Abstract
The mucolytic drug ambroxol hydrochloride reduces the production of pro-inflammatory cytokines and the frequency of exacerbation in patients with chronic obstructive pulmonary disease (COPD). However, the inhibitory effects of ambroxol on rhinovirus infection, the major cause of COPD exacerbations, have not been studied. We examined the effects of ambroxol on type 14 rhinovirus (RV14) infection, a major RV group, in primary cultures of human tracheal epithelial cells. RV14 infection increased virus titers and cytokine content in the supernatants and RV14 RNA in the cells. Ambroxol (100 nM) reduced RV14 titers and cytokine concentrations of interleukin (IL)-1β, IL-6 and IL-8 in the supernatants and RV14 RNA in the cells after RV14 infection, in addition to reducing susceptibility to RV14 infection. Ambroxol also reduced the expression of intercellular adhesion molecule-1 (ICAM-1), the receptor for RV14, and the number of acidic endosomes from which RV14 RNA enters the cytoplasm. In addition, ambroxol reduced the activation of the transcription factor nuclear factor kappa B (NF-κB) in the nucleus. These results suggest that ambroxol inhibits RV14 infection partly by reducing ICAM-1 and acidic endosomes via the inhibition of NF-κB activation. Ambroxol may modulate airway inflammation by reducing the production of cytokines in rhinovirus infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhinoviruses (RVs) are associated with acute exacerbations of chronic obstructive pulmonary disease (COPD) (Seemungal et al. 2000). RV infection induces the production of cytokines, including interleukin (IL)-1, IL-6 and IL-8 (Subauste et al. 1995; Zhu et al. 1996; Suzuki et al. 2002). These cytokines exert pro-inflammatory effects and may be related to the pathogenesis of RV infection and COPD exacerbations (Akira et al. 1990; Subauste et al. 1995; Zhu et al. 1996; Seemungal et al. 2001; Suzuki et al. 2002; Sethi 2004).
The members of the major RV group enter the cytoplasm of infected cells after binding to their receptor, intercellular adhesion molecule (ICAM)-1 (Greve et al. 1989; Casasnovas and Springer 1994). The entry of the RNA of a major group RV, RV14, into the cytoplasm of infected cells is thought to be mediated by destabilization induced by receptor binding and endosomal acidification (Casasnovas and Springer 1994). Various agents, including glucocorticoids, the macrolide antibiotics bafilomycin and erythromycin, the β2 agonist procaterol, and the anti-cholinergic agent tiotropium, inhibit RV14 infection by reducing ICAM-1 expression or increasing endosomal pH (Pérez and Carrasco 1993; Suzuki et al. 2000, 2001, 2002; Yamaya et al. 2011, 2012).
A mucolytic agent, ambroxol, prevents COPD exacerbations (Olivieri et al. 1987; Malerba et al. 2004). Ambroxol reduces reactive oxygen species in bronchoalveolar lavage fluid in COPD patients (Teramoto et al. 1999) and inhibits the release of cytokines from human leukocytes (Gibbs et al. 1999). These findings suggest that ambroxol inhibits COPD exacerbation partly through anti-inflammatory effects. However, the anti-inflammatory effects of ambroxol in airway epithelial cells in RV infections have not been studied.
Ambroxol reduces the frequency of acute upper respiratory disease (Nobata et al. 2006), including the common cold and influenza, and it inhibits influenza virus infection in mouse airways (Yang et al. 2002). The mucolytic agents N-acetylcysteine and l-carbocisteine reduce the expression of the ICAM-1 in cells (Blesa et al. 2003; Yasuda et al. 2006), and l-carbocisteine inhibits RV infection in airway epithelial cells by reducing ICAM-1 expression or increasing endosomal pH (Yasuda et al. 2006). However, the inhibitory effects of ambroxol on RV infection are unknown.
In the present study, we examined the effects of ambroxol on infection with RV14 and the mechanisms in human airway epithelial cells.
Materials and methods
Human tracheal epithelial cell culture
Human tracheal surface epithelial cells were isolated and cultured in plastic tubes (Becton–Dickinson, Franklin Lakes, NJ, USA) or on coverslips in Petri dishes as described previously (Yamaya et al. 2011).
The cells for culture studies were derived from tracheas obtained after death from 33 patients (age 71 ± 3 years; 13 female, 20 male). None of the patients were being treated with ambroxol at the time of death. This study was approved by the Tohoku University Ethics Committee.
Human embryonic fibroblast cell culture
Human embryonic fibroblast cells (HFL-III cells, Riken Bio Resource Center Cell Bank, Cell No: RCB0523; Tsukuba, Japan) were cultured as described previously (Yamaya et al. 2011).
