Abstract
It is generally accepted that mitochondrial deficits cause many common age-associated diseases including type 2 diabetes. However, it has not been understood what causes mitochondrial damages and how to interrupt the development of the diseases in patients. Recent epidemiologic studies demonstrated a positive correlation between serum concentrations of environmental pollutants and insulin resistance/diabetes. Emerging data strongly suggest that some synthetic pollutants disturb the signaling pathway critical for energy homeostasis and insulin action. The synthetic chemicals are possibly involved in pathogenesis of insulin resistance and diabetes as mitochondria-disturbing agents. In this review, we present a molecular scheme to address the contribution of environmental synthetic chemicals to this metabolic catastrophe. Efforts to identify synthetic chemicals with mitochondria-damaging activities may open a new era to develop effective therapeutic interventions against the worldwide-spreading metabolic disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is classified as two different diseases of type 1 (T1DM) and type 2 (T2DM). Although these two diseases share similar metabolic phenotypes and complications, T1DM is a largely genetic disorder linked to autoimmunity and T2DM is metabolic disorder linked with obesity and sedentary lifestyle. The prevalence of diabetes for all age-groups has exploded over the last several decades: the worldwide average was estimated to be 2.8 % in 2000 and 4.4 % in 2030 (Wild et al. 2004). Diabetes affects approximately 5 and 8.3 % of the Korean and the U.S. populations, respectively. In general, almost 20 % of the adult population has been diagnosed with diabetes and/or insulin resistance. The “diabetic epidemic” is predicted to continue even though the levels of obesity remain constant.
In order to mitigate the deleterious impact of diabetes, great efforts have been made to disclose the factors underlying this metabolic disaster. But researchers failed to fully explain why some people get the DM and others do not. T2DM usually occurs when peripheral tissues like muscle and fat respond abnormally to insulin, defined as insulin resistance. The correlation between T2DM and high-calorie diet plus sedentary lifestyle is well established, but it is not clear yet whether- or how- these factors initiate the insulin resistance. After all, 75–80 % of obese people never develop T2DM. Therefore, some genetic factors are suggested to play a role in developing T2DM and more than 40 genes have been reported as genetic factors associated with T2DM by genome-wide association studies (GWAS). However, most genetic factors are related to β-cell function and account for only about 10 % of T2DM (Jonietz 2012).
Besides diet, life style and genetic factors, synthetic chemicals are also considered as one of the causal factors for obesity, insulin resistance, and T2DM. The chemicals currently used in a variety of industrial and agricultural applications are leading to widespread contamination of the environment. Epidemiological studies suggest that persistent organic pollutants (POPs), such as polychlorinated biphenyl (PCB) congeners, organochlorine (OC) pesticides, brominated diphenyl ether (BDE), and dioxin, are strongly correlated with abdominal obesity and T2DM (Lee et al. 2012; Tanaka et al. 2011). These exogenous chemicals, so-called environmental endocrine disrupting chemicals (EDCs), interfere with endogenous hormonal axes and induce metabolic disruption, leading to the chemical-induced diabetes and the insulin action changes in vitro and in vivo. Unlike the early studies of EDCs focused on identifying chemicals to modulate sex steroid and thyroid hormone signaling, emerging data strongly suggest that they disturb the signaling pathway critical for energy homeostasis. Here, we summarized some chemicals that destroy normal mitochondrial activities and insulin signaling. We present their roles in the pathogenesis of insulin resistance/diabetes and a molecular scheme to address the contribution of environmental synthetic chemicals to this metabolic catastrophe.
Mitochondrial deficits cause the age-associated degenerative diseases including T2DM
“Happy families (mitochondria) are all alike; every unhappy family (mitochondrion) is unhappy in its own way.” in Anna Karenina by Leo Tolstoy.
