatic cancer cells (Li et al. 2009). MiR-153 has also been implicated in the regulation of drug response to platimun-based agents, oxaliplatin and cisplatin, in CRC patients (Zhang et al. 2013). Because drug resistance accounts for most of the tumor relapses in chemotherapy-treated patients, understanding the role of miRNAs and their mechanism in chemosensitivity may aid in the development of therapeutic strategies aimed at overcoming drug resistance and limited efficacy.
Previously, we revealed the changes in miRNA expression in pancreatic TSs, and a potential association with 3D drug resistance has been suggested (Yeon et al. 2013). In this study, we focused on miR-96, as its up-regulation has been reported in various cancers including CRC (Mattie et al. 2006; Hamfjord et al. 2012). Additionally, low expression levels of miR-96 have been associated with poor clinical outcomes in CRC patients (Ress et al. 2014). Furthermore, miR-96 has been shown to promote sensitivity to cisplatin and has been suggested as a potential therapeutic agent (chemosensitizer) for cancer (Wang et al. 2012). However, this mechanism has not been fully analyzed.
Compared to conventional 2D monolayer cultures, tumor spheroids (TSs), a 3D tumor culture model, closely mimic in vivo microenvironmental features of solid tumors, such as cell–cell interaction, cell–extracellular matrix interaction, hypoxia, and limited penetration of drugs (Tredan et al. 2007). Recently, 3D cell culture models have been accepted for the study of pharmacokinetics and pharmacodynamics of anticancer agents as valid in vitro models representing many clinically relevant cues (Hicks et al. 2006; Mikhail et al. 2013). We and others have demonstrated the in vivo relevance of TSs with regard to drug resistance compared to monolayers (Minchinton and Tannock 2006; Kim et al. 2009; Yeon et al. 2013; Lee et al. 2014).
In this study, we studied the correlation between miR-96 and 5-FU sensitivity in colorectal cancer cells. Introduction of a miR-96 mimic into cells resulted in the reduced expression of its putative targets, such as the anti-apoptotic regulator XIAP and the p53 stability regulator UBE2N (ubiquitin-conjugating enzyme E2N), and the opposite effect was observed with miR-96 inhibitors. The proliferation rate and sensitivity to 5-FU were elevated in cells transfected with the miR-96 mimic, which was observed in parallel with enhanced apoptosis signaling mediated by caspase-3 activation and PARP cleavage. Although presenting 5-FU resistance, 3D TSs showed partial agreement with the effect of miR-96 expression, i.e., only XIAP, but not UBE2N, was induced by miR-96 in CRC cells.
Materials and methods
Reagents and cell culture
5-FU was purchased from Sigma-Aldrich (St. Louis, MO). HT-29, DLD-1 and HCT-116 human colorectal cancer cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were maintained in RPMI1640 (Gibco BRL, Grand Island, NY) supplemented with 100 μg/mL streptomycin, 100 units/mL penicillin, 250 ng/mL amphotericin B and 10 % fetal bovine serum (FBS) (WELGENE, Daegu, Korea) in a humidified atmosphere (5 % CO2/95 % air) at 37 °C.
Formation of 3D tumor spheroids
TSs were prepared using HT-29, DLD-1 or HCT-116 cells using the liquid overlay technique as previously described (Kobayashi et al. 1993). Briefly, 103 cells were seeded onto round-bottom 96-well plates, which were coated with 55 μL of 1.5 % SeaPlaque® Agarose (Lonza, Basel, Switzerland). TSs were maintained under normal cell culture conditions with daily media change, and the growth of spheroids was monitored by size.
MicroRNA transfection
Transfection of miRNA mimic or inhibitor was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Mock miRNAs (negative control (NC), miR-96 and Anti-96) were purchased from Genolution Pharmaceuticals, Inc. (Seoul, Korea). NC values were used as a universal control: UCACAACCUCCUAGAAAGAGUAGA. Anti-96, a miR-96 inhibitor, was chemically synthesized.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen). For qRT-PCR of miR-96, reverse transcription was performed using the Mir-X miRNA First-strand Synthesis Kit (Clontech Laboratories, Inc., Mountain View, CA) according to the manufacturer’s instruction. For qRT-PCR of XIAP, UBE2N, MDM2 and p53, reverse transcription was performed using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Otsu, Japan) according to the manufacturer’s instructions. U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as housekeeping genes for standardization of the initial miRNA and mRNA content of each sample. Triplicate quantitative PCR reactions were performed using the Stratagene Mx3000P QPCR system with SYBR Premix Ex Taq II (Takara Bio Inc.) according to the manufacturer’s instructions. Quantification of relative gene expression was performed using the ΔΔCt method (2−ΔΔCt).