Viral stock
RV14 (a major group RV) stock was prepared from a patient with a common cold by infecting human embryonic fibroblast cells as previously described (Numazaki et al. 1987; Yamaya et al. 2011). We used RV14 stock that had been passaged 3–5 times.
Detection and titration of viruses
RV14 in the supernatants (cell-culture medium) was detected and titrated using human embryonic fibroblast cells with endpoint methods (Condit 2006; Yamaya et al. 2011). The rates of RV14 release into the supernatants are expressed as TCID50 (tissue culture infective dose) units/ml/24 h (Numazaki et al. 1987; Yamaya et al. 2011).
Quantification of rhinoviral RNA
To quantify the RV14 RNA and ribosomal RNA (18S, rRNA) expression in human tracheal epithelial cells after RV14 infection, a two-step real-time quantitative reverse transcription (RT)-PCR using the TaqMan technique (Roche Molecular Diagnostic Systems) was performed using TaqMan® Gene Expression Master Mix (Applied Biosystems, Bedford, CA, USA) as described by Nolan et al. (2006) and previously reported by Yamaya et al. (2011).
Viral infection of epithelial cells
Infection of the human tracheal epithelial cells with RV14 (100 μl in each tube, 1.0 × 104 TCID50 units/100 μl, 5.0 × 10−2 TCID50 units/cell) was performed using previously described methods (Yamaya et al. 2011). The cells were cultured at 33 °C with rolling.
Treatment with ambroxol
The mean peak human plasma concentration has been reported to be 81.5 ng/ml (=0.2 μM) at 1.7 h after oral ingestion of 60 mg of ambroxol (Su et al. 2007). Furthermore, the concentration of ambroxol is approximately 20-fold higher in the lungs than in the serum (Mezzetti et al. 1990). Therefore, to examine the effects of ambroxol, cultured human tracheal epithelial cells were treated with either ambroxol (0.1 μM, Sigma) or vehicle (0.1 % double distilled water), as previously described (Severina et al. 2000). The cells were treated with ambroxol for 3 days (72 h) before infection with RV14 and until the end of the experimental period after the RV14 infection, as described previously (Yamaya et al. 2011).
To examine the concentration-dependent effects of ambroxol on RV14 infection, cells were treated with ambroxol at concentrations ranging from 0.01 to 100 μM.
To examine the effects of ambroxol on ICAM-1 mRNA expression in the cells and on the concentration of the soluble form of ICAM-1 (sICAM-1) in the supernatants, the cells were pretreated with ambroxol (0.1 μM) for 3 days before RV14 infection.
Collection of supernatants for measurements
We collected the supernatants at 1 or 12 h after RV infection to examine the viral release. Furthermore, to examine the effects of ambroxol on the viral release and the secretion of IL-1β, IL-6, and IL-8, we collected supernatants at 1 day (24 h), 3 days (72 h), 5 days (120 h), and/or 7 days (168 h) after infection using previously described methods (Yamaya et al. 2011).
Effects of ambroxol on susceptibility to rhinovirus infection
Epithelial cells were pretreated with ambroxol (0.1 μM) or vehicle for 3 days prior to infection, and the effects of ambroxol on the cellular susceptibility to RV14 infection were evaluated as previously described (Yamaya et al. 2011).
Measurement of ICAM-1 expression
The level of ICAM-1 mRNA was examined using two-step real-time RT-PCR analysis using the methods described above (quantification of rhinoviral RNA), with a forward primer designed for ICAM-1 (Yamaya et al. 2011). The concentration of sICAM-1 in the supernatants was measured with an enzyme immunoassay (EIA) (Yamaya et al. 2011).
Measurement of changes in acidic endosomes
The distribution and the fluorescence intensity of acidic endosomes in the cells were measured, as previously described, with LysoSensor DND-189 dye (Molecular Probes, Eugene, OR, USA) (Suzuki et al. 2001; Yamaya et al. 2011).
Measurement of cytokine production
We measured IL-1β, IL-6, and IL-8 levels in the supernatants with specific enzyme-linked immunosorbent assays (ELISAs) in human tracheal epithelial cells (Yamaya et al. 2011).
NF-kappa B assay
The presence of the p50, p65, and c-Rel subunits in nuclear extracts was assayed using a TransFactor Family Colorimetric Kit-NF-κB (BD Bioscience/CLONTECH) according to the manufacturer’s instructions, as previously described (Yamaya et al. 2011).
Statistical analysis
The results are expressed as the mean ± SEM. Statistical analysis was performed using a one-way analysis of variance (ANOVA). Subsequent post hoc analysis was performed using Bonferroni’s method. For all analyses, values of p < 0.05 were assumed to be significant. The number of donors (tracheae) from which cultured epithelial cells were used is referred to as n.
