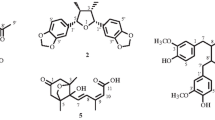
A new norneolignan derivative, clitorternalactone (1), has been isolated from the stems of Clitoria ternatea, together with six known compounds, clitorienolactone A (2), myricetin 3-glucoside (3), quercetin 3-glucoside (4), kaempferol 3-glucoside (5), taraxerol (6), and taraxerone (7). The structure of the new compound 1 was determined through spectroscopic and MS analyses. Among the isolates, compound 1 exhibited cytotoxicities with IC50 values of 2.54 ± 0.23, 3.68 ± 0.17, and 4.05 ± 0.43 μM, respectively, against DLD-1, CCRF-CEM, and IMR-32 cell lines. In addition, clitorternalactone (1), clitorienolactone A (2), quercetin 3-glucoside (4), and kaempferol 3-glucoside (5) showed potent inhibition with IC50 values of 3.05 ± 0.12, 5.44 ± 0.46, 10.20 ± 0.69, and 13.57 ± 0.36 μM, respectively, against LPS-induced NO generation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Clitoria ternatea L. (Fabaceae) is a perennial twining herb, distributed in Indochina, the Philippine Islands, Madagascar, and Taiwan [1]. Previous chemical studies of this plant have reported the isolation of several components including norneolignans [2], triterpenoids [2, 3], flavonol glycosides [4, 5], steroids [4, 6], anthocyanins [7, 8], fatty acid [9, 10], and their derivatives. This plant also exhibits diverse biological activities, including memory enhancing [2], antiacetylcholinesterase [2], antiasthmatic [11], anti-inflammatory [12], antipyretic [12], and antiamnesic [13] activities. The current phytochemical investigation of the stems of this plant has led to the isolation of a new norneolignan derivative, clitorternalactone (1), along with seven known compounds. The structural elucidation of 1 and the cytotoxic and anti-inflammatory properties of 1–7 are described herein.
Extensive fractionation of the CH2Cl2 soluble portion of a MeOH extract of stems of Clitoria ternatea using silica gel column chromatography (CC) and preparative TLC afforded compounds 1–7.
Clitorternalactone (1) was isolated as yellowish prisms. The HR-ESI-MS gave an [M + Na]+ ion at m/z 367.07934 (calcd for C18H16O7Na, 367.07937), consistent with a molecular formula of C18H16O7. IR absorptions for carbonyl (1716 cm–1) and hydroxyl (3356, 3273 cm–1) functions were observed. Comparison of the 1H and 13C NMR data of 1 with those of clitorienolactone A (2) [2] suggested that their structures were closely related, except that the 3,5-dihydroxy groups [δC 147.1 (C-3), 147.1 (C-5)] of 1 replaced MeO-3 [δH 3.75, δC 56.2] and H-5 [δH 6.51, δC 115.8] of clitorienolactone A [2]. This was supported by (1) HMBC correlations observed between H-2 (δ 6.24) and C-1 (δ 128.5), C-4 (δ 133.1), and C-7 (δ 39.7); and (2) HMBC correlations observed between H-6 (δ 6.24) and C-1 (δ 128.5), C-4 (δ 133.1), and C-7 (δ 39.7). The absolute configuration of 1 was proved by the similar CD Cotton effects [209 ([θ] = +2393), 220 ([θ] = –1930), 231 ([θ] = +1778), 251 ([θ] = –2739), and 286 ([θ] = +5313) nm] compared with analogous norneolignan derivatives [2]. The full assignment of 1H and 13C NMR resonances was supported by 1H–1H COSY, DEPT, HSQC, NOESY, and HMBC (Table 1) spectral analyses. On the basis of the above data, the structure of 1 was elucidated as (S)-4-(4-hydroxy-2-methoxyphenyl)-5-(3,4,5- trihydroxybenzyl)furan-2(5H)-one and was named clitorternalactone.

