Abstract
5-Fluorouracil (5-FU) as a chemotherapeutic drug is used to treat colorectal cancer (CRC). However, 5-FU is associated with acquired CRC resistance, which decreases the therapeutic potential of 5-FU. Several studies indicated that miR-200c is also involved in chemotherapeutic drug resistance, but the exact mechanism of miR-200c mediated chemoresistance has not yet been fully understood. In this study, we examined the effect of inhibition of miR-200c on the sensitivity of HCT-116 cells to 5-FU. HCT-116 cells were transfected with LNA-anti- miR-200c for 48 h. mRNA expression of miR-200c was investigated by qRT-PCR. The protein expression of phosphatase and tensin homolog (PTEN) and E-cadherin were evaluated by western blotting. Annexin V/ PI staining and caspase 3 activity were used to detect apoptosis. LNA-anti-miR-200c inhibited the miR-200c expression in the transfected cells compared with that in the control group. LNA-anti-miR-200c suppressed the expression of PTEN and E-cadherin independent of the presence of the chemotherapeutic drug 5-FU. LNA-anti-miR-200c reduced the 5-FU-induced apoptosis and caspase 3 activity. miR-200c, as a novel prognostic marker in CRC, can be a potential therapeutic approach to overcome chemoresistance during 5-FU chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC), the fourth leading cause of cancer-related mortality, leads to about 50, 000 deaths each year worldwide [1]. Despite overall advances in treatment of diseases, CRC remains a main health issue leading to a mortality rate of approximately 5% in the developed countries [2].
5-Fluorouracil (5-FU) is widely used to treat CRC and is still the main chemotherapy [3]. 5-FU, as a precursor of UTP and dTTP, interferes with the metabolism of DNA and RNA. Prolonged exposure of 5FU to cancer cells may cause chemoresistance. Resistance to 5-FU is a major clinical complication following the successful treatment of CRC [4–7]. It has not yet been clearly explained why cancer treatment becomes ineffective over time; however, it was shown that cancer cells become resistant via certain mechanisms such as alteration in drug target, drug inactivation, influx and efflux of drugs in the cells, processing of drug-induced damage, and evasion of apoptosis [8].
miRNAs are non-coding single strand RNAs with a length of 18–24 nucleotides. The first products of microRNAs, pri-microRNAs, are cleaved by Drosha to release pre-microRNAs in the nuclear compartment. The complex is translocated into the cytoplasm by exportin-5. Pre-microRNAs are cleaved by Dicer into the mature microRNAs [9]. These small RNAs play important roles in various biological processes and downregulate the expression of gene at the post-transcriptional level. The complementary base-pairing between miRNA and their target mRNA, which causes degradation of mRNA or translation disturbance, depends on the degree of miRNA-mRNA complementarity [10].
The miR-200 family has five members consisting of miR-200a, miR-200b, miR- 200c, miR-429, and miR-141.
miR-200c was demonstrated to be downregulated in a variety of human cancers [11–13]. Cochrane et al. found that chemosensitivity to paclitaxel considerably increased following the transfection of miR-200c into endometrial cancer cells [14]. Similarly, Ceppi et al. demonstrated that the upregulation of miR-200c maintained the sensitivity of non-small cell lung cancer (NSCLC) cells to cisplatin and cetuximab [15]. miR-200c decreases during tumor progression and serves as a key inhibitor for tumor cell invasion, metastasis, and epithelial-to-mesenchymal transition (EMT) [16]. miR-200c is associated with cancer progression and chemotherapeutic drug resistance, but the exact mechanism remains unclear [17].
Our aim was to evaluate the effect of LNA-anti-miR-200c transfection on the expression of E-cadherin and PTEN, as downstream targets of miRNA-200c, as well as the sensitivity of HCT-116 cells to 5-FU.
Materials and Methods
Cell Culture
HCT-116 cell line (human colorectal cancer) was purchased from the Pasteur Institute (Tehran, Iran). The cells were cultured in DMEM medium (Gibco, Invitrogen, USA) containing 15% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Gibco, Invitrogen). HCT-116 cells were incubated in a humidified environment at 37 °C and 5% CO2.