Results
Effects of ambroxol on rhinoviral infection in human tracheal epithelial cells
No virus was detected at 1 h after infection; however, RV14 was detected in the supernatants at 12 h. The viral content progressively increased between 1 and 12 h after infection and increased significantly with time for the first 3 days (p < 0.05 by ANOVA) (Fig. 1a).
a The time course of viral release into the supernatants of human tracheal epithelial cells obtained at different times after exposure to 5.0 × 10−2 TCID50 units/cell of RV14 in the presence of ambroxol (0.1 μM) (closed circles) or vehicle (double distilled water) (open circles). The epithelial cells isolated from each donor were treated with ambroxol or vehicle beginning 3 days prior to the initiation of infection and until the end of the experimental period. To examine whether the supernatants contained a significant amount of RV14, human embryonic fibroblast cells were observed for evidence of cytopathic changes for 7 days (168 h) after exposing the fibroblasts to the supernatants. The rates of change in RV14 concentration in the supernatants are expressed as TCID50 units/ml/24 h. The results are reported as the mean ± SEM from five different tracheae (two ex-smokers and three non-smokers). Significant differences compared with treatment with the vehicle alone are indicated by *p < 0.05. b Concentration-dependent effects of ambroxol on viral release into supernatants collected between 1 day (24 h) and 3 days (72 h) after the infection. The cells were treated with ambroxol (closed circles) or vehicle (control; open circle) beginning 3 days (72 h) before RV14 infection until the end of the experimental period. The epithelial cells isolated from each donor were treated with ambroxol or vehicle. The rates of change in the RV14 concentration in the supernatants are expressed as TCID50 units/ml/24 h. The results are reported as the mean ± SEM from five different tracheae. Significant differences compared with treatment with the vehicle alone (control) are indicated by *p < 0.05 and **p < 0.01
Pretreatment of the cells with ambroxol (0.1 μM) resulted in significantly lower viral titers of RV14 in the supernatants from 12 h after infection (Fig. 1a) and reduced RV14 titers in a concentration-dependent manner (Fig. 1b). Pretreatment of the cells with ambroxol at concentrations of 0.1 μM or greater reduced the viral titers of RV14 (Fig. 1b).
RV14 titers in the supernatants collected from the cells of the 13 ex-smokers did not differ from those of the 20 patients who had never smoked (data not shown). Likewise, the RV14 titers in the supernatants from the three patients with COPD did not differ from those of the 30 patients without COPD (data not shown).
The cell numbers in the confluent sheets cultured in the medium supplemented with ambroxol (0.1 μM, 72 h) did not differ from cell numbers in the unsupplemented medium (data not shown). Treatment of the cells with ambroxol did not change cell viability, as assessed using a trypan blue exclusion assay, and LDH concentration in the supernatants (data not shown).
Effects of ambroxol on viral RNA replication
RV14 RNA was consistently observed in the cells from 1 day after infection onward, and the levels increased with time after infection (Fig. 2). The maximum level of RV14 RNA replication was observed at 2 or 3 days after infection. Pretreatment with ambroxol (0.1 μM) resulted in a lower level of RV14 RNA at 1 and 3 days after infection (Fig. 2).
Replication of viral RNA in human tracheal epithelial cells at 1 day (24 h) or 3 days (72 h) after infection with RV14 in the presence of ambroxol (0.1 μM) (RV+Ambroxol) or vehicle (RV) or after infection with ultraviolet-inactivated RV14 in the presence of vehicle of ambroxol (UV-RV), as detected by real-time quantitative RT-PCR. The epithelial cells isolated from each donor were treated with ambroxol or vehicle (double distilled water). The results are expressed as the relative amount of RNA expression (%) compared with that of maximal RV14 RNA levels on day 3 (72 h) in the cells treated with vehicle. The results are reported as the mean ± SEM from five samples (two ex-smokers and three non-smokers). Significant differences compared with treatment with the vehicle alone (RV) at each time point are indicated by *p < 0.05. RV14 RNA was not detected at significant levels after UV-RV infection
Effects of ambroxol on susceptibility to rhinovirus infection
Pretreatment of the cells with ambroxol (0.1 μM, 72 h) decreased the susceptibility of the cells to infection with RV14. The minimum dose of RV14 necessary to cause infection of the cells treated with ambroxol was significantly higher than that for the cells treated with the vehicle alone (Table 1).