The known isolates were readily identified by comparison of physical and spectroscopic data (UV, IR, 1H NMR, and MS) with corresponding authentic samples or literature values, and they included clitorienolactone A (2) [2], myricetin 3-glucoside (3) [14], quercetin 3-glucoside (4) [14], kaempferol 3-glucoside (5) [14], taraxerol (6) [15], and taraxerone (7) [16].
Nitric oxide (NO) is a mediator in the inflammatory response involved in host defense. The anti-inflammatory effects of the compounds isolated from the stems of C. ternatea were evaluated by suppressing lipopolysaccharide (LPS)-induced NO generation in murine macrophage cell line RAW264.7. The inhibitory activity data of the isolates 1–7 on NO generation by macrophages are shown in Table 2. Quercetin was used as positive control. Among the isolates, clitorternalactone (1), clitorienolactone A (2), quercetin 3-glucoside (4), and kaempferol 3-glucoside (5) showed potent inhibition with IC50 values of 3.05 ± 0.12, 5.44 ± 0.46, 10.20 ± 0.69, and 13.57 ± 0.36 μM, respectively, against LPS-induced NO generation.
Isolates from the stems of C. ternatea were tested in vitro against DLD-1, CCRF-CEM, HL-60, and IMR-32 cell lines, with cytotoxicity data shown in Table 3. The anticancer agent doxorubicin was used as a positive control. Among the isolates, clitorternalactone (1) exhibited cytotoxicities with IC50 values of 2.54 ± 0.23, 3.68 ± 0.17, and 4.05 ± 0.43 μM, respectively, against DLD-1, CCRF-CEM, and IMR-32 cell lines. Taraxerone (7) showed cytotoxic effects, with IC50 values of 18.15 ± 1.57 and 21.86 ± 2.33 μM, respectively, against DLD-1 and HL-60 cell lines.
Experimental
General Experimental Procedures. UV spectra were obtained on a Jasco UV-240 spectrophotometer. CD spectra were obtained on a Jasco J-815 spectropolarimeter (Jasco Co.). IR spectra (neat or KBr) were recorded on a Perkin Elmer 2000 FT-IR spectrometer. NMR spectra, including COSY, NOESY, HMBC, HSQC experiments, were recorded on a Varian Inova 500 spectrometer operating at 500 MHz (1H) and 125 MHz (13C), with chemical shifts given in ppm (δ) using tetramethylsilane (TMS) as an internal standard. ESI and HR-ESI-mass spectra were recorded on a Bruker APEX II mass spectrometer. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography (CC). Silica gel 60 F-254 (Merck) was used for thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC). High-performance liquid chromatography (HPLC) was performed using a silica column (Thermo Hypersil-100 silica, 10 mm i.d. × 250 mm; detector RI) and an ODS column (Biosil PRO-ODS-U, 10 mm i.d. × 250 mm; detector RI).
Plant Material. The stems of Clitoria ternatea were collected from Pingtung City, Pingtung County, Taiwan, in June 2018, and a voucher specimen (CT 201806) was deposited in the Faculty of Pharmacy, National Yang-Ming University, Taipei, Taiwan.