Mitochondria are organelles that act as powerhouses of cells. They produce ATP, which is a main source of energy for all activities in cells, and are the locations for glucose, fatty acid, and protein metabolisms. Because of their important metabolic functions, damaged mitochondria can theoretically cause diseases in any organ at any age. This characteristic has earned mitochondria the title of “powerhouses of disease” (Lane 2006). Previously, we have described several molecular players in controlling mitochondrial biogenesis and in developing various mitochondria-related diseases (Pak and Jeong 2010). The diseases include obesity, T2DM, metabolic syndrome, cardiovascular diseases, and neurodegenerative diseases. Mitochondria consist of more than 1,500 proteins encoded by nuclear DNA (nDNA) and 13 mitochondrial DNA (mtDNA)-encoded proteins in oxidative phosphorylation (OXPHOS) complex. A single-base mutation in mtDNA and/or nDNA causes the rare but devastating childhood genetic diseases, and over 50 different mutations have been reported (DiMauro and Schon 2003). Similarly, the damaged mitochondrial proteins may induce quantitative declines of OXPHOS activity and efficiency leading to age-associated degenerative diseases. Probably, it would not be important which protein is hampered in OXPHOS physiology, reducing ATP synthetic capacity or transcription of mitochondrial genes. In various complex degenerative diseases, the resulting “unhappy” mitochondria would be similar in physiological phenotypes although the identity of damaged protein or gene might be different each other. To reverse the “unhappy” mitochondria to “happy” mitochondria, targeting on a specific mitochondrial component responsible for the disease development may not be critical in developing therapeutic agents. Rather, it may be necessary to normalize “unhappy” mitochondria by antagonizing consequent effects of mitochondrial damages, such as reduction of ATP and membrane potential, and increased oxidative stress.
Mitochondria are susceptible to exogenous stress
MtDNA exists in multiple copies and is tightly associated with a number of proteins to form a nucleoid structure (Choi et al. 2011). Electrons leaked from OXPHOS complex react with oxygen to generate reactive oxygen species (ROS) in mitochondrial matrix, where mtDNAs are present. Since proteins, lipids, or mtDNA in mitochondria are constantly exposed to mitochondrial ROS which creates a higher oxidative environment in mitochondria than in nuclei, mitochondrial components are more susceptible to exogenous chemical-mediated ROS damages than those in other locations. Furthermore, damages on mtDNA may not be repaired as properly as those of nDNA because mtDNA repair system seems to be inefficient or absent in mitochondria (Larsen et al. 2005). Improper repair of mtDNA damages have been presented by age-dependent accumulation of point mutations or large-scale deletions of mtDNA in tissues of aged subjects and patients (Pak and Jeong 2010). Thus, mitochondrial damages should be greater than nuclear or cytoplasmic damages and precedes the changes in nucleus and cytoplasm as cells are exposed to hazardous chemicals.
POPs, obesity, and T2DM
A complex combination of many genetic and environmental factors causes T2DM and other chronic diseases. Epidemiologic studies demonstrated that high serum concentrations of various POPs are strongly associated with T2DM and insulin resistance prevalence (Lee et al. 2012, 2011, 2006). POPs are lipophilic chemicals that accumulate in adipose tissue and are hard to eliminate from contaminated environments. Polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), PCBs, and OC pesticides have been highlighted as being POPs of concern. Obesity may be caused by simultaneous exposure to various POPs in the general population (Lee et al. 2011), which posited environmental “obesogen hypothesis”. This hypothesis explains that certain environmental pollutants induce weight gain (Neel and Sargis 2011). It is important to note that POPs in fat tissue, not obesity itself, may contribute to the development of insulin resistance and T2DM.
In contrast, “obesity paradox” has been documented, in which the overweight and obese subjects have a better prognosis (less mortality) than those with normal body weight. The obesity paradox was strongly observed among the subjects with high levels of POPs. For example, when the elderly had relatively high POPs, those with high fat mass showed a lower risk of mortality than those with low fat mass (Hong et al. 2012). This may reflect the relative safety of storing the harmful POPs in adipose tissue rather than in other critical organs. On the other hand, POPs storage in adipose tissue may increase the stability of POPs, leading to enhanced exposure periods. The relationships between obesity and POPs themselves are not simple. Long-term exposure to low-level POPs can cause obesity while high-dose POPs can lead to weight loss and cell deaths. In addition, weight loss releases POPs into serum from fat, whereas weight gain decreases serum POPs by sequestration in fat. The dynamics of POPs in the body may be affected by obesity over time, age-related body composition and fat distribution changes. It would be desirable to disentangle these complicated relationships by identifying intermediate factors involved in fat mass expansion, serum POPs, and tissue damage.
A comprehensive Environmental-Wide Association Study (EWAS) demonstrated that the organochlorine pesticide-derivative heptachlor epoxide [odds ratio (OR) = 0.7, p < 0.001], the vitamin γ-tocopherol (OR = 1.5, p < 0.001), dioxins and furans (OR = 1.8–5, p < 0.001), and PCBs (OR = 2.2–5.5, p < 0.001) were harmful, but β-carotene (OR = 0.6, p < 0.001) was protective in T2DM development (Patel et al. 2010). Reflecting the OR of most risk genetic loci identified by Genome-Wide Association Study (GWAS) was 1.3–1.7, environmental factors have greater contributions than genomic factors in T2DM pathogenesis. Because these lipophilic POPs are persistent in the environment and bio-accumulate in fat tissues through the food chain, the physiological action by chronic exposure of low dose POPs is complex and the molecular mechanism underlying T2DM development need to be intensively studied.
Some POPs induce toxic and biological effects via AhR
Persistent organic pollutants are known to induce adverse effects on various biological systems via binding to aryl hydrocarbon receptor (AhR), a cytosolic receptor present in most vertebrate tissues (Schlezinger et al. 2010). AhR is a ligand-activated transcription factor belonging to the basic helix-loop-helix (bHLH)/Per-Arnt-Sim family (Beischlag et al. 2008). The interaction of AhR ligands with AhR activates the transcription of multiple genes including cytochrome P450 (CYP) members, CYP1A1/2 and CYP1B1, and ligand metabolizing enzymes. Increment of CYP members enhances the clearance of AhR ligands by metabolizing them in liver. The most famous AhR ligand with high affinity is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and therefore, AhR is a so-called dioxin receptor. AhR-mediated reporter gene assays identified exogenous AhR agonists covering diverse synthetic chemicals (Denison et al. 2002), some pesticides, insecticides (carbaryl), herbicides (diuron), or naturally occurring compounds [indole-3-carbinol (I3C), flavonoids and glucosinolates] (Kiss et al. 2011; Li et al. 2011). I3C dietary AhR ligand provided from vegetables protects the host by an induction of active immunologic tolerance in intestine (Li et al. 2011). The tryptophan catabolite kynurenine has been identified as an endogenous AhR ligand that is constitutively generated in human brain tumor cells (Opitz et al. 2011). Table 1 summarizes the exogenous and endogenous AhR ligands and their characteristics that are exogenous and endogenous.
Aryl hydrocarbon receptor ligands like dioxins mediate a various range of biological and toxic effects, including tumor promotion, teratogenicity, immuno-, hepato-, cardio-, and dermal toxicity, wasting, lethality, and alteration of endocrine homeostasis. For a better understanding of the molecular mechanisms underlying AhR ligand action, complex AhR-dependent or independent modulations of target gene expression have been suggested through multiple molecules interacting with AhR (Denison et al. 2011). In classical mechanism of AhR-dependent gene activation, the cytosolic AhR forms a complex with HBV X-associated protein 2 (XAP2), Hsp90 and p23 (Shetty et al. 2003), which are displaced by Arnt after binding to the ligand. The resulting AhR/Arnt dimer binds to a drug response element (DRE, 5′-TNGCGTG-3′) on the promoters of target genes such as CYPs, aldehyde dehydrogenase 3 (ALDH3), AhR repressor (AhRR), p27kip1, and etc. Here, AhRR is another bHLH/Per-Arnt-Sim transcription factor. Unlike other target genes, the transactivation domain-deficient AhRR functions as a naturally occurring dominant negative factor by competing with AhR for heterodimer formation with Arnt and subsequently binding to DRE sequence of targets (Evans et al. 2008). AhRR expression is induced by AhR/Arnt complex upon binding on DRE of AhRR promoter. Therefore, AhRR and AhR constitute a regulatory loop affecting each other’s transcriptions to maintain a homeostasis. In various human malignant tissues AhRR mRNA was down-regulated due to methylation of DRE sites of the promoter, suggesting that AhRR might play a role as a tumor suppressor (Zudaire et al. 2008).
Interaction of auxiliary proteins to AhR may be important because they are able to alter AhR-mediated transcription events or toxicological process. A number of co-activators and nuclear proteins have been reported to bind AhR/Arnt dimer directly. They include p300, CREB-binding protein (CBP), steroid receptor coactivators 1/2 (SRC1/2), receptor-interacting protein 140 (RIP140), and NcoA2 (Beischlag et al. 2002). Because these co-activators are also used by other nuclear receptors (NRs)-mediated transcription, AhR activation may suppress the transcriptional activity of other NRs with lower affinity for co-activators. On the other hand, AhR is able to bind to alternative DRE elements as a dimer with either Arnt or other transcription factors in a non-classical mechanism of action. Estrogen receptor bound to AhR is subjected to proteasomal degradation to quench ER response (Denison et al. 2011). RelA (p65) and RelB subunits of NF-κB interact with AhR, resulting in direct activation of inflammatory signaling (Tian et al. 2002). Another interesting partner is ATP5α1, a subunit of ATP synthase complex. AhR interacts with ATP5α1 in the absence of ligand in mitochondria (Tappenden et al. 2011). Exposure to TCDD broke this interaction and diminished portion of mitochondrial AhR. It was speculated that AhR–ATP5α1 interaction might be important to maintain ATP synthesis efficiency.
Enigmatic activation of AhR
Like a POPs-obesity paradox, AhR responses are promiscuous. First, AhR is able to bind other secondary proteins in either ligand-dependent or -independent manner. Second, the AhR-mediated dysmetabolic responses show the U-shaped dose–response relationships with POPs concentration. The AhR autoregulatory loop of endogenous negative feedback regulation may explain the AhR response complexity. AhR ligands activate the gene expressions of CYP1A1/2 and CYP1B1, which enhance the clearance of AhR ligands. The tightly bound endogenous agonist 6-formylindolo[3,2-b]carbazole (FICZ) induced CYP1A1 mRNA and enzyme activity, keeping its own steady-state concentration very low. Stimulation by oxidative stress, AhR antagonists, or AhR ligands with low affinity, reduced the FICZ-mediated CYP1A1 induction, elevated FICZ levels, and subsequently activated AhR responses (Wincent et al. 2012). Therefore, a prolonged induction of AhR activity disturbs the feedback regulation of FICZ levels, resulting in detrimental consequences. Rannug research group (Wincent et al. 2012) provided a list of compounds that may prevent the FICZ-mediated inactivation to activate AhR indirectly. The list includes endogenous substances, clinical drugs, food additives, industrial compounds, pesticides, metals, phytochemicals, and oxidants. On the contrary, AhR agonist-induced ligand-metabolizing enzymes might enhance AhR ligand clearance, and eventually AhR agonists would act like AhR antagonists. The AhR-mediated responses would be entangled by another feedback loop of AhRR which counteracts the AhR-dependent gene expression to balance the AhR activity (Zudaire et al. 2008). Enigmatic activation of AhR-dependent gene activation is summarized and illustrated in Fig. 1. It is not clear whether synthetic compounds might induce mitochondrial deficits directly or indirectly via AhR.
Illustration of enigmatic activation of AhR. Ligand binding to AhR induces the formation of AhR/Arnt dimer complex to activate gene expressions of CYPs and ligand metabolizing enzymes. Transcription of AhRR, which built an autoregulatory feedback loop, is also activated. Chemicals in box are AhR ligands: grey box, conventional AhR ligand agonists; white box, low affinity agonist or protective AhR ligands; dotted box, inhibitors of metabolizing enzymes. The precise mechanism of AhR ligand- or AhR-mediated mitochondrial damages is necessary to define
Little has been known about the physiological role of AhR or which ligands activate AhR under normal and healthy conditions. Several studies on AhR knock-out mice suggested that AhR might be involved in liver development and intestinal immune function (Li et al. 2011). Dietary compound I3C derived from cruciferous vegetables such as broccoli is converted into high affinity AhR ligand, indole[3,2-b]carbazole (ICZ), by acids in stomach. The resulting activation of AhR signaling in epithelial cells regulated intestinal immune function through unidentified target gene induction. Further studies are necessary for better understanding on physiological roles of AhR ligands, whether the chemicals function systemically as agonist or antagonist at the end point, and which organs, liver or intestine, they target to control whole body function.
Synthetic chemicals disrupt mitochondria
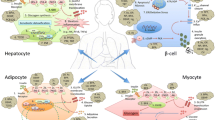
There are many different ways to damage mitochondria. Although various AhR responses to exogenous ligands have been widely studied, the role of AhR in mitochondrial function and insulin resistance has not been defined. As shown in Fig. 2, humans are exposed to synthetic chemicals—POPs, pesticides, herbicides, food additives, pharmaceuticals, and cosmetics via agricultural goods consumer products, soil, water, and/or air. Some pollutant chemicals disturb ecosystem and generate mutant animals like hermaphrodite frogs found in Mississippi river, while others contaminated human’s food chain like dairy products, meat, and fish. Then, humans would be exposed chronically to a low-dose of persistent and lipophilic chemicals through food consumption. Bio-accumulation of the chemicals in adipose tissue and other organs could possibly result in mitochondrial damages in affected organs. Eventually, insulin-sensitive organs such as adipose tissue, pancreas, liver, muscles might fail to function properly leading to age-associated chronic diseases, T2DM and insulin resistance.
Scheme of synthetic chemical exposure to human disease development. Acute exposure to high dose chemicals directly induces necrotic cell death in tissue. Contaminated chemicals are present persistently in the environment and food chain. Persistent chemicals are bio-accumulated in animal tissues. Chronic exposure to chemicals induces mitochondrial dysfunction and organ failure, leading to human diseases. PD Parkinson’s disease, AD Alzheimer’s disease
Only a few studies have been reported regarding to the relationships among three players: synthetic compounds, AhR, and mitochondrial dysfunction. TCDD treatment in vivo activated death receptor and mitochondrial pathway of apoptosis through NF-κB (Camacho et al. 2005). AhR knock-out mouse study showed that the level of mitochondrial ROS generation was depending on AhR without affecting CYP1A1/2 (Senft et al. 2002). TCDD-induced ROS concomitantly evoked an increase in mitochondrial thiol reduction state (Shen et al. 2005). Proteomic analysis of TCDD-treated rat hepatoma cells revealed the AhR-dependent up-regulation of VDAC2, a mitochondrial outer membrane pore protein (Sarioglu et al. 2008). Tandem affinity purification analysis demonstrated that ATP5α1 subunit of ATP synthase was strongly interacted with mitochondrial AhR in the absence of ligand (Tappenden et al. 2011). TCDD treatment damaged mitochondrial OXPHOS and ATP production and disrupted cristae structure in cardiomyocytes (Neri et al. 2011). TCDD at 3 nM also induced oxidative damages in mtDNA as well as the caspase-3-dependent apoptosis in trophoblast-like cells (Chen et al. 2010). On the other hand, TCDD-treated mice at 30 μg/kg dose differentially alter the hepatic mRNA levels of OXPHOS complex subunits. Several genes of OXPHOS subunits (Ndufa10, Cyc1, Cox7b, Atp5g3, Atp5I, Ucp2, Ucp5 and Sco2) may contain DRE sequence on their promoters (Forgacs et al. 2010). It is not clear whether the differential expression of OXPHOS subunits is secondary responses to TCDD-mediated oxidative stress.
Besides TCDD, the interesting and direct evidence for POPs-mediated mitochondrial disturbance were presented using salmon oil diet. Ruzzin et al. administered rats for 28 days with salmon oil contaminated with POPs. POPs-fed rats developed insulin resistance, abdominal obesity, and hepatosteatosis as well as the reduced expression of mitochondrial gene regulator, PGC-1α (Ruzzin et al. 2010). They showed that OC pesticides, DDT, and mono-ortho-substituted PCB mixtures (1–100 nM), reduced insulin action of 3T3-L1 adipocytes, but dioxins and dioxin-like PCBs (0.2–20 nM) did not. We have reported that atrazine, a widely used herbicide, induced obesity in vivo by destroying mitochondria through direct inhibition of coenzyme Q10 in OXPHOS (Lim et al. 2009). Our study suggested that the cause of mitochondrial damages may be the direct accumulation of POPs or chemicals in mitochondrial OXPHOS.
There are many candidate chemicals reported that may be detrimental to mitochondria. A cell-based analysis system has been utilized to list mitochondrial toxins by screening thousands of exogenous small chemicals (Wagner et al. 2008). Protein synthesis inhibitors (emetine, anisomycin, cycloheximide) decoupled coordination of nDNA- and mtDNA transcription, and a subset of HMG-CoA inhibitors (fluvastatin, lovastatin, simvastatin) strongly decreased ATP levels and complex I activity. Statins are the number-one selling medicines in the world to lower blood cholesterol, but induce muscle-related side effects, muscle weakness, cramps, or myopathy, in patients. Inhibition of mevalonate pathway decreased de novo synthesis of cholesterol as well as isoprenoid, which provides isoprenyl units of ubiquinone (CoQ10). Maintenance of isoprenoid intermediates, farnesyl pyrophosphate and geranylgeranyl pyrophosphate, by inhibiting metabolizing enzymes recovered statin-induced ATP reduction in C2C12 cells (Wagner et al. 2011).
Monitoring of mitochondrial damage by food products, harmaline and sodium benzoate
Harmaline is a derivative of the harman family, present in everyday-life food products such as coffee, cheese, and beer. The first attraction of this chemical was its structural similarity to 1-methyl-4-phenylpyridinium (MPP+) (Storch et al. 2004). MPP+ is a metabolized form of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in glial cells that gets transported to dopaminergic neurons via the dopamine transporter. Once in the neurons, MPP+ inhibits the activity of complex I of OXPHOS, eventually leading to mitochondrial damage and dopaminergic neuronal cell death (Murphy et al. 1995; Nicklas et al. 1985). Similar to the effects of MPP+, when brain cells were treated with 50 μM of harmaline, mitochondria released cytochrome c to initiate apoptosis, indicating mitochondrial damage (Lee et al. 2002). Also, the intraperitoneal injection of harmaline induced tremor in rats, a symptom of Parkinson’s disease (PD) (Tariq et al. 2002).
Sodium benzoate is a widely used preservative for various food and cosmetic products. It is mainly known to be present in carbonated drinks, fruit juices, pickles, jelly, medical products, and energy drinks, all of which are products that we encounter very frequently. Sodium benzoate damaged mtDNA in yeast (Piper 1999), and decreased the survival rate of dopaminergic neurons by decreasing the expression of tyrosine hydroxylase and dopamine transporter in vitro and in vivo (Chen et al. 2009). Also, it has been reported that the exposure to sodium benzoate decreases the survival rates of zebrafish embryos in a dose- and exposure time-dependent manner (Tsay et al. 2007). After such reports, many companies have been limiting their use of sodium benzoate in their products, but there are no specific reports about sodium benzoate-mediated mitochondrial damage.
We have screened several chemicals which reduced mitochondrial activities such as ATP levels and ROS generation using cell-based mitochondrial activity profiling assays (CMAPS) as previously described (Piao et al. 2012). Harmaline and sodium benzoate reduced mitochondrial activities of C2C12 cells in a dose-dependent manner (Fig. 3). The parameters we have checked were cell membrane integrity by calcein assay, complex I activity (NADH dehydrogenase activity) by tetrazolium precipitation of MTT, intracellular ATP contents, mitochondrial membrane potential by TMRE assay, and ROS generation by DCF-DA. Harmaline treatment damaged complex I, ATP generation, and mitochondrial membrane potential in a dose-dependent manner, but did not generate ROS significantly (Fig. 3a). In contrast, sodium benzoate induced all mitochondrial dysfunction and ROS increment (Fig. 3b).
Mitochondrial activity (CMAPS) analysis of harmaline and sodium benzoate using C2C12 myoblasts. The C2C12 myoblast cells in phenol-red-free media containing 0.5 % FBS were treated with harmaline or sodium benzoate as indicated. Parameters are calcein for cell viability, MTT for complex I activity, ATP contents, TMRE for mitochondrial membrane potential, and DCFDA for ROS. Incubation periods are shown. *p < 0.05, **p < 0.01, ***p < 0.001 versus control vehicle
Because all mitochondria are undergoing fusion and fission constantly and dynamically, it is difficult to identify the shape of mitochondrial network. However, when zoomed in from the fixed images of dsRed2-mito-transfected cells (Piao et al. 2012), the shape of the organelle becomes visible, allowing us to compare the specific shape of the organelle with that of other test groups. As shown in Fig. 4, the magnified images of DMSO-treated samples (negative control) display both long and short shapes of mitochondria, indicating a well-balanced interaction of fission and fusion. On the other hand, the magnified images of chemical-treated samples show only fragmented forms of mitochondria, implying either unbalanced fusion over fission or damaged mitochondria. Also, if inspected closely, the magnified images of oligomycin-treated samples (positive control) and harmaline-treated samples both show a significant amount of punctured or ring-structured mitochondria. This change in mitochondrial shape may only be a coincidence or a process of mitophagy, an auto-removal of damaged mitochondria. The MPP+- and sodium benzoate-treated mitochondria exhibited rather condensed or destroyed shape of mitochondria. Further study is necessary to define the change of mitochondrial morphology, and specifically the molecular process of mitophagy may be an interesting topic for future investigation.
Fixed confocal images of DsRed2-labeled mitochondria treated with various chemicals. All images are representations of SK-Hep1 cells transfected with DsRed2-mito vectors. Each image represents the effects of harmaline and sodium benzoate on mitochondrial morphology and dynamics. MPP+ and oligomycin are positive controls, whereas DMSO is the negative control. Inset represents magnification of the boxed area. Scale bars are indicated on the images
Clinical biomarkers to monitor the levels of synthetic chemicals and AhR ligands to induce mitochondrial dysfunction
Research in this area is hampered by a lack of tools to measure early markers of mitochondrial dysfunction in human studies, particularly with respect to ambient exposures of synthetic chemicals. Current clinical markers for mitochondrial dysfunction typically detect only advanced symptoms of tissue injury and disease. There is an urgent need for reliable, informative markers of early mitochondrial dysfunction associated with synthetic chemicals and environmental stressors, as these will enhance the mechanistic understanding of chemically-induced mitochondrial toxicity and disease and enable prevention and intervention in the subclinical stages of disease. For application to large-scale population studies, biomarkers should be developed in easily accessible tissues including blood, mucosa, and urine.
We have demonstrated that CMAPS is a useful system to monitor mitochondrial function in cells, not in isolated mitochondria, as it probes OXPHOS function coupling to other cellular processes (Piao et al. 2012; Ahn et al. 2010). This system is applicable to monitor the effects of clinical serum samples on mitochondrial activities of C2C12 cells. Measurement of oxygen consumption conjunct with mitochondrial dynamics or morphology would provide additional information of clinical samples on mitochondrial physiology. It would be necessary to determine the direct relationship between serum concentrations of AhR ligands in sum (ΣAhR agonists and antagonists) and parameters of metabolic syndrome. To our knowledge, no study had ever demonstrated the direct linear relation between them. Conventional analytical methods, high resolution GC/MS, determine the concentrations of individual POP congeners, but do not measure unknown AhR-agonists or ligands. Epidemiological studies utilized data generated by GC/MS, which is expensive and hard to be applicable for large-scale clinical study. Thus, the cell-based assay system for AhR activity using clinical human serum in small volume would be valuable to evaluate effects of AhR ligands which are present or contaminated in human serum. Systemic profiling of data from cell-based analyses of human serum, such as CMAPS, mitochondrial morphology, oxygen consumption, and AhR ligands, as well as clinical metabolism parameters may shed light on how synthetic chemicals and POPs in serum are perturbing cells and mitochondrial function in human body. We hope that the systemic profiling of mitochondrial physiology and synthetic chemicals in human serum could become a clinical biomarker in establishing the cause-and-effect relationship between exposure to chemicals and the development of human diseases including obesity, T2DM and metabolic syndrome.
Conclusion
We have enumerated several reports and experimental data which seem to be irrelevant each other. Current diabetic pandemic is possibly associated with chronic exposure to synthetic chemical at low concentration. The synthetic chemicals target various proteins and genes through AhR-dependent or independent pathway. Lipid-soluble chemicals accumulated in human tissues may destroy mitochondrial activities of organs. AhR ligand levels and read-outs of mitochondrial physiology on target cells are possible clinical biomarkers in serum. The assays for AhR ligands and mitochondrial function need to be finely developed and validated to adapt to large-scale human population. A deeper, more mechanistic understanding of how synthetic chemicals induce mitochondrial dysfunction is also required. Meanwhile, clinical biomarkers for mitochondria and AhR ligands can be applied to screen environmental compounds, chemicals, or therapeutics with regulating mitochondrial activity. We are anticipating with beating heart that all puzzle pieces find their places connecting the enumerated factors in near future.
Abbreviations
- T2DM:
-
Type 2 diabetes
- GWAS:
-
Genome-Wide Association Study
- EWAS:
-
Environmental-Wide Association Study
- OR:
-
Odds ratio
- POPs:
-
Persistent organic pollutants
- PCB:
-
Polychlorinated biphenyl
- OC:
-
Organochlorine
- EDC:
-
Endocrine disrupting chemicals
- mtDNA:
-
Mitochondrial DNA
- Ndna:
-
Nuclear DNA
- OXPHOS:
-
Oxidative phosphorylation
- AhR:
-
Aryl hydrocarbon receptor
- AhRR:
-
AhR repressor
- TCDD:
-
2,3,7,8-tetrachlorodibenzo-p-dioxin
- ROS:
-
Reactive oxygen species
- FICZ:
-
6-formylindolo[3,2-b]carbazole
- MPP+ :
-
1-methyl-4-phenylpyridinium
- PD:
-
Parkinson’s disease
- CMAPS:
-
Cell-based mitochondrial activity profiling assays
References
Ahn, S.Y., Y.S. Choi, H.J. Koo, J.H. Jeong, W.H. Park, M. Kim, Y. Piao, and Y.K. Pak. 2010. Mitochondrial dysfunction enