Abstract
Bioassay-guided fractionation of the MeOH extract of Magnolia grandiflora seeds resulted in the isolation of a new dimeric neolignan, named bishonokiol A (1), as well as two known neolignans magnolol (2) and honokiol (3). The structures of the compounds were determined on the basis of data obtained using NMR and MS. Bishonokiol A (1) showed potent anti-proliferative activities in four human cancer cell lines, with IC50 values ranging from 5.1 to 7.5 µM. Additionally, bishonokiol A (1) induced apoptosis, as well as down-regulated the expression of the anti-apoptotic protein Bcl-2 and caspase-3 cleavage in HepG2 cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
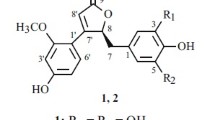
Natural products are considered as rich sources for the discovery of new therapeutic agents (Baker et al. 2007; Ngo et al. 2013). In particularly, plants contain a variety of functionally relevant secondary metabolites that have been shown to prevent the development of malignancies (Surh et al. 2003). Magnolia grandiflora L., a traditional medicine widely used for the treatment of fever, diarrhea, abdominal diseases, and rheumatic arthritis (Schühly et al. 2001), contains alkaloids, tyramine, flavonoids, terpenes, phenolic alcohols, glycosides, and sesquiterpene lactones (Mohamed et al. 2010; Del Valle Mondragón et al. 2004; Feltenstein et al. 2004). In our search for biologically active agents from medicinal plants, we examined the properties of M. grandiflora seed extracts. The EtOAc-soluble extracts (10 μg/mL) exhibited cytotoxic activity in the human liver hepatocellular carcinoma cell line, HepG2 (inhibition rate, 85 %). Bioassay-guided fractionation of the MeOH extract of M. grandiflora seeds resulted in the isolation of one new dineolignan, bishonokiol A (1), together with two known neolignans magnolol (2) and honokiol (3) (Fig. 1). In this study, we report the isolation, identification, and biological activities of compound 1.
Materials and methods
General experimental procedures
Optical rotation was measured with a JASCO P-1020 digital polarimeter (Jasco, Japan). UV spectrum was recorded on an UV-1601 spectrometer (Shimadzu, Japan), and IR spectrum was obtained on a FT-IR-650 spectrometer (Gangdong, China). NMR spectra were recorded using a Varianunity Ionva-400 NMR instrument (VARI, USA). ESI-MS and HR-MS spectra were recorded using a Finnigan navigator LC/MS/DS system (Finnigan, USA) and a JMS-HX/HX 110A tandem mass spectrometer (JEOL, Japan), respectively. A Waters 2535Q HPLC system fitted with a 2998 photodiode array detector (Waters, USA) was used for the semi-preparative separations.
Plant material
Fresh seeds of M. grandiflora L. were collected in October 2012 from a farm located in Bengbu city, Anhui, China. A voucher specimen (DM. BY0158) has been deposited in our laboratory. The authenticity of the plant was confirmed by Prof. Huizi Lv, College of Pharmacy, Yanbian University.
Extraction and isolation
Magnolia grandiflora L. seeds (1.4 kg) were extracted with MeOH (2 × 10 L, 72 h each). MeOH was evaporated in vacuo in order to yield MeOH-soluble extract. The MeOH-soluble extract (100 g) was suspended in H2O, and sequentially partitioned with n-hexane, and EtOAc. The bioactive EtOAc-soluble material (13.2 g) was subjected to silica gel column chromatography (Merck Kieselgel 60, 500 g; 230–400 mesh; 4.0 × 60 cm). The column was eluted with a stepwise gradient using mixtures of n-hexane-EtOAc (100:1, 1,000 mL; 90:1, 1,000 mL; 80:1, 2,000 mL; 75:25, 2,000 mL; 60:40, 2,000 mL; 50:50, 2,000 mL) and 250 mL fractions were collected. The active fraction 4 (150 mg, eluted 80:20 Hexane/EtOAc; cytotoxicity) was further purified by preparative reversed phase HPLC by using a 65 % CH3CN in H2O over 40 min (YMC-J’sphere ODS-H80; 10 × 25 mm; 5 μm particle size; absorbance 254 nm; flow rate 3 mL/min; 0.05 % trifluoroacetic acid) to yield bishonokiol A (1, 19.1 mg) and magnolol (2, 37.7 mg). The active fraction 5 (0.9 g, eluted 75:25 n-hexane/EtOAc; cytotoxicity) was subjected to silica gel column chromatography (Merck Kieselgel 60, 80 g; 230–400 mesh; 2.0 × 30 cm) using elution mixtures of n-hexane-EtOAc (85:15, 2,000 mL), and honokiol (3, 536.2 mg) was identified.