Luciferase assay
Partial 3′ untranslated regions (UTRs) of XIAP (2550–2772 bp) and UBE2N (826–1571 bp) containing the predicted miR-96 binding site were cloned into psiCHECK renilla/firefly luciferase vectors. These psiCHECK vectors were co-transfected with NC or miR-96 mimic into HT-29 cells. Cells were incubated for 24 h, and the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI) was used to measure changes in luciferase activity. Relative luciferase activity was calculated by normalizing the ratio of renilla/firefly luciferase.
Western blotting analysis
For protein extraction, cells were lysed in RIPA buffer containing 25 mM Tris, pH 7.6, 150 mM NaCl, 1 % NP-40, 5 mM EDTA, 1 % sodium deoxycholate, 0.1 % SDS supplemented with Halt™ Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, Rockford, IL). Total protein in lysates was quantified using the Pierce® BCA Protein Assay Kit (Thermo Scientific). Twenty micrograms of total protein was resolved on 15 % SDS-PAGE gel under reducing conditions and transferred onto Immun-Blot® PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 5 % skim milk in TBS with 0.05 % Tween-20 (TBS-T) at room temperature and incubated with antibodies against caspase-3 (1:2,000, ABclonal, Cambridge, MA), PARP (1:1,000, Cell Signaling, Danvers, MA), and β-actin (1:5,000, Thermo Scientific) at 4 °C overnight. After exposure to horseradish peroxidase-conjugated secondary antibodies, the proteins were visualized using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific).
Acid phosphatase (APH) assay for drug response
Drug sensitivity was determined using the acid phosphatase (APH) assay. Briefly, cells were plated at 3 × 103 cells/well, and TSs cultured for 2 days were transferred onto new 96-well plates (Friedrich et al. 2007). After 48 or 72 h of drug exposure, the APH assay was performed as previously described (Yeon et al. 2013), i.e., cells or TSs were carefully washed with PBS and incubated in 100 μL of assay buffer (0.1 M sodium acetate, 0.1 % Triton-X 100 in deionized water supplemented with p-nitrophenyl phosphate (Pierce, Rockford, IL)) at 37 °C for 90 min. Ten microliters of 1 N NaOH was then added to each well, and absorption at 405 nm was measured on an ELISA reader within 15 min. Cell viability was expressed as % of control, and the IC50 was calculated using the Emax model (Park et al. 2004) with SigmaPlot 12.5 software (Point Richmond, CA).
Sulforhodamine B (SRB) and wound healing assay for cell proliferation
Changes in the proliferative activity of HT-29 cells after transfection were determined using the SRB assay (Vichai and Kirtikara 2006). Briefly, cells were harvested and plated into 96-well plates and cultured until 72 h. At predetermined times, 150 μL of 10 % trichloroacetic acid was added to terminate the growth, and the plates were then incubated at 4 °C for one hour. The precipitated cells were washed, air-dried and stained using SRB solution (100 μL, 0.4 % in 1 % acetic acid) at room temperature for 10 min. After washing with 1 % acetic acid and air-drying, Tris–HCl (150 μL, 10 mM, pH 7.1) was added and the optical density was measured at 540 nm.
The wound healing assay was also used to determine changes in the proliferative activity of HT-29 cells after transfection. A wound was made in the monolayers of confluent cells in a 6-well plate by scraping the central region with a P10 plastic pipette tip and rinsing twice with PBS to remove floating cells. The underside of the dish was marked for the wounded area, and the images were taken initially and after 48 h at the same location using a microscope equipped with a camera. Cell motility was evaluated by comparing the reduction in distance between wound edges.
Statistical analysis
Statistical analyses were performed using Microsoft Excel 2010. Student’s t test and analysis of variance (ANOVA), followed by Tukey’s test, were performed to test the statistical significance, where p < 0.05 was considered statistically significant.
Results
Effect of miR-96 level on the expression of apoptosis- and proliferation-related genes
To characterize the function of miR-96, we first performed a target search. We listed putative miR-96 target genes from Miranda (http://www.microrna.org/), PicTar (http://pictar.mdc-berlin.de/) and Target Scan Human (http://www.targetscan.org/). From this list, we selected UBE2N, MDM2 and XIAP, which are associated with cell death and survival pathways as putative targets of miR-96 conferring 5-FU sensitivity.