Cell Transfection
Sequences of miR-200c nucleotide were obtained from www.mirbase.org as TCCATCATTACCCGGCAGTA (accession number MIMAT0000617). miRCURY LNA microRNA Inhibitor™ for hsa-miR-200c and the scrambled LNA were purchased from the Exiqon (Copenhagen, Denmark). The 5′ ends of both oligonucleotides were labeled with fluorescent dye, 6-FAM. Transfection of HCT-116 cells was conducted according to the manufacturer’s instructions by the X-treme GENE siRNA Transfection Reagent ™ (Roche, Mannheim, Germany). Briefly, 5 × 105 cells were cultured in six-well culture plates (Spl, Korea) containing 1.8 ml DMEM per well without antibiotic and FBS. Five μL of X-treme GENE siRNA Transfection Reagent™ was mixed with 50 pmol miRCURY LNA microRNA Inhibitor™ in 200 μl Opti-MEMI Medium™ (Gibco, Paisley, UK) and then incubated at room temperature for 15 min. The mixture was then added to the cells and shaken carefully to ensure even spreading over the entire plate surface. After an 8-h incubation, the antibiotics and FBS were added and the cells were incubated for the 24, 48 and 72 h [18]. This method was applied for cells transfected with scrambled-LNA. Assessment of the transfection was conducted by fluorescent microscopy and Flow cytometry.
Cell Viability Assay
The inhibitory effects of 5-FU on cell growth were investigated using the 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich). Five thousand HCT-116 cells were seeded in 96-well tissue culture plates, and various concentrations of 5-FU (0–20 μM) added. After 24 and 48-h incubation, 10 μl of MTT solution was added to each well and incubated for 4 h at 37 °C. Cell culture medium was removed and then 100 μl of DMSO (Sigma-Aldrich) added. The optical density (OD) was measured by an ELISA reader at 570-nm wavelength. To evaluate the effect of miR-200c on 5-FU sensitivity of HCT-116, the cells were transfected with LNA-anti-miR-200c, 5-FU was added to the wells, and cell viability was analyzed by MTT assay.
Real Time PCR
Total RNA was extracted from HCT-116 cells using the RNX- Plus solution (Sinaclone, Iran). RNA concentration and purity was measured by nanodrop. Two μg of total RNA was used for complementary DNA (cDNA) synthesis from miRNA according to the manufacturer (Parsgenom, Tehran, Iran) instructions. Real time-PCR was conducted with SYBR green master mix and specific miR-200c primers. Thermal cycle for mixture consisted of an initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 5 s, annealing at 62 °C for 20 s, and an extension for 30 s at 72 °C. The levels of mRNA expression were normalized to 5S rRNA. Relative expression was reported by 2−ΔΔCT method.
Western Blot Analysis
PTEN and E-cadherin protein expression was investigated by Western blotting. HCT-116 cells were lysed in 0.2 ml of RIPA buffer (Santa Cruz, USA) and centrifuged at 13,000 g for 20 min at 4 °C. The protein concentration was measured by Bradford assay. For immunoblotting, 50 μg of each sample was loaded into wells and separated by SDS-PAGE. Then, the proteins were transferred onto a nitrocellulose membrane. The membrane was incubated with primary antibodies: Rabbit monoclonal anti- PTEN (ab32199, 1:7000) and Rabbit monoclonal anti- E-cadherin (ab40772, 1:7000). Then, the membranes were incubated at room temperature for 1 h with the secondary antibody (anti-rabbit antibody conjugated with horse-radish peroxidase). β-Actin was used as a loading control. Immunoreactivity was visualized using an ECL kit (Amersham, USA) and quantified by densitometric analysis using ImageJ software (National Institute of Health, Betheseda, MD, USA) [19].
Apoptosis Assay
Annexin V-FITC/PI assay was used to detect apoptosis induced by LNA-anti-miR-200c in HCT-116 cancer cells. HCT-116 cells were seeded in 6-well plates at a 200,000 cells/well density. After treatment procedure, the adherent cells were trypsinized and centrifuged at 1500 g for 5 min. Afterward, the cells were stained with annexin V and PI according to the manufacturer’s instructions. Annexin V-FITC detects the Phosphatidylserine during apoptosis, and PI distinguishes necrotic cells. The cells were analyzed using a FACS caliber cytometer (Becton Dickinson, San Diego, CA, USA) and CellQuest software.
Caspase 3 Activity Assay
The activation of caspases 3 was evaluated using caspase 3 colorimetric assay kit (Cambridge, MA, USA). Briefly, the treated HCT-116 cells were trypsinized and centrifuged at 1800 g for 10 min. The cell pellet was washed in ice-cold PBS and lysed in 50 ul of lysis buffer and centrifuged at 10000 g for 1 min at 4 °C. After the protein concentration was determined by a Bradford assay, 100 μg of the proteins from each sample was added to 50 μl of reaction buffer containing DTT and caspase-3 substrate (DEVD-pNA), and incubated at 37 °C for 1–2 h. The activity of caspase-3 in cell lysate was measured at 405-nm wavelength in the plate reader.