Bishonokiol A (1)
Purple oil; [α] d 25 +1.7 (c, 0.1, MeOH); UV (MeOH) λmax (log ε) 225 (2.764), 264 (2.768), 304 (2.953) nm; IR \( {\text{v}}_{ \hbox{max} }^{\text{KBr}} \) cm−1 3,018, 1,521, 1,476, 1,215, 1,046; ESI-MS m/z 557 [M−H]−, 559 [M+H]+; HRESI-MS m/z 559.2835 calculated 559.2848 for C38H39O4. NMR data are shown in Table 1.
Magnolol (2)
ESI-MS m/z [M−H]− 265. 1H-NMR (400 MHz, CDCl3), δ 7.12–6.76 (6H, m, Ar–H), 5.99 (2H, m, H-8, 8′), 5.09 Ar–OH, 5.12 (4H, m, H-9, 9′), 3.38 (4H, d, J = 6.4 Hz, H-7, 7′) ppm (Lee et al. 2008).
Honokiol (3)
ESI-MS m/z [M−H]− 265. 1H-NMR (400 MHz, CDCl3), δ 7.23–6.91 (6H, m, Ar–H), 6.02 (2H, m, H-8, 8′), 5.13 (4H, m, H-9, 9′), 3.48 (2H, d, J = 6.4 Hz, H-7), 3.37 (2H, d, J = 6.4 Hz, H-7′) ppm (Lee et al. 2008).
Cell viability assay
The human liver hepatocellular carcinoma (HepG2), breast cancer (MDA-MB-231 and MDA-MB-435), and nasopharyngeal carcinoma (HNE1) cell lines were obtained from the Shanghai cell line bank (China). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DEME, Hyclone, USA) containing 10 % fetal bovine serum (FBS, Hyclone, USA) and 1 % penicillin/streptomycin (Gibco, USA) in a humidified 5 % CO2 atmosphere at 37 °C. Upon reaching confluence, the cells were detached using a 0.25 % trypsin–EDTA solution. The cells were seeded in 96-well plate at a density of 8,000 cells/well, 1 day before the start of treatment. We treated the cells with various concentrations of compounds 1–3 for 48 h. Cell viability was evaluated using a MTT assay as described previously. The amount of formazan formed was calculated from the absorbance (A) measured at 570 nm by using a microplate reader. Cell viability (%) = (Asample−Ablank)/(Acontrol−Ablank) × 100 %.
PI staining
Human liver hepatocellular carcinoma HepG2 cells were seeded at a density of 5 × 105 cells/well in six-well plates in order to reach exponential growth for 24 h before treatment. Whole cell lysates from HepG2 cells were treated with drugs for 48 h by using propidium iodide (PI) staining, which was then quantified by using flow cytometry (BD Accuri C6, USA).
Western blot analysis
The HepG2 cells were seeded at a density of 5 × 105 cells/well in six-well plates. After incubation of the drugs, the cells were harvested, washed twice in phosphate-buffered saline (PBS), and lysed using a lysis buffer [50 mM Tris–HCl pH = 7.4, 1 % Triton X-100, 150 mM NaCl, and a cocktail of protease inhibitors (Sigma, USA)]. Lysates were loaded onto 12 % SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5 % skim milk, and were incubated overnight with anti-Bcl-2 (Proteintech, USA), anti-Caspase 3 (Abcam, UK), and anti-β-actin (Santa Cruz Biotechnology, USA) antibodies. Mouse and rabbit horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, USA) were used in TBST/5 % skim milk. The amount of bound antibodies was visualized using an enhanced chemiluminescence (ECL) substrate (Intron Biotechnology, Korea) and imaged with gel imaging equipment (Bio-Rad, CA, USA).