Effects of ambroxol on the expression of ICAM-1
Ambroxol (0.1 μM, 72 h) reduced baseline ICAM-1 mRNA expression in the cells by approximately 30 % compared with the cells treated with the vehicle alone prior to RV14 infection (Fig. 3a). Furthermore, the concentrations of sICAM-1 in the supernatants from cells treated with ambroxol were significantly lower than those from the cells treated with the vehicle alone (Fig. 3b).
a The expression of ICAM-1 mRNA before the RV14 infection in human tracheal epithelial cells treated with ambroxol (0.1 μM, 72 h, Ambroxol) or vehicle (control), as detected by real-time quantitative RT-PCR. The epithelial cells isolated from each donor were treated with ambroxol or vehicle. ICAM-1 mRNA levels were normalized to the constitutive expression of ribosomal RNA (rRNA). The expression of ICAM-1 mRNA in the cells treated with vehicle was set to 1.0. The results are reported as the mean ± SEM from five different tracheae (two ex-smokers and three non-smokers). Significant differences compared with treatment with the vehicle alone are indicated by *p < 0.05. b Concentration of sICAM-1 in supernatants before RV14 infection of human tracheal epithelial cells treated with ambroxol (0.1 μM, 72 h, ambroxol) or vehicle (control) as detected by enzyme immunoassay. The sICAM-1 concentrations in the supernatants are expressed in ng/ml. The results are reported as the mean ± SEM from five different tracheae (two ex-smokers and three non-smokers). Significant differences compared with control values are indicated by *p < 0.05
Effects of ambroxol on the acidification of endosomes
Treatment with the vehicle for 3 days did not change the number of fluorescent acidic endosomes in the cells (Fig. 4a, b) or the fluorescence intensity of the acidic endosomes compared with the cells prior to any treatment (Fig. 4d). In contrast, treatment with ambroxol (0.1 μM, 72 h) reduced the number of acidic endosomes displaying green fluorescence (Fig. 4c) and reduced the fluorescence intensity of the acidic endosomes compared with cells treated with vehicle and cells prior to any treatment (Fig. 4d).
a–c Changes in the distribution of acidic endosomes (green fluorescence) in human tracheal epithelial cells before treatment with ambroxol (a) and 3 days (72 h) after treatment with ambroxol (0.1 μM) (c) or vehicle (b). Data are representative of five different experiments (two ex-smokers and three non-smokers) (Bar 100 μm). d The fluorescence intensity of acidic endosomes 3 days after treatment with ambroxol (0.1 μM) or vehicle (vehicle) and before treatment (before). The results are reported as the fluorescence intensity divided by the fluorescence intensity before treatment (expressed as a percentage). The results are reported as the mean ± SEM from five samples (two ex-smokers and three non-smokers). Significant differences compared with the values before treatment (before) are indicated by *p < 0.05
Effects of ambroxol on cytokine production
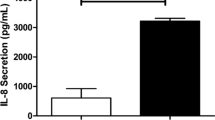
Ambroxol (0.1 μM) pretreatment reduced the baseline secretion of IL-1β, IL-6, and IL-8 for 24 h before the RV14 infection compared with the secretion from cells treated with vehicle alone (Fig. 5). RV14 infection increased the secretion of IL-1β, IL-6, and IL-8, and ambroxol (0.1 μM) also reduced the RV14 infection-induced secretion of IL-1β, IL-6, and IL-8 (Fig. 5).
a–c Time course for cytokine release (A, IL-1β; B, IL-6; C, IL-8) into the supernatants of human tracheal epithelial cells before (time 0) and after infection with RV14 in the presence of ambroxol (0.1 μM) or the ambroxol vehicle (water). The epithelial cells isolated from each donor were treated with ambroxol or vehicle. The rates of change in the concentrations of cytokines in the supernatants are expressed in pg/ml/24 h. The results are reported as the mean ± SEM from eight different tracheae (four ex-smokers and four non-smokers). Significant differences compared with values before RV14 infection (time 0) in the presence of vehicle are indicated by *p < 0.05 and **p < 0.01. Significant differences compared with the RV14 infection alone (RV+vehicle) at each time point after infection are indicated by +p < 0.05 and ++p < 0.01. Exposure to culture medium used for the RV14 stock (vehicle) and infection with ultraviolet-inactivated RV14 (UV-RV) did not change the concentrations of cytokines in the supernatants
Effects on NF-kappa B
Ambroxol (0.1 μM, 72 h) treatment significantly reduced the levels of the p50 and p65 subunits of nuclear factor kappa B (NF-κB) in the nuclear extracts of cells cultured prior to RV14 infection compared with the cells treated with vehicle alone (Fig. 6a, b). In contrast, ambroxol treatment did not alter the levels of the c-Rel subunit of NF-κB (0.264 ± 0.011 OD at 655 nm in control vs. 0.241 ± 0.010 OD at 655 nm in ambroxol, n = 5, p > 0.20), although a decreasing trend was observed.