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). One-way ANOVA and Tukey’s posthoc tests were used to determine the statistical significance of inter-group differences. P < 0.05 was considered statistically significant.
Results
Effect of miR-200c on 5-FU Sensitivity of HCT-116 Cells
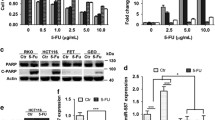
We investigated the effect of various concentrations of 5-FU (1–20 μM) on HCT-116 cells viability by MTT assay. As illustrated in Fig. 1, 5-FU decreased the percentage of viable cells compared with the untreated cell in a dose and time-dependent manner. 5-FU displayed significant cytotoxicity at 5-μM concentration for 48 h (p < 0.001). The 50% inhibition concentration (IC50) values were derived 10 μM after 48 h.
Effect of LNA-anti-miR-200c on 5-FU sensitivity of HCT-116 cells. The HCT-116 cells were treated with different concentrations of 5-FU for 48 h. The viability of cells was inhibited by 5-FU compared to the control group (P < 0.001). Transfection of the HCT-116 cells with anti-miR-200c decreased their sensitivity to 5-FU treatment. Data are represented as mean ± SEM (n = 8). *P < 0.05, **P < 0.01 and ***P < 0.001 versus cells treated with 5-FU
In addition, the sensitivity of HCT-116 cells transfected with LNA-anti-miR-200c to 5-FU decreased compared with that of the untransfected cells. The IC50 of HCT-116 cells transfected with anti-miR-200c was 1.50 times higher (p < 0.01) than that of untransfected HCT-116 cells.
Effect of LNA-Anti-miR on miR200c Expression
For inhibition of miR-200c, HCT-116 cells were transfected with the miRCURY LNA miR inhibitor. The transfection efficiency was determined using flow cytometry and fluorescence microscopy. We obtained transfection efficiency of HCT-116 cells with LNA-anti miR-200c to be about 70% (data not shown). The expression of miR-200c was evaluated at 12, 24, 48, and 72 h after transfection by reverse transcriptase microRNA real time-PCR in HCT-116 cells transfected with LNA-anti-miR, scrambled LNA, and untreated HCT-116 cells. In all four time intervals, the expression of miR-200c was considerably lower in the LNA-anti-miR group than the untreated groups. However, there was no significant difference between scrambled LNA-transfected group and the untreated cells (Fig. 2).
Effect of LNA-anti-miR on miR200c expression. Expression level of miR-200c was assessed by quantitative real time PCR. LNA-anti-miR-200c reduced the expression of miR-200c in HCT-116 cells. All data were normalized to levels of 5S rRNA. Data are represented as mean ± SEM. ***P < 0.001 versus control group
Effect of miR-200c Inhibition on PTEN and E-Cadherin Protein Expression
Then, we examined the expression of PTEN and E-cadherin, two downstream targets of miR-200c, by western blot. Immunoblotting analysis revealed that the levels of PTEN and E-cadherin were reduced by knockdown of miR-200c in HCT-116 cells. Figure 3 shows that depletion of miR-200c decreases 5-FU-stimulated PTEN and E-cadherin expression.
Effect of miR-200c on Apoptosis
To investigate the effect of miR-200c inhibition on apoptosis, the HCT-116 cells were stained with Annexin-V. We examined 5-FU-induced apoptosis in HCT-116 cells following transfection with LNA-anti-miR-200c. We found that miR-200c inhibition decreased the apoptosis following treatment with 5-FU, but apoptosis in 5-FU-treated cells was higher than that in the control group (Fig. 4).
Effect of miR-200c inhibition on HCT-116 cells apoptosis. HCT-116 cells were treated with 10 μM of 5-FU in the presence and absence of miR-200c. Apoptosis was quantified using Annexin V-PI staining. LNA-anti-miR-200c decreased the apoptosis rate. Data are presented as the mean ± SEM. *** p < 0.001 versus control and # P < 0.001 versus 5-FU
Effect of miR-200c Inhibition on Caspase 3 Activity:
The effect of miR-200c on 5-FU–mediated apoptosis was confirmed by caspase 3 activity assay. miR-200c inhibition reduced the caspase 3 activity following treatment with 5-FU compared with 5-FU-treated HCT-116 cells (Fig. 5).