Results and discussion
Chromatographic purification of the EtOAc-soluble fraction led to the isolation of compounds 1–3. The molecular formula of compound 1, C38H38O4 was determined from HR-ESIMS data (m/z 559.2835 calculated 559.2848 for C38H39O4) in conjunction with NMR data. The 1H-NMR spectrum showed two ABX type protons with a signals at δH 7.28 (1H, dd, J = 8.4, 2.4 Hz), 7.23 (1H, d, J = 2.4 Hz), and 6.96 (1H, d, J = 8.4 Hz), as well as 7.05 (1H, dd, J = 8.4, 2.4 Hz), 7.03 (1H, d, J = 2.4 Hz), and 6.90 (1H, d, J = 8.4 Hz), vinyl group signals at δH 6.0 (m) and 5.07 or 5.11 (m), O-methyl signals at δH 3.88 (3H, s), and two aliphatic proton signals at δH 3.43 (2H, d, J = 6.8 Hz) and 3.35 (2H, d, J = 6.8 Hz). The 13C-NMR and distortionless enhancement by polarization transfer (DEPT) experiments only showed the following nineteen carbon signals, which indicated that compound 1 had complete symmetry: seven aromatic or olefinic quaternary, seven aromatic or olefinic methine, two aromatic or olefinic methane, two aliphatic methane, and one O-methyl carbons (Table 1). The physicochemical properties and NMR data suggested that compound 1 was very similar to the known neolignan 4-O-methylhonokiol (Lee et al. 2008; Ito et al. 1982; Oh et al. 2009). These results suggested that compound 1 is a dimeric molecule containing two 4-O-methylhonokiol moieties. The connectivity between the two 4-O-methylhonokiol units through a peroxide bridge (C-2′ OH and C-2′′′ OH) was verified from MS and NMR data. The 1H-1H COSY showed important correlations between protons at H-5/H-6, H-7/H-8, H-8/H-9, H-3′/H-4′, H-7′/H-8′, and H-8′/H-9′ (Fig. 2). Meanwhile, heteronuclear multiple-bond correlation (HMBC) spectroscopy experiments showed key 1H–13C long range correlations (4-OCH3/C-4, H-7/C-3, C-4, C-8, C-9, H-7′/C-5′, C-8′, C-9′, C-2/C-1′, and C-6/C-1′) (Fig. 2). Therefore, the structure of the new dimeric neolignan, bishonokiol A (1), was assigned as shown in Fig. 1.
Magnolia species are considered rich sources of lignans (Ito et al. 1982; Schuehly et al. 2010; Schühly et al. 2009; Clark et al. 1981; Shen et al. 2009). Neolignans such as obovatol, honokiol, and magnolol have been previously shown to possess various biological activities including antitumor, antiviral, anxiolytic-like, and anti-inflammatory effects (Oh et al. 2009; Arora et al. 2012; Yu et al. 2012). To determine the anti-proliferative activity of the isolated compounds 1–3, MTT assays were carried out using human liver hepatocellular carcinoma HepG2, breast cancer MDA-MB-231 and MDA-MB-435, and nasopharyngeal carcinoma HNE1 cell lines. Our results showed that 48 h after administration, compound 1 exhibited potent anti-proliferative avtivities against four human cancer cell lines with IC50 values ranging from 5.1 to 7.5 µM, whereas the two other compounds had lower potencies (Table 2).
Apoptosis is an important programmed cell death (PCD) process that occurs in order to maintain the homeostasis between cell proliferation and cell death. We investigated whether compound 1 induced cell death involved apoptosis, and our result indeed showed that 1 was a potent pro-apoptotic compound. We analyzed the effect of compound 1 on apoptosis in the HepG2 cell line by using propidium iodide (PI) staining in combination with flow cytometry. After exposure to 10, 20, and 30 µM compound 1 for 48 h, the apoptosis rate were 5.9, 21.4, and 75.8 %, respectively (Fig. 3). To explore the mechanisms underlying the proapoptotic action of compound 1, we also examined the expression level of the antiapoptotic protein Bcl-2, as well as the cleavage of caspase 3, a key down-stream effector of apoptosis. As shown in Fig. 4, western blot analysis indicated that compound 1 down-regulated the expression of Bcl-2 and potentiated the cleavage of caspase-3 in a concentration dependent manner.
Effect of bishonokiol A (1) on the expression of apoptosis related proteins. HepG2 cells were treated for 24 h with vehicle or with various concentrations of bishonokiol A (1). The ability of bishonokiol A (1) to regulate the expression of apoptosis-related proteins was evaluated as described in the materials and methods
Taken together, the findings suggest that newly identified dimeric neolignan (1) exerted anti-cancer effects through the induction of apoptosis in human cancer cells. Further studies will be required to investigate the in vivo anti-tumor activity of the new compound 1 and to confirm the cellular mechanisms involved.
References
Arora, S., S. Singh, G.A. Piazza, C.M. Contreras, J. Panyam, and A.P. Singh. 2012. Honokiol: A novel natural agent for cancer prevention and therapy. Current Molecular Medicine 10: 1244–1252.
Baker, D.D., M. Chu, U. Oza, and V. Rajgarhia. 2007. The value of natural products to future pharmaceutical discovery. Natural Product Reports 24: 1225–1244.
Clark, A.M., A.S. EI-Feraly, and W.S. Li. 1981. Antimicrobial activity of phenolic constituents of Magnolia grandiflora L. Journal of Pharmaceutical Sciences 70(8): 951–952.
Del Valle Mondragón, L., F.A. Tenorio López, J.C. Torres Narváez, G.G. Zarco Olvera, and G. Pastelín Hernández. 2004. Estudio de los extractos de Magnolia grandiflora sobreel músculo cardíacode cobayo. Archivos de Cardiologia de Mexico 74: 108–117.
Feltenstein, M.W., W. Schühly, J.E. Warnick, N.H. Fischer, and K.J. Sufka. 2004. Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear’s Foot. Pharmacology Biochemistry and Behavior 79: 299–302.
Ito, K., T. Iida, K. Ichino, M. Tsunezuka, M. Hattori, and T. Namba. 1982. Obovatol and obovatal, novel biphenyl ether lignans from the leaves of Magnolia obovata Thunb. Chemical & Pharmaceutical Bulletin 30(9): 3347–3353.
Lee, S.K., H.N. Kim, Y.R. Kang, C.W. Lee, H.M. Kim, D.C. Han, J. Shin, K.H. Bae, and B.M. Kwon. 2008. Obovatol inhibits colorectal cancer growth by inhibiting tumor cell proliferation and Inducing apoptosis. Bioorganic & Medicinal Chemistry 16: 8397–8402.
Mohamed, S.M., E.M. Hassan, and N.A. Ibrahim. 2010. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Natural Product Reports 24(15): 1395–1402.
Ngo, L.T., J.I. Okogun, and W.R. Folk. 2013. 21st century natural product research and drug development and traditional medicines. Natural Product Reports 30(4): 584–592.
Oh, J.H., L.L. Kang, J.O. Ban, Y.H. Kim, K.H. Kim, S.B. Han, and J.T. Hong. 2009. Anti-inflammatory effect of 4-O-methylhonokiol, a novel compound isolated from Magnolia officinalis through inhibition of NF-κΒ. Chemico-Biological Interactions 190: 506–514.
Schuehly, W., W. Voith, H. Teppner, and O. Kunert. 2010. Substituted dineolignans from Magnolia garrettii. Journal of Natural Products 73: 1381–1384.
Schühly, W., I. Khan, and N.H. Fischer. 2001. The ethnomedicinal uses of Magnoliaceae from the Southeastern United States as leads in drug discovery. Pharmaceutical Biology 39: 63–69.
Schühly, W., S.I. Khan, and N.H. Fischer. 2009. Neolignans from north American Magnolia species with cyclooxygenase 2 inhibitory activity. Inflammopharmacology 17: 106–110.
Shen, C.C., C.L. Ni, Y.C. Shen, Y.L. Huang, C.H. Kuo, T.S. Wu, and C.C. Chen. 2009. Phenolic constituents from the stem bark of Magnolia officinalis. Journal of Natural Products 72: 168–171.
Surh, Y.J. 2003. Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer 3(10): 768–780.
Yu, J.Y., J.J. Lee, J.K. Jung, Y.K. Min, J.Y. Ma, T.J. Kim, M.Y. Lee, and Y.P. Yun. 2012. Anti- platelet activity of diacetylated obovatol through regulating cyclooxygenase and lipoxygenase activities. Archives of Pharmacal Research 5(12): 2191–2198.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China under Grant (no. 81302671, 81372899); the Natural Science Foundation of Anhui Province under Grant (no. 1408085QH162); the Natural Science Foundation of Bengbu Medical College under Grant (Bykf1326).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong-Mei Li and Su-Rong Zhao have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, HM., Zhao, SR., Huo, Q. et al. A new dimeric neolignan from Magnolia grandiflora L. seeds. Arch. Pharm. Res. 38, 1066–1071 (2015). https://doi.org/10.1007/s12272-014-0476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0476-4