Amount of p50 (a) and p65 (b) in the nuclear extracts of human tracheal epithelial cells treated with ambroxol (0.1 μM) or vehicle (control) for 3 days (72 h) prior to the RV14 infection. The results are expressed as the optical density (OD) and are reported as the mean ± SEM from five different tracheae (two ex-smokers and three non-smokers). Significant differences compared with control values (control) prior to RV14 infection are indicated by *p < 0.05
Effects of an NF-kappa B inhibitor on ICAM-1 and acidic endosomes
We also studied the role of NF-κB on the expression of ICAM-1 and acidic endosomes. Treatment of the cells with an NF-κB inhibitor, caffeic acid phenethyl ester (CAPE, Calbiochem, La Jolla, CA, USA; 10 μM, 72 h) (Natajaran et al. 1996), reduced sICAM-1 concentrations in the supernatants compared with the concentrations in cells treated with vehicle (0.05 % ethanol) (Table 2). Treatment of the cells with CAPE also reduced the fluorescence intensity of the acidic endosomes in the cells compared with the intensity in the cells treated with vehicle (Table 2).
Discussion
In the present study, we have demonstrated that the mucolytic drug ambroxol reduced the titers of type 14 rhinovirus (RV14), a major group rhinovirus, in supernatants and decreased the RNA replication of the virus in primary cultures of human tracheal epithelial cells. Pretreatment with ambroxol reduced expression of mRNA and protein levels for ICAM-1, the receptor for the major group RVs (Greve et al. 1989), before RV14 infection. The minimum dose of RV14 necessary to cause infection in the cells treated with ambroxol was significantly higher than that in the cells treated with the ambroxol vehicle. These findings suggest that ambroxol might inhibit RV14 infection partly through reducing the production of its receptor, ICAM-1.
Furthermore, treatment with ambroxol reduced the number and fluorescence intensity of acidic endosomes from which RV RNA enters the cytoplasm (Casasnovas and Springer 1994; Turner and Couch 2006). Therefore, the reduction in the number of acidic endosomes might inhibit RNA entry into the cytoplasm and thereby reduce the number of virions that enter the cytoplasm. Ambroxol might also inhibit RV14 infection partly through inhibiting RV RNA entry from the acidic endosomes into the cells.
The major group of RVs enters the cytoplasm of infected cells after binding to the receptor ICAM-1 (Greve et al. 1989). In the present study, ambroxol reduced ICAM-1 expression in human tracheal epithelial cells. These findings are consistent with those of previous reports on the inhibitory effects of other mucolytic agents demonstrated that N-acetylcysteine and l-carbocisteine reduce the expression of ICAM-1 in lung and human tracheal epithelial cells, respectively (Blesa et al. 2003; Yasuda et al. 2006). The inhibitory effects of ambroxol on ICAM-1 expression in human tracheal epithelial cells might be associated with the inhibitory effects of ambroxol on RV14 infection, as has been previously reported for various agents, including glucocorticoids (Pérez and Carrasco 1993; Suzuki et al. 2000, 2001, 2002; Yamaya et al. 2011, 2012).
ICAM-1 is shed by the cell membrane by proteolytic cleavage and detected in plasma and culture supernatants of cells as sICAM-1 (van de Stolpe and van der Saag 1996). Soluble form of ICAM-1 (sICAM-1) consists of most of the extracellular portion of ICAM-1 (van de Stolpe and van der Saag 1996). To demonstrate the effects of ambroxol on the protein expression of airway epithelial cells, in addition to the effects on mRNA expression in the cells, we measured sICAM-1 in the supernatants. In the present study, we demonstrated that reduced ICAM-1 mRNA expression by ambroxol might be associated with reduced ICAM-1 protein expression in the cells and with the inhibition of RV infection.
In the present study, treatment of the cells with ambroxol did not change viability or LDH concentrations in the supernatants, indicating no apparent cytotoxicity from ambroxol, which has been previously demonstrated (Hong et al. 2003).
RVs are associated with the exacerbation of COPD (Seemungal et al. 2000). Neutrophilic inflammation, which takes place during the exacerbation of COPD, has been suggested to be associated with a variety of mediators, including IL-6, after RV infection (Seemungal et al. 2001). In the present study, ambroxol reduced RV14 infection-induced production of mediators, including IL-6 and IL-8. These findings are consistent with previous findings by Yasuda et al. (2006), who found that the mucolytic agent l-carbocisteine reduces production of mediators after RV14 infection. Furthermore, ambroxol also reduces the production of IL-6 and tumor necrosis factor (TNF)-α induced by lipopolysaccharide (Jang et al. 2003; Su et al. 2004), suggesting inhibitory effects on the inflammatory response caused by Gram-negative bacilli, which are also associated with COPD exacerbations (Sethi 2004).