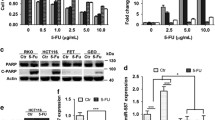
To evaluate the effect of miR-96 on putative target genes, we determined their expression in HT-29 cells transfected with miR-96 mimic or inhibitor using qRT-PCR. In addition, expression of FOXO3, a known direct target of miR-96, was also determined as a reference (Lin et al. 2010). The level of miR-96 in HT-29 cells increased approximately 45-fold by the miR-96 mimic and its endogenous level decreased approximately 52 % by anti-96 inhibitor transfection (Fig. 1a). Expression of FOXO3 showed decreased levels upon miR-96 mimic transfection and increased levels upon inhibitor transfection, which indicates the validity of the transfection experiment. Among the target genes, UBE2N and XIAP were negatively regulated by miR-96 expression, whereas MDM2 did not show significant changes (Fig. 1b).
Changes in the expression of apoptosis-related genes such as XIAP and UBE2N in HT-29 cells transfected with miR-96 mimic or anti-96 inhibitor. The expression level of miR-96 was significantly increased or decreased by transfection with 50 nM miR-96 mimic or anti-96 inhibitor, respectively (a). FOXO3, a known target gene of miR-96, was measured as a reference. The caspase inhibitor XIAP and p53 stability regulator UBE2N showed parallel changes with miR-96 transfection, whereas no changes were observed in MDM2 (b). Cells were transfected with miR-96 mimic or inhibitor, and the expression levels were determined using qRT-PCR. Data are expressed as the mean ± SD. *p < 0.05, and **p < 0.01
To test the direct interaction of miR-96 with the XIAP and UBE2N mRNA 3′UTR regions, a dual luciferase reporter assay was performed; however, no changes in relative luciferase activity were observed (Fig. 2), suggesting no direct interaction between miR-96 and these genes.
XIAP and UBE2N are not direct targets of miR-96. Putative miR-96 binding sites in the transcripts of XIAP and UBE2N (a). No changes in the luciferase activity of the 3′UTR-luciferase mRNA constructs of target genes (XIAP and UBE2N) after co-transfection with the miR-96 mimic in HT-29 cells were observed (b). Data are expressed as the mean ± SD
Taken together, these results suggest that miR-96 may affect apoptosis signaling by altering the expression levels of XIAP and UBE2N in an indirect manner in HT-29 cells.
Effects of miR-96 in 5-FU sensitivity and cell proliferation
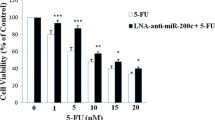
The effect of the miR-96 expression level on sensitivity to 5-FU was examined using viability assay and by measuring apoptosis induction in HT-29 cells. Increased levels of miR-96 sensitized HT-29 cells to 5-FU treatment via an increase in apoptosis, as shown by a left shift of the dose–response curve and significant increase in PARP cleavage (Fig. 3a). It is important to note that no significant changes were observed for caspase-3 activation despite increased PARP cleavage. Caspase-3 activation was seen only for control versus mimic transfected cells without drug treatments. However, decreased levels of miR-96 protected HT-29 cells from 5-FU treatment, albeit subtly within a limited range of concentrations, as shown by differences in the dose–response curve and slightly reduced levels of cleaved caspase-3 but not of PARP cleavage (Fig. 3b). These data suggest that changes in the expression level of miR-96 might modulate drug-induced apoptosis signaling and contribute to sensitivity to 5-FU in HT-29 cells.
MiR-96 affects sensitivity to 5-FU and cellular proliferation in HT-29 cells. Overexpression of miR-96 significantly increased sensitivity to 5-FU with the induction of cleaved PARP (c-PARP) (a). Down-regulation of miR-96 caused a subtle decrease in sensitivity to 5-FU with a minimal decrease in caspase activation (b). Drug sensitivity was measured using the APH assay, and the level of caspase activation and PARP cleavage were assessed using western blotting analyses following 48 h of exposure to 5-FU in HT-29 cells transfected with miR-96 mimic or the inhibitor anti-96. Increased proliferation was observed in cells overexpressing miR-96, as determined using the SRB assay and wound healing assay (c). Scale bar 500 μm. Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001
To confirm the effect of miR-96 on cellular proliferation, we performed cell viability assays. As shown in Fig. 3c, overexpression of miR-96 accelerated cell proliferation. The same changes were observed in the wound healing assay, i.e., twofold increase in migration distance by mimic-transfected cells, whereas cells transfected with the anti-96 inhibitor showed no changes in either the proliferation assay or wound healing assay. These data indicate that miR-96 may positively regulate cellular proliferation in HT-29 cells. Collectively, these results suggested that miR-96 may exert effect on sensitivity to 5-FU as well as on cellular proliferation.