Discussion
CRC-associated morbidity and mortality are high worldwide. Metastasis is the main cause of death in CRC patients [20]. Therefore, for successful treatment, surgery or radiotherapy can be done alongside chemotherapy. 5-FU is considered the main chemotherapy drug of choice to treat CRC and reduce the annual probability of recurrence and mortality. However, resistance to chemotherapy drugs is a major obstacle to efficient treatment of cancer [21]. The role of miRNAs in chemoresistance was demonstrated [22]. Emerging evidence suggests that the loss of miR-200c expression is involved in the cancer cell resistance to chemotherapy drugs [23].
The role of miR-200c in chemoresistance in esophageal and breast cancer was demonstrated, but its role in chemoresistance in CRC has not yet been explained [16, 23]. In this study for the first time, we showed that the inhibition of miR-200c correlated with the acquired resistance of colorectal cancer cells (HCT-116) to 5-FU. To achieve this purpose, we used LNA-anti miR-200c to knockdown miR-200c in order to evaluate the HCT-116 colon cancer cells response to 5-FU. In the current study, we investigated the effect of miR-200c inhibition on apoptosis and the expression of PTEN and E-cadherin protein. Our results showed that LNA-anti-miR-200c suppressed the expression of miR-200c, PTEN, and E-cadherin compared with the control cells. Furthermore, we observed that LNA-anti-miR-200c could promote cell proliferation through inhibition of caspase 3 activity and reduction of apoptosis that is mediated by PTEN expression.
PTEN is a tumor suppressor gene located in the cytoplasm and is responsible for lipid and protein dephosphorylation [24]. PTEN is an essential factor in the suppression of tumor cell proliferation, cell migration, and apoptosis [10]. Downregulation of the PTEN gene is prevalent in various tumors, such as those in brain, endometrium, colorectal, skin, prostate, and breast cancers [25]. A study has shown that miR-200c decreases the expression of PTEN in pituitary adenoma [26]. In contrast, a study by Chen et al. revealed that miR-200c inhibited doxorubicin resistance in MCF-7 breast cancer cells by suppressing Akt signaling via upregulation of PTEN and E-cadherin [23]. It seems that miR-200c can upregulate the expression of PTEN indirectly through enhancing E-cadherin expression.
Furthermore, it should be noted that PTEN inactivates the AKT signaling pathway by phosphatidylinositol triphosphate dephosphorylation and subsequent inhibition of AKT phosphorylation [27–29].
E-cadherin as a transmembrane protein plays a critical role in cell adhesion and is involved in transmitting intracellular chemical signals. It is one of the major downstream regulators of miRNA-200c contributing to EMT, which is also important to inhibit tumor invasion and proliferation as well as to induce cell apoptosis [9, 30, 31]. Li et al. demonstrated that restoring E-cadherin-mediated cell-cell adhesion enhanced PTEN protein levels by increasing PTEN protein stability and suppressing degradation in human breast cancer cells [10].
Consistent with our findings, a study has shown that ectopic expression of miR-200c inhibits EMT, invasion, and metastasis through blocking ZEB1 expression and increasing the E-cadherin/TA-p73/p63 expression in lung carcinoma cell lines [32].
Evidence suggests that ZEB1 plays an important role in the EMT of CRC cells. miR-200c is able to prevent EMT process through suppression of several targets, such as ZEB1/2. miR-200 suppresses the expression of ZEB by binding to its 3′ UTRs and inhibits cell invasion [33, 34].
It has also been shown that PTEN level decreases in cetuximab-resistant cell lines (HCC827-CR), leading to the activation of PI3K/Akt signaling, while upregulation of PTEN significantly caused reduction in Akt activity and restored drug sensitivity. PTEN downregulation seems to provoke the acquired resistance of NSCLC to cetuximab by activation of PI3K/Akt signaling [35]. Moreover, upregulation of miRNA-200c restored the sensitivity of non-small cell lung cancer (NSCLC) cells to cisplatin and cetuximab [15]. miRNA-200c is important in the EMT and metastasis in different types of cancers. Several studies showed that downregulation of miRNA-200c occurs in a variety of human cancer types [12, 13, 23, 36]. miR-200c inhibits EMT, proliferation, metastasis, resistance to chemotherapy drugs and also contributes significantly to the development of drug resistance in cancer cells [37–39].