Extraction and Separation of Compounds. The dried stems (1.3 kg) of C. ternatea were extracted three times with MeOH (5 L each) for 3 days. The methanol extract (97 g) was partitioned between CH2Cl2 and H2O (1:1) to afford CH2Cl2-soluble (Fr. A, 33 g) and H2O-soluble (Fr. B, 64 g) fractions. The CH2Cl2-soluble fraction (33 g) was chromatographed on silica gel (70–230 mesh, 1.5 kg), eluting with CH2Cl2 and gradually increasing the polarity with MeOH to give 12 subfractions (Subfrs. A1–A12). Subfraction A3 (2.5 g) was separated by column chromatography on silica gel (230–400 mesh, 112 g), eluting with CH2Cl2–MeOH (10:1–0:1) to yield 10 subfractions (Subfrs. A3-1–A3-10). Part (125 mg) of Subfr. A3-5 was purified by preparative TLC (silica gel, n-hexane–acetone, 7:1) to give taraxerone (7) (6.6 mg). Part (88 mg) of Subfr. A3-7 was purified by preparative TLC (silica gel, CH2Cl2–EtOAc, 5:1) to afford taraxerol (6) (5.2 mg). Subfraction A6 (2.1 g) was separated by column chromatography on silica gel (230–400 mesh, 95 g), eluting with CH2Cl2–MeOH (5:1–0:1) to yield 11 fractions (Subfrs. A6-1–A6-11). Part (145 mg) of Subfr. A6-8 was purified by preparative TLC (silica gel, CH2Cl2–MeOH, 4:1) to afford clitorienolactone A (2) (4.3 mg). Subfraction A9 (3.0 g) was separated by column chromatography on silica gel (230–400 mesh, 135 g), eluting with CH2Cl2–MeOH (3:1–0:1) to yield nine subfractions (Subfrs. A9-1–A9-9). Subfraction A9-6 (280 mg) was purified by MPLC (13 g of SiO2, 230–400 mesh; CH2Cl2–MeOH, 3:1–0:1, 150-mL fractions) to give eight subfractions (A9-6-1–A9-6-8). Subfraction A9-6-6 (39 mg) was purified by HPLC (ODS column, MeOH–H2O, 3:1, 2.0 mL/min) to obtain clitorternalactone (1) (4.2 mg) and kaempferol 3-glucoside (5) (3.8 mg). Subfraction A9-6-7 (42 mg) was purified by HPLC (ODS column, MeOH–H2O, 4:1, 2.0 mL/min) to obtain myricetin 3-glucoside (3) (5.9 mg) and quercetin 3-glucoside (4) (6.4 mg).
Clitorternalactone (1). Yellowish prisms, mp 217–219°C (CH2Cl2–MeOH). UV (MeOH, λmax, nm): 324. CD (MeOH, [θ], nm): 209 (+2393), 220 (–1930), 231 (+1778), 251 (–2739), 286 (+5313). IR (KBr, νmax, cm–1): 3356 (OH), 3273 (OH), 1716 (C=O). 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1. ESI-MS m/z 367 [M + Na]+. HR-ESI-MS m/z 367.07934 [M + Na]+ (calcd for C18H16O7Na, 367.07937).
Cytotoxic Assay. The cell lines used in this study were DLD-1 cells (human colorectal carcinoma), CCRF-CEM cells (human lymphoblastic leukemia), HL-60 cells (human myeloid leukemia), and IMR-32 cells (human neuroblastoma). The above cell lines were purchased from the Bioresource Collection and Research Center (BCRC), Food Industry Research and Development Institute (FIRDI), Hsinchu 300, Taiwan.
The cytotoxic activities of compounds 1–7 against DLD-1, CCRF-CEM, HL-60, and IMR-32 were assayed by a modification of the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method [17]. To measure the cytotoxic activities of the purified compounds against the above tumor cells, each cell line began with 5 × 105 cells/well in 96-well microtiter plates (Falcon). Eight concentrations (triplicate) of test compounds (dissolved in 0.5% DMSO) encompassing a 128-fold range were added to each cell line. Each tumor cell was enumerated using MTT (Sigma) after exposure to the test compounds for 3 days. Then 15 μL of 1 mg/mL MTT were added to each well, and the plates were incubated at 37°C for a further 4 h. Formazan crystals were redissolved in DMSO (Merck) for 10 min with shaking, and the plate was read immediately on a microtiter plate reader (Dynatech) at a wavelength of 570 nm. The IC50 value was defined as the concentration of the test compound necessary to inhibit the growth to 50% of the control in the MTT assay. The anticancer agent doxorubicin and 0.5% DMSO were used as positive control and solvent control, respectively. The assays were repeated three times.