In the present study, ambroxol increased the endosomal pH, although we were not able to determine whether ambroxol inhibits vacuolar H+-ATPase or the Na+/H+ exchangers that regulate endosomal pH (Mellman et al. 1986; Marshansky and Vinay 1996; Nass and Rao 1998). On the other hand, ambroxol inhibits the β2 agonist terbutaline-dependent chloride ion secretion (Hasegawa et al. 2006). H+-ATPase activity is reduced by chloride channel blockers (Fernandez et al. 1997), and inactivating mutations of the chloride channel are associated with modifications of H+-ATPase activity in the cells (Moulin et al. 2003). These findings suggest that the inhibitory effects of ambroxol on chloride channels might be associated with vacuolar H+-ATPase functions.
NF-κB increases the expression of genes for ICAM-1 and various pro-inflammatory cytokines (Zhu et al. 1996; Papi and Johnston 1999), and the mucolytic agent, l-carbocisteine, reduces NF-κB activation (Yasuda et al. 2006). In the present study, ambroxol reduced the expression of ICAM-1 before RV14 infection and the secretion of pro-inflammatory cytokines in supernatants before and after RV14 infection. RV14 infection increases the activation of NF-κB as previously reported (Suzuki et al. 2002). Ambroxol reduced the baseline expression of the p50 and p65 subunits of NF-κB before RV14 infection, although we did not observe a significant inhibitory effect on the c-Rel subunit of NF-κB. We demonstrated that treatment of the cells with an NF-κB inhibitor, CAPE (Natajaran et al. 1996), reduced sICAM-1 concentrations in the supernatants. These findings suggest that ambroxol might reduce expression of ICAM-1 on the cells and secretion of pro-inflammatory cytokines partly through the inhibition of NF-κB activation. Treatment of the cells with CAPE also reduced the fluorescence intensity from acidic endosomes in the present study, demonstrating the relation between NF-κB and acidic endosomes. The reduction of NF-κB activation by ambroxol might also be associated with the increased pH of acidic endosomes, although the precise mechanisms are uncertain.
In summary, this is the first report demonstrating that the mucolytic drug ambroxol reduces the release of RV14 into human tracheal epithelial cell supernatants, reduces the replication of RV14 RNA, and decreases the susceptibility of these cells to RV14 infection. These effects may occur, in part, through a reduction in the expression of ICAM-1, the receptor for RV14, and a reduction in the number of acidic endosomes, through which RV14 RNA enters the cytoplasm. Pretreating cells with ambroxol reduced the baseline and RV14 infection-induced release of IL-1β, IL-6, and IL-8 into the supernatants. Therefore, ambroxol may inhibit RV14 infection and modulate the inflammatory responses in the airways after RV infection.
References
Akira, S., T. Hirano, T. Taga, and T. Kishimoto. 1990. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). The FASEB Journal 4: 2860–2867.
Blesa, S., J. Cortijo, M. Mata, A. Serrano, D. Closa, F. Santangelo, J.M. Estrela, J. Suchankova, and E.J. Morcillo. 2003. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. European Respiratory Journal 21: 394–400.
Casasnovas, J.M., and T.A. Springer. 1994. Pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): An intermediate in which ICAM-1 is bound and RNA is released. Journal of Virology 68: 5882–5889.
Condit, R.C. 2006. Principles of virology. In Fields virology, 5th ed, ed. D.M. Knipe, and P.M. Howley, 25–57. Philadelphia: Lippincott Williams and Wilkins.
Fernandez, R., J.R. Bosqueiro, A.C. Cassola, and G. Malnic. 1997. Role of Cl− in electrogenic H+ secretion by cortical distal tubule. Journal of Membrane Biology 157: 193–201.
Gibbs, B.F., W. Schmutzler, I.B. Vollrath, P. Brosthardt, U. Braam, H.H. Wolff, and G. Zwadlo-Klarwasser. 1999. Ambroxol inhibits the release of histamine, leukotrienes and cytokines from human leukocytes and mast cells. Inflammation Research 48: 86–93.
Greve, J.M., G. Davis, A.M. Meyer, C.P. Forte, S.C. Yost, C.W. Marlor, M.E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56: 839–847.
Hasegawa, I., N. Niisato, Y. Iwasaki, and Y. Marunaka. 2006. Ambroxol-induced modification of ion transport in human airway Calu-3 epithelia. Biochemical and Biophysical Research Communications 343: 475–482.
Hong, J.S., H.H. Ko, E.S. Han, and C.S. Lee. 2003. Inhibition of bleomycin-induced cell death in rat alveolar macrophages and human lung epithelial cells by ambroxol. Biochemical Pharmacology 66: 1297–1306.
Jang, Y.Y., J.H. Song, Y.K. Shin, E.S. Han, and C.S. Lee. 2003. Depressant effects of ambroxol and erdosteine on cytokine synthesis, granule enzyme release, and free radical production in rat alveolar macrophages activated by lipopolysaccharide. Pharmacology and Toxicology 92: 173–179.
Malerba, M., A. Ponticiello, A. Radaeli, G. Bensi, and V. Grassi. 2004. Effect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST Trial). Pulmonary Pharmacology & Therapeutics 17: 27–34.
Marshansky, V., and P. Vinay. 1996. Proton gradient formation in early endosomes from proximal tubes. Biochimica et Biophysica Acta 1284: 171–180.
Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annual Review of Biochemistry 55: 663–700.
Mezzetti, M., L. Colombo, M.G. Marini, V. Crusi, P. Pierfederici, and E. Mussini. 1990. A pharmacokinetic study on pulmonary tropism of ambroxol in patients under thoracic surgery. The European Journal of Emergency Surgery and Intensive Care 13: 179–185.
Moulin, P., T. Igarashi, P. Van der Smissen, J.P. Cosyns, P. Verroust, R.V. Thakker, S.J. Scheinman, P.J. Courtoy, and O. Devuyst. 2003. Altered polarity and expression of H+-ATPase without ultrastructural changes in kidneys of Dent’s disease patients. Kidney International 63: 1285–1295.
Nass, R., and R. Rao. 1998. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. Journal of Biological Chemistry 273: 21054–21060.
Natajaran, K., S. Singh, T.R. Burke Jr, D. Grunberger, and B.B. Aggarwal. 1996. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proceedings of the National Academy of Sciences of the United States of America 93: 9090–9095.
Nobata, K., M. Fujimura, Y. Ishiura, S. Myou, and S. Nakao. 2006. Ambroxol for the prevention of acute upper respiratory disease. Clinical and Experimental Medicine 6: 79–83.
Nolan, T., R.E. Hands, and S.A. Bustin. 2006. Quantification of mRNA using real-time RT-PCR. Nature Protocols 1: 1559–1582.
Numazaki, Y., T. Oshima, A. Ohmi, A. Tanaka, Y. Oizumi, S. Komatsu, T. Takagi, M. Karahashi, and N. Ishida. 1987. A microplate methods for isolation of viruses from infants and children with acute respiratory infections. Microbiology and Immunology 31: 1085–1095.
Olivieri, D., G. Zavatti, G. Tomasini, S. Daniotti, G. Bonsignore, G. Ferrara, N. Carnimero, R. Chianese, E. Catena, S. Marcatili, M. Del Donno, C. Grassi, E. Pozzi, V. Grassi, C. Tantucci, M. Lucchesi, G. Schimid, C.F. Marchioni, S. Penitenti, A. Mistretta, N. Crimi, L. Casali, R. Cabiddu, C. Donner, A. Patessio, V. Massei, C.M. Sanguinetti, O. Orlandi, S. Bruna, C. Serra, and A. Giacopelli. 1987. Ambroxol for the prevention of chronic bronchitis: Exacerbations: Long-term multicenter trial. Respiration 51: 42–51.
Papi, A., and S.L. Johnston. 1999. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NF-κB and GATA transcription factors. Journal of Biological Chemistry 274: 30041–30051.
Pérez, L., and L. Carrasco. 1993. Entry of poliovirus into cells does not require a low-pH step. Journal of Virology 67: 4543–4548.
Seemungal, T., R. Harper-Owen, A. Bhowmik, D.J. Jeffries, and J.A. Wedzicha. 2000. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. European Respiratory Journal 16: 677–683.
Seemungal, T., R. Harper-Owen, A. Bhowmik, I. Moric, G. Sanderson, S. Message, P. Maccallum, T.W. Meade, D.J. Jeffries, S.L. Johnston, and J.A. Wedzicha. 2001. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 164: 1618–1623.
Sethi, S. 2004. New developments in the pathogenesis of acute exacerbations of chronic obstructive pulmonary disease. Current Opinion in Infectious Diseases 17: 113–119.
Severina, I.S., O.G. Bussygina, N.V. Pyatakova, Y.V. Khropov, and R.A. Krasnoperov. 2000. Ambroxol as an inhibitor of nitric oxide-dependent activation of soluble guanylate cyclase. European Journal of Pharmacology 407: 61–64.
Su, F., F. Wang, W. Gao, and H. Li. 2007. Determination of ambroxol in human plasma by high performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-MS/ESI). Journal of Chromatography B 853: 364–368.
Su, X., L. Wang, Y. Song, and C. Bai. 2004. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Medicine 30: 133–140.
Subauste, M.C., D.B. Jacoby, S.M. Richards, and D. Proud. 1995. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. Journal of Clinical Investigation 96: 549–557.
Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, K. Nakayama, M. Yanai, Y. Numazaki, and H. Sasaki. 2001. Bafilomycin A1 inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. American Journal of Physiology 280: L1115–L1127.
Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, A. Yoshino, H. Yasuda, H. Takahashi, H. Nishimura, and H. Sasaki. 2002. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. American Journal of Respiratory and Critical Care Medicine 165: 1113–1118.
Suzuki, T., M. Yamaya, K. Sekizawa, N. Yamada, K. Nakayama, S. Ishizuka, M. Kamanaka, T. Morimoto, Y. Numazaki, and H. Sasaki. 2000. Effects of dexamethasone on rhinovirus infection in cultured human tracheal epithelial cells. American Journal of Physiology 278: L560–L571.
Teramoto, S., M. Suzuki, E. Ohga, H. Ishii, T. Matsuse, and Y. Ouchi. 1999. Effects of ambroxol on spontaneous or stimulated generation of reactive oxygen species by bronchoalveolar lavage cells harvested from patients with or without chronic obstructive pulmonary diseases. Pharmacology 59: 135–141.
Turner, R.B., and R.B. Couch. 2006. Rhinoviruses. In Fields Virology, 5th ed, ed. D.M. Knipe, and P.M. Howley, 895–909. Philadelphia: Lippincott Williams and Wilkins.
van de Stolpe, A., and P.T. van der Saag. 1996. Intercellular adhesion molecule-1. Journal of Molecular Medicine 74: 13–33.
Yamaya, M., H. Nishimura, Y. Hatachi, H. Yasuda, X. Deng, T. Sasaki, H. Kubo, and R. Nagatomi. 2012. Inhibitory effects of tiotropium on rhinovirus infection in human airway epithelial cells. European Respiratory Journal 40: 122–132.
Yamaya, M., H. Nishimura, Y. Hatachi, M. Yoshida, H. Fujiwara, M. Asada, K. Nakayama, H. Yasuda, X. Deng, T. Sasaki, H. Kubo, and R. Nagatomi. 2011. Procaterol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. European Journal of Pharmacology 650: 431–444.
Yang, B., D.F. Yao, M. Ohuchi, M. Ide, M. Yano, Y. Okumura, and H. Kido. 2002. Ambroxol suppresses influenza-virus proliferation in the mouse airway by increasing antiviral factor levels. European Respiratory Journal 19: 952–958.
Yasuda, H., M. Yamaya, T. Sasaki, D. Inoue, K. Nakayama, M. Yamada, M. Asada, M. Yoshida, T. Suzuki, H. Nishimura, and H. Sasaki. 2006. Carbocisteine inhibits rhinovirus infection in human tracheal epithelial cells. European Respiratory Journal 28: 51–58.
Zhu, Z., W. Tang, A. Ray, Y. Wu, O. Einarsson, M.L. Landry, J. Gwaltney Jr, and J.A. Elias. 1996. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor κB-dependent transcriptional activation. Journal of Clinical Investigation 97: 421–430.
Acknowledgments
This study was supported by Health, Labour and Welfare Sciences Research Grants for Research on Measures for Intractable Diseases from the Japanese Government, and Teijin Pharma Co. Ltd.
Conflict of interest
All authors have no conflict of interest. Mutsuo Yamaya is a professor and Hiroshi Kubo is an associate professor in the Department of Advanced Preventive Medicine for Infectious Disease, Tohoku University Graduate School of Medicine. This department is funded by eleven pharmaceutical companies: Teijin Pharma Co., Ltd., Kyorin Pharmaceutical Co. Ltd., Abbott Japan, Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., AstraZeneca K.K., Daiichi Sankyo Co. Ltd., Otsuka Pharmaceutical Co. Ltd., GlaxoSmithKline K.K. Co., Ltd., Tanabe Mitsubishi Pharmaceutical Co., Ltd., Ono Yakuhin Co. Ltd., and Nippon Boehringer-Ingelheim Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaya, M., Nishimura, H., Nadine, L.K. et al. Ambroxol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Arch. Pharm. Res. 37, 520–529 (2014). https://doi.org/10.1007/s12272-013-0210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0210-7