Recently, it was demonstrated that the cells that lack miR-200c expression are more prone to invasion into the lymphatic system and blood. Naïve CCL227 cell-derived exosomes contain high levels of miR-200c, while the 5-FU-resistant CCL227 exosomes are free of miR-200c and accelerated circular chemorepellent-induced defects (CCIDs) formation in the blood endothelial cell (BEC) compared to naïve CCL227. miR-200c inhibits CCIDs formation through downregulation of SNAI, ZEB2, and TWIST expression in the BECs [40].
Another study has shown that the serum levels of miR-155, miR-200c, and miR-201 can predict relapse and distant metastasis, chemoresistance, and poor prognosis in colon cancers [41]. It was demonstrated that curcumin chemosensitizes CRC cells to 5-FU via the upregulation of miR-200c in 5-FUR cell lines [42]. Soubani et al. reported that the loss of miR-200 family and PTEN expression led to aggressive behavior of pancreatic cancer cells [43].
Overall, the inhibition of miR-200c correlated with the acquired resistance of colorectal cancer cells (HCT-116) to 5-FU with decreasing the levels of E-cadherin and PTEN protein. This study suggests that miR-200c can be a possible therapeutic agent, particularly in combination with anti-cancer chemotherapy agents.
References
Deng J, Lei W, Fu J-C, Zhang L, Li J-H, Xiong J-P (2014) Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem Biophys Res Commun 443(3):789–795
Draht MX, Riedl RR, Niessen H, Carvalho B, Meijer GA, Herman JG et al (2012) Promoter CpG island methylation markers in colorectal cancer: the road ahead. Epigenomics 4(2):179–194
Davoodi H, Hashemi SR, Seow HF (2013) 5-fluorouracil induce the expression of TLR4 on HCT116 colorectal cancer cell line expressing different variants of TLR4. Iran J Pharm Res:IJPR 12(2):453
Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD et al (2004) Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res 10(6):2158–2167
Hsu H-C, Liu Y-S, Tseng K-C, Hsu C-L, Liang Y, Yang T-S et al (2013) Overexpression of Lgr5 correlates with resistance to 5-FU-based chemotherapy in colorectal cancer. Int J Color Dis 28(11):1535–1546
Wang W, Cassidy J, O’Brien V, Ryan KM, Collie-Duguid E (2004) Mechanistic and predictive profiling of 5-fluorouracil resistance in human cancer cells. Cancer Res 64(22):8167–8176
Leichman L, Lenz H-J, Leichman C, Groshen S, Danenberg K, Baranda J et al (1995) Quantitation of intratumoral thymidylate synthase expression predicts for resistance to protracted infusion of 5-fluorouracil and weekly leucovorin in disseminated colorectal cancers: preliminary report from an ongoing trial. Eur J Cancer 31(7):1306–1310
Lau M, Klausen C, Leung P (2011) E-cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via β-catenin-Egr1-mediated PTEN expression. Oncogene 30(24):2753–2766
Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205
Li Z, Wang L, Zhang W, Fu Y, Zhao H, Hu Y et al (2007) Restoring E-cadherin-mediated cell–cell adhesion increases PTEN protein level and stability in human breast carcinoma cells. Biochem Biophys Res Commun 363(1):165–170
Slaby O, Jancovicova J, Lakomy R, Svoboda M, Poprach A, Fabian P et al (2010) Expression of miRNA-106b in conventional renal cell carcinoma is a potential marker for prediction of early metastasis after nephrectomy. J Exp Clin Cancer Res 29(1):1
Philippidou D, Schmitt M, Moser D, Margue C, Nazarov PV, Muller A et al (2010) Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res 70(10):4163–4173
Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D (2013) Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog 52(4):297–303
Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK (2009) MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther 8(5):1055–1066
Ceppi P, Mudduluru G, Kumarswamy R, Rapa I, Scagliotti GV, Papotti M et al (2010) Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non–small cell lung cancer. Mol Cancer Res 8(9):1207–1216
Feng X, Wang Z, Fillmore R, Xi Y (2014) MiR-200, a new star miRNA in human cancer. Cancer Lett 344(2):166–173
Chen J, Wang W, Zhang Y, Hu T, Chen Y (2014) The roles of miR-200c in colon cancer and associated molecular mechanisms. Tumor Biol 35(7):6475–6483
Sharifi M, Salehi R, Gheisari Y, Kazemi M (2014) Inhibition of microRNA miR-92a induces apoptosis and inhibits cell proliferation in human acute promyelocytic leukemia through modulation of p63 expression. Mol Biol Rep 41(5):2799–2808