Measurement of Nitric Oxide/Nitrite. The murine macrophage cell line RAW264.7 was cultured in Dulbecco′s modified Eagle′s medium (DMEM, Gibco BRL Life Technologies, Inc.) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and incubated at 37°C in a humidified 5% CO2 atmosphere with a 96-well flat-bottomed culture plate. After 24 h, the medium was replaced with fresh DMEM and FBS. Then compounds 1–7 (2.5, 5, 10, 20, and 40 μM) were added, respectively, in the presence of lipopolysaccharide (LPS; 1 μg/mL; Sigma, Cat No. L-2654) and the whole incubated under the same conditions for 24 h. The cultured cells were then centrifuged, and the supernatants were used for NO-production measurement. The supernatant was mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% N-(naphthalen-1-yl)ethylenediamine dihydrochloride in 2.5% H2PO4 solution) and incubated for 10 min at room temperature. The nitrite concentration was determined by measuring the absorbance at 540 nm using an ELISA plate reader (μ Quant) [18]. The percentage of NO inhibition of the test compound was calculated as follows:
The data are expressed as the mean of four experiments. The software SigmaPlot was used for determining the IC50 values.
Statistical Analysis. Results are expressed as the mean ± SEM, and comparisons were made using Tukey′s HSD test. A probability of 0.05 or less was considered significant. The software SigmaPlot was used for the statistical analysis.
References
T. C. Huang and H. Ohashi, Leguminosae in Flora of Taiwan, Vol. 3, 2nd Ed., Editorial Committee of the Flora of Taiwan, Taipei, Taiwan, 1993, pp. 160–396.
K. Vasisht, M. Dhobi, S. Khullar, S. K. Mandal, and M. Karan, Tetrahedron Lett., 57, 1758 (2016).
S. K. Banerjee and R. N. Chakravarti, Bull. Calcutta Sch. Trop. Med., 11, 106 (1963).
R. K. Gupta and L. B. Lal, Ind. J. Pharm., 30, 167 (1968).
K. Kazuma, N. Noda, and M. Suzuki, Phytochemistry, 64, 1133 (2003).
H. Ripperger, Pharmazie, 33, 82 (1978).
N. Saito, K. Abe, T. Honda, C. F. Timberlake, and P. Bridle, Phytochemistry, 24, 1583 (1985).
B. K. Srivastava and C. S. Pande, Planta Med., 32, 138 (1977).
S. S. Joshi, R. K. Shrivastava, and D. K. Shrivastava, J. Am. Oil Chem. Soc., 58, 714 (1981).
S. Husain and K. S. Devi, J. Oil Technol. Assoc. India, 30, 162 (1998).
D. J. Taur and R. Y. Patil, J. Ethnopharmacol., 136, 374 (2011).
B. Parimaladevi, R. Boominathan, and S. C. Mandal, Fitoterapia, 74, 345 (2003).
A. D. Taranalli and T. C. Cheeramkuzhy, Pharm. Biol., 38, 51 (2000).
K. Kazuma, N. Noda, and M. Suzuki, Phytochemistry, 62, 229 (2003).
K. N. Sangeetha, S. Sujatha, V. S. Muthusamy, S. Anand, N. Nithya, D. Velmurugan, A. Balakrishnan, and B. S. Lakshmi, Biochim. Biophys. Acta, 1800, 359 (2010).
S. Chunhakant and C. Chaicharoenpong, Molecules, 24, 2798 (2019).
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
M. Johansson, B. Kopcke, H. Anke, and O. Sterner, J. Antibiot., 55, 104 (2002).
Acknowledgment
This research was supported by a grant from the Ministry of Science and Technology, Taiwan (MOST 106-2320-B-010-033-MY3), awarded to Prof. J.-J. Chen. This work was also supported by grants from Far Eastern Memorial Hospital–National Yang-Ming University Joint Research Program (108DN22), Medical Research Fund (No. 108-22) of Kaohsiung Armed Forces General Hospital, and Yuan’s General Hospital (YGH 19-027). Fu-Sen Wu, Ching-Ju Hung, and Chien-Liang Lin have contributed equally to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2020, pp. 863–866.
Rights and permissions
About this article
Cite this article
Wu, FS., Hung, CJ., Lin, CL. et al. New Norneolignan and Bioactive Constituents of Clitoria ternatea. Chem Nat Compd 56, 1000–1004 (2020). https://doi.org/10.1007/s10600-020-03213-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03213-w