Tumor spheroid culture induced down-regulation of miR-96 and decreased sensitivity to 5-FU
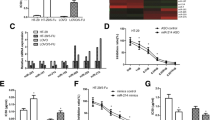
We evaluated the endogenous expression level of miR-96 and 5-FU sensitivity in the CRC cell lines HT-29, DLD-1 and HCT-116, when grown as either 2D monolayers or 3D TSs (Fig. 4), confirming the results obtained from transfection studies in 2D monolayers of HT-29 cells. When miR-96 expression levels were compared between monolayers and TSs using qRT-PCR, a significant decrease was observed in all three TSs (approximately 93, 74, and 67 % decreases for HT-29, DLD-1, and HCT-116, respectively), and that the level in HT-29 TSs was lower than that in monolayers transfected with 50 nM of anti-96 inhibitor. Treatment with 5-FU caused a dose-dependent decrease in cell viability in all three cell lines. HCT-116 cells showed the greatest sensitivity to 5-FU compared to the other two cell lines, as demonstrated by less than 20 % survival at 10 μM, the lowest concentration tested. TSs of all three cell lines showed significant resistance to 5-FU compared to 2D culture conditions. HT-29 cells showed the smallest changes in sensitivity but showed more than a tenfold increase in IC50 in TSs compared to monolayers, i.e., 31.3 μM; 3D: 466 μM in 2D and TSs, respectively. DLD-1 cells showed the greatest changes in sensitivity, i.e., less than 10 % reduction was shown at 10 μM in 3D viability, whereas there was more than 50 % killing in the 2D condition. These data suggested that the 3D culture condition displays drug resistance due to molecular changes related to drug-induced apoptosis signaling, which may be associated with downstream signaling of miR-96.
Comparison of miR-96 expression level and sensitivity to 5-FU in three colorectal cancer cell lines grown as 2D monolayers or 3D TSs. All three cell lines, HT-29 (a), DLD-1 (b) and HCT-116 (c), showed a significantly decreased level of miR-96 as measured using qRT-PCR. Sensitivity to 5-FU was also significantly reduced in TSs of all three cell lines when exposed to 5-FU for 72 h, and cell viability was measured using the APH assay. Inhibition (%) was expressed as the % viability compared to control (no drug treatment). Data are expressed as the mean ± SD. *p < 0.05
Changes in the expression levels of putative target genes of miR-96 in tumor spheroids of HT-29 cells
As miR-96 showed a dramatic reduction in spheroid cultures, we determined changes in the expression of putative target genes in 2D and 3D cultures of HT-29 cells. As expected, the level of FOXO3 showed a significant up-regulation in TSs (Fig. 5a). Interestingly, UBE2N and MDM2 showed a significant down-regulation in TSs compared to 2D cultures in contrast to the elevated levels of XIAP (Fig. 5b). These results in 3D culture were inconsistent with those from 2D monolayer cells after transfection (i.e., changes in UBE2N and MDM2), which indicate that gene regulation by upstream miRNA expression might be subjected to different cellular contexts present in 2D and 3D culture conditions, with particular regard to genes related to cell cycle regulation, such as UBE2N and MDM2.
Differential expression of miR-96-related genes in HT-29 cells grown as 2D monolayers or 3D TSs. The mRNA levels of the transcription factor FOXO3 showed significantly increased levels when cells were grown in 3D TSs (a). Among the putative target genes of miR-96, the caspase inhibitor XIAP showed a significant increase when cells were grown in 3D TSs, whereas a significant decrease was observed in genes regulating p53 stability, such as UBE2N and MDM2 (b). Gene expression levels were determined using qRT-PCR. Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001
Discussion
In the present study suggests that miR-96 may play an important drug-sensitizing role via deregulation of XIAP and UBE2N expression, resulting in enhanced apoptotic cell death as illustrated in Fig. 6. The additional effect of enhanced proliferation by miR-96 may act synergistically with cell-cycle-dependent drugs, such as 5-FU, in inducing apoptosis in CRC cells (Fig. 3c). In 3D TSs, a significantly lower level of miR-96 and resistance to 5-FU was observed, but changes in target gene expression showed partial agreement with the findings obtained in the 2D condition, i.e., only XIAP, but not UBE2N, showed increased expression. Since miR-binding regions predicted in silico by available databases do not guarantee direct binding of miRs, a dual luciferase reporter assay was used to confirm the binding, however, a negative result was obtained. Similar negative results were found when only one of two putative binding sites for miR-96 or miR-203 showed a direct targeting interaction (Wang et al. 2012; Zhou et al. 2014). Indirect effects on putative targets and a partial agreement between the 2D and 3D conditions warrants further studies in order to determine the mechanism underlying the 5-FU resistance induced by the suppression of miR-96 in CRC cells.
A schematic illustration for the modulation of 5-FU-induced apoptosis by miR-96. MiR-96 may suppress the expression of anti-apoptotic genes such as UBE2N and XIAP, resulting in enhancement of 5-FU-induced apoptosis in HT-29 cells grown as monolayers. Under 3D culture condition, suppressed miR-96 expression was observed in addition to increased levels of XIAP. Nevertheless, UBE2N showed decreased levels. Although expression of MDM2 was not modulated in monolayer cells transfected with the miR-96 mimic or inhibitor, significant down-regulation of MDM2 was measured in 3D TSs, suggesting differential regulation of gene expression by the 3D cellular context and microenvironmental signals. Overall, miR-96 might have a role in modulating 5-FU sensitivity by promoting apoptosis signaling; however, differential gene regulation in the 3D condition should be considered for extrapolation in vivo
Conflicting results have been reported on the role of miR-96 with regard to different cancer types. In pancreatic cancer in vitro and in vivo, miR-96 has been shown to function as a tumor suppressor gene that directly targets the KRAS oncogene and human ether-a-go-go-related potassium channel (HERG1), and down-regulation of miR-96 has been proposed as an early marker of pancreatic carcinogenesis and a potential therapeutic target (Tanaka et al. 2014; Yu et al. 2010; Feng et al. 2014). On the other hand, miR-96 has been shown to be up-regulated in various types of cancer; thus, it is recognized as an oncogenic miRNA with a potential role in metastasis in breast cancer (Guttilla and White 2009; Lin et al. 2010), prostate cancer (Haflidadottir et al. 2013), hepatocellular carcinoma (Chen et al. 2012; Xu et al. 2013), bladder cancer (Guo et al. 2012), gastric cancer (Tang et al. 2014) and CRC (Xu et al. 2012). MiR-96 has also been implicated as a potential therapeutic agent (chemosensitizer) for certain types of cancer. Support for this assertion was derived from studies that demonstrated repression of the DNA repair proteins REV1 and RAD51 by miR-96 resulted in enhanced responses to cisplatin and a PARP inhibitor in osteocarcinoma, cervical cancer, hepatocarcinoma, and breast cancer cells (Wang et al. 2012). In the present study, overexpression of miR-96 significantly enhanced responses to 5-FU with increased cleavage of PARP (Fig. 3a) in human CRC cells. Our data suggest the possibility that overexpression of miR-96, even in the HT-29 cells expressing a mutant form of p53 (R273H mutation), may reduce XIAP and UBE2N expression in an indirect manner, resulting in enhanced 5-FU-induced apoptosis (Figs. 1b, 3a, 6). Thus, further detailed mechanistic studies are required in order to assess the therapeutic value of miR-96 agonists in CRC treatment.
In this study, cells transfected with miR-96 mimic showed significantly increased proliferation (Fig. 3c) despite down-regulation of XIAP and UBE2N. Increased cellular proliferation may be caused by down-regulation of FOXO3, a known target of miR-96 (Fig. 1b). Suppression of FOXO3 by miR-96 in breast cancer cells has also been shown to promote cellular proliferation (Lin et al. 2010). It has been speculated that miR-96 may control cellular proliferation mainly by modulating FOXO3 levels whereas it down-regulates XIAP and UBE2N and promotes apoptosis under cytotoxic insult conditions.
XIAP, a member of the inhibitor of apoptosis protein family, is known for its role as a negative regulator of apoptosis via selective binding and inhibition of caspases-3, -7 and -9 (Eckelman et al. 2006). Overexpression of XIAP confers resistance to chemotherapeutic drugs including cisplatin and other drugs via mechanisms such as modulation of the p53-mediated pathway (Kashkar 2010). Thus, inhibition of XIAP signaling may synergistically enhance the activity of anticancer agents, which exert activity via activation of apoptosis signaling and cell death (Arlt et al. 2013). The same rationale was also applied to the development of antisense oligonucleotides that have similar functions to an endogenous XIAP inhibitor, and a phase 1/2 trial has shown promising results (Schimmer et al. 2006, 2009). According to the caspase-3 inhibitory activity of XIAP, cleavage of caspase-3 and PARP as a hallmark of traditional apoptosis was expected after transfection of miR-96 mimic and down-regulation of XIAP (Fig. 1b). However, no significant changes were observed for caspase-3 activation despite increased PARP cleavage (Fig. 3a), suggesting the involvement of caspase-3-independent pathway in our experiment. Evidence for caspase-independent cell death has been found, and several models have been proposed, such as paraptosis, mitotic catastrophe, slow cell death and autophagy (Broker et al. 2005). A serine protease, HtrA2, showed the ability to inhibit XIAP, as well as caspase-independent cell death (Suzuki et al. 2001).
An E2 ubiquitin-conjugating enzyme, UBE2N, also known as Ubc13, directly regulates the localization and activity of p53 (Laine et al. 2006). Recently, a small molecule inhibitor against UBE2N has shown therapeutic potential by demonstrating strong cytotoxic effects on neuroblastoma cells by wild type p53-mediated or p53-independent apoptosis (Cheng et al. 2014; Xia et al. 2010). Our result showed that down-regulation of UBE2N by transfection of miR-96 enhanced apoptosis in the HT-29 monolayer known to express a mutant type of p53, which may suggest the involvement of a p53-independent pathway.
Strategies to enhance drug-induced apoptosis may represent an efficient way to overcome drug resistance of retractable cancers. We examined the effect of miR-96, which has been previously reported as an inhibitor of DNA repair and apoptosis inducer (Wang et al. 2012) on chemosensitivity in monolayers as well as in 3D TSs. Over-expression of miR-96 increased apoptosis induction when exposed to 5-FU via the suppressed expression of UBE2N and XIAP (Figs. 1b, 3a). These two genes showed increased expression when the level of miR-96 was partially inhibited; however, the resistance to 5-FU was not prominent (Figs. 1b, 3b). Under 3D TS culture conditions, a significantly reduced level of miR-96 was observed with an increased level of XIAP (Figs. 4a, 5b); however, other factors including UBE2N and MDM2 showed changes that were inconsistent with the results obtained in the 2D culture conditions (Figs. 1b, 5b). Overall, the different findings obtained in the 2D and 3D conditions suggested the miR-mediated signaling pathway were dependent on both cellular context and microenvironmental signals. Thus, caution should be taken when extrapolating miR-mediated signal modulation data from conventional 2D culture models to in vivo tissues.
References
Arlt, A., S.S. Muerkoster, and H. Schafer. 2013. Targeting apoptosis pathways in pancreatic cancer. Cancer Letters 332: 346–358.
Broker, L.E., F.A. Kruyt, and G. Giaccone. 2005. Cell death independent of caspase: A review. Clinical Cancer Research 11: 3155–3162.
Chen, R.X., Y.H. Xia, T.C. Xue, and S.L. Ye. 2012. Suppression of microRNA-96 expression inhibits the invasion of hepatocellular carcinoma cells. Molecular Medicine Reports 5: 800–804.
Cheng, J., Y.H. Fan, X. Xu, H. Zhang, J. Dou, Y. Tang, X. Zhong, Y. Rojas, Y. Yu, Y. Zhao, S.A. Vasudevan, H. Zhang, J.G. Nuchtern, E.S. Kim, X. Chen, F. Lu, and J. Yang. 2014. A small-molecule inhibitor of UBE2N induces neuroblastoma cell death via activation of p53 and JNK pathways. Cell Death and Disease 5: e1079.
Croce, C.M. 2009. Causes and consequences of microRNA dysregulation in cancer. Nature Reviews Genetics 10: 704–714.
Eckelman, B.P., G.S. Salvesen, and F.L. Scott. 2006. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Reports 7: 988–994.
Feng, J., J. Yu, X. Pan, Z. Li, Z. Chen, W. Zhang, B. Wang, L. Yang, H. Xu, G. Zhang, and Z. Xu. 2014. HERG1 functions as an oncogene in pancreatic cancer and is downregulated by miR-96. Oncotarget 5: 5832–5844.
Friedrich, J., W. Eder, J. Castaneda, M. Doss, E. Huber, R. Ebner, and L.A. Kunz-Schughart. 2007. A reliable tool to determine cell viability in complex 3-d culture: The acid phosphatase assay. Journal of Biomolecular Screening 12: 925–937.
Guo, Y., H. Liu, H. Zhang, C. Shang, and Y. Song. 2012. miR-96 regulates FOXO1-mediated cell apoptosis in bladder cancer. Oncology Letters 4: 561–565.
Guttilla, I.K., and B.A. White. 2009. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. Journal of Biological Chemistry 284: 23204–23216.
Haflidadottir, B.S., O. Larne, M. Martin, M. Persson, A. Edsjo, A. Bjartell, and Y. Ceder. 2013. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS One 8: e72400.
Hamfjord, J., A.M. Stangeland, T. Hughes, M.L. Skrede, K.M. Tveit, T. Ikdahl, and E.H. Kure. 2012. Differential expression of miRNAs in colorectal cancer: Comparison of paired tumor tissue and adjacent normal mucosa using high-throughput sequencing. PLoS One 7: e34150.
Hicks, K.O., F.B. Pruijn, T.W. Secomb, M.P. Hay, R. Hsu, J.M. Brown, W.A. Denny, M.W. Dewhirst, and W.R. Wilson. 2006. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. Journal of the National Cancer Institute 98: 1118–1128.
Karasawa, H., K. Miura, W. Fujibuchi, K. Ishida, N. Kaneko, M. Kinouchi, M. Okabe, T. Ando, Y. Murata, H. Sasaki, K. Takami, A. Yamamura, C. Shibata, and I. Sasaki. 2009. Down-regulation of cIAP2 enhances 5-FU sensitivity through the apoptotic pathway in human colon cancer cells. Cancer Science 100: 903–913.
Kashkar, H. 2010. X-linked inhibitor of apoptosis: A chemoresistance factor or a hollow promise. Clinical Cancer Research 16: 4496–4502.
Kim, S.H., S.J. Choi, Y.C. Kim, and H.J. Kuh. 2009. Anti-tumor activity of noble indirubin derivatives in human solid tumor models in vitro. Arch Pharm Res 32: 915–922.
Kobayashi, H., S. Man, C.H. Graham, S.J. Kapitain, B.A. Teicher, and R.S. Kerbel. 1993. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proceedings of the National Academy of Sciences of the United States of America 90: 3294–8.
Laine, A., I. Topisirovic, D. Zhai, J.C. Reed, K.L. Borden, and Z. Ronai. 2006. Regulation of p53 localization and activity by Ubc13. Molecular and Cellular Biology 26: 8901–8913.
Lee, S.H., J.K. Nam, J.K. Park, J.H. Lee, S. Min Do, and H.J. Kuh. 2014. Differential protein expression and novel biomarkers related to 5-FU resistance in a 3D colorectal adenocarcinoma model. Oncology Reports 32: 1427–1434.
Li, Y., T.G. Vandenboom 2nd, D. Kong, Z. Wang, S. Ali, P.A. Philip, and F.H. Sarkar. 2009. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Research 69: 6704–6712.
Lin, H., T. Dai, H. Xiong, X. Zhao, X. Chen, C. Yu, J. Li, X. Wang, and L. Song. 2010. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS One 5: e15797.
Manoochehri, M., A. Karbasi, M. Bandehpour, and B. Kazemi. 2014. Down-regulation of BAX gene during carcinogenesis and acquisition of resistance to 5-FU in colorectal cancer. Pathology & Oncology Research 20: 301–307.
Mattie, M.D., C.C. Benz, J. Bowers, K. Sensinger, L. Wong, G.K. Scott, V. Fedele, D. Ginzinger, R. Getts, and C. Haqq. 2006. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Molecular Cancer 5: 24.
Mikhail, A.S., S. Eetezadi, and C. Allen. 2013. Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: A comparison of docetaxel-loaded block copolymer micelles and Taxotere(R). PLoS One 8: e62630.
Minchinton, A.I., and I.F. Tannock. 2006. Drug penetration in solid tumours. Nature Reviews Cancer 6: 583–592.
Park, J.K., S.H. Lee, J.H. Kang, K. Nishio, N. Saijo, and H.J. Kuh. 2004. Synergistic interaction between gefitinib (Iressa, ZD1839) and paclitaxel against human gastric carcinoma cells. Anti-Cancer Drugs 15: 809–818.
Ress, A.L., V. Stiegebauer, E. Winter, D. Schwarzenbacher, T. Kiesslich, S. Lax, S. Jahn, A. Deutsch, T. Bauernhofer, H. Ling, H. Samonigg, A. Gerger, G. Hoefler, and M. Pichler. 2014. MiR-96-5p influences cellular growth and is associated with poor survival in colorectal cancer patients. Molecular Carcinogenesis. doi:10.1002/mc.22218.
Schimmer, A.D., S. Dalili, R.A. Batey, and S.J. Riedl. 2006. Targeting XIAP for the treatment of malignancy. Cell Death and Differentiation 13: 179–188.
Schimmer, A.D., E.H. Estey, G. Borthakur, B.Z. Carter, G.J. Schiller, M.S. Tallman, J.K. Altman, J.E. Karp, J. Kassis, D.W. Hedley, J. Brandwein, W. Xu, D.H. Mak, E. Lacasse, C. Jacob, S.J. Morris, J. Jolivet, and M. Andreeff. 2009. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. Journal of Clinical Oncology 27: 4741–4746.
Schmidt, W.M., M. Kalipciyan, E. Dornstauder, B. Rizovski, G.G. Steger, R. Sedivy, M.W. Mueller, and R.M. Mader. 2004. Dissecting progressive stages of 5-fluorouracil resistance in vitro using RNA expression profiling. International Journal of Cancer 112: 200–212.
Suzuki, Y., Y. Imai, H. Nakayama, K. Takahashi, K. Takio, and R. Takahashi. 2001. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Molecular Cell 8: 613–621.
Tanaka, M., H.I. Suzuki, J. Shibahara, A. Kunita, T. Isagawa, A. Yoshimi, M. Kurokawa, K. Miyazono, H. Aburatani, S. Ishikawa, and M. Fukayama. 2014. EVI1 oncogene promotes KRAS pathway through suppression of microRNA-96 in pancreatic carcinogenesis. Oncogene 33: 2454–2463.
Tang, X., D. Zheng, P. Hu, Z. Zeng, M. Li, L. Tucker, R. Monahan, M.B. Resnick, M. Liu, and B. Ramratnam. 2014. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the beta-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Research 42: 2988–2998.
Tredan, O., C.M. Galmarini, K. Patel, and I.F. Tannock. 2007. Drug resistance and the solid tumor microenvironment. Journal of the National Cancer Institute 99: 1441–1454.
Vichai, V., and K. Kirtikara. 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols 1: 1112–1116.
Wang, Y., J.W. Huang, P. Calses, C.J. Kemp, and T. Taniguchi. 2012. MiR-96 downregulates REV1 and RAD51 to promote cellular sensitivity to cisplatin and PARP inhibition. Cancer Research 72: 4037–4046.
Xia, Y., and K. Lee. 2010. Targeting multidrug resistance with small molecules for cancer therapy. Biomolecules & Therapeutics 18: 375–385.
Xu, D., X. He, Y. Chang, C. Xu, X. Jiang, S. Sun, and J. Lin. 2013. Inhibition of miR-96 expression reduces cell proliferation and clonogenicity of HepG2 hepatoma cells. Oncology Reports 29: 653–661.
Xu, X.M., J.C. Qian, Z.L. Deng, Z. Cai, T. Tang, P. Wang, K.H. Zhang, and J.P. Cai. 2012. Expression of miR-21, miR-31, miR-96 and miR-135b is correlated with the clinical parameters of colorectal cancer. Oncology Letters 4: 339–345.
Yeon, S.E., Y. Da No, S.H. Lee, S.W. Nam, I.H. Oh, J. Lee, and H.J. Kuh. 2013. Application of concave microwells to pancreatic tumor spheroids enabling anticancer drug evaluation in a clinically relevant drug resistance model. PLoS One 8: e73345.
Yu, S., Z. Lu, C. Liu, Y. Meng, Y. Ma, W. Zhao, J. Liu, J. Yu, and J. Chen. 2010. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Research 70: 6015–6025.
Zhang, L., K. Pickard, V. Jenei, M.D. Bullock, A. Bruce, R. Mitter, G. Kelly, C. Paraskeva, J. Strefford, J. Primrose, G.J. Thomas, G. Packham, and A.H. Mirnezami. 2013. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Research 73: 6435–6447.
Zhang, N., Y. Yin, S.J. Xu, and W.S. Chen. 2008. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 13: 1551–1569.
Zhou, Y., G. Wan, R. Spizzo, C. Ivan, R. Mathur, X. Hu, X. Ye, J. Lu, F. Fan, L. Xia, G.A. Calin, L.M. Ellis, and X. Lu. 2014. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Molecular Oncology 8: 83–92.
Acknowledgments
This work was supported by grants obtained from the National Research Foundation of Korea (NRF), which is funded by the Korean government (MEST) (NRF-2012R1A5A2051477 and NRF-2012R1A2A2A01003361).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SA., Kim, I., Yoon, S.K. et al. Indirect modulation of sensitivity to 5-fluorouracil by microRNA-96 in human colorectal cancer cells. Arch. Pharm. Res. 38, 239–248 (2015). https://doi.org/10.1007/s12272-014-0528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0528-9