Mirzamohammadi S, Aali E, Najafi R, Kamarul T, Mehrabani M, Aminzadeh A et al (2015) Effect of 17β-estradiol on mediators involved in mesenchymal stromal cell trafficking in cell therapy of diabetes. Cytotherapy 17(1):46–57
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt L, Ramanathan R, Hansen TB et al (2011) Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer 128(6):1327–1334
Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F et al (2012) Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 31(16):2062–2074
Magee P, Shi L, Garofalo M (2015) Role of microRNAs in chemoresistance. Ann Transl Med 3(21):332. doi:10.3978/j.issn.2305-5839.2015.11.32
Chen Y, Sun Y, Chen L, Xu X, Zhang X, Wang B et al (2013) miRNA-200c increases the sensitivity of breast cancer cells to doxorubicin through the suppression of E-cadherin-mediated PTEN/Akt signaling. Mol Med Rep 7(5):1579–1584
Yin Y, Shen W (2008) PTEN: a new guardian of the genome. Oncogene 27(41):5443–5453
Lu Y-X, Yuan L, Xue X-L, Zhou M, Liu Y, Zhang C et al (2014) Regulation of colorectal carcinoma Stemness, growth, and metastasis by an miR-200c-Sox2–negative feedback loop mechanism. Clin Cancer Res 20(10):2631–2642
Liao C, Chen W, Fan X, Jiang X, Qiu L, Chen C et al (2014) MicroRNA-200c inhibits apoptosis in pituitary adenoma cells by targeting the PTEN/Akt signaling pathway. Oncol Res 21(3):129–136
Berlanga P, Muñoz L, Piqueras M, Sirerol JA, Sánchez-Izquierdo MD, Hervás D et al (2016) miR-200c and phospho-AKT as prognostic factors and mediators of osteosarcoma progression and lung metastasis. Mol Oncol
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nat Rev Cancer 2(7):489–501
Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M et al (2005) Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer 117(3):376–380
Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J et al (2013) MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 62(9):1315–1326
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):–402, 8
Boominathan L (2010) The tumor suppressors p53, p63, and p73 are regulators of microRNA processing complex. PLoS One 5(5):e10615
Sun J, Ding W, Zhi J, Chen W (2015) MiR-200 suppresses metastases of colorectal cancer through ZEB1. Tumor Biol 1–7
Karimi Dermani F, Saidijam M, Amini R, Mahdavinezhad A, Heydari K, Najafi R (2016) Resveratrol inhibits proliferation, invasion, and epithelial–Mesenchymal transition by increasing miR-200c expression in HCT-116 colorectal cancer cells. J Cell Biochem
Kim SM, Kim JS, Kim J-H, Yun C-O, Kim EM, Kim HK et al (2010) Acquired resistance to cetuximab is mediated by increased PTEN instability and leads cross-resistance to gefitinib in HCC827 NSCLC cells. Cancer Lett 296(2):150–159
Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H et al (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64(11):3753–3756
Gotanda K, Hirota T, Matsumoto N, Ieiri I (2013) MicroRNA-433 negatively regulates the expression of thymidylate synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa cells. BMC Cancer 13(1):369
Hewish M, Lord CJ, Martin SA, Cunningham D, Ashworth A (2010) Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat Rev Clin Oncol 7(4):197–208
Bronckaers A, Gago F, Balzarini J, Liekens S (2009) The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med Res Rev 29(6):903–953
Holzner S, Senfter D, Stadler S, Staribacher A, Nguyen CH, Gaggl A et al (2016) Colorectal cancer cell-derived microRNA200 modulates the resistance of adjacent blood endothelial barriers in vitro. Oncol Rep 36(5):3065–3071
Chen J, Wang W, Zhang Y, Chen Y, Hu T (2014) Predicting distant metastasis and chemoresistance using plasma miRNAs. Med Oncol 31(1):1–7
Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C et al (2015) Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 36(3):355–367
Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L et al (2008) The miR-15a–miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14(11):1271–1277
Acknowledgements
This study was funded by the Hamadan University of Medical Sciences (grant no.: 9407073722).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Heydari, K., Saidijam, M., Sharifi, M.r. et al. The Effect of miR-200c Inhibition on Chemosensitivity (5- FluoroUracil) in Colorectal Cancer. Pathol. Oncol. Res. 24, 145–151 (2018). https://doi.org/10.1007/s12253-017-0222-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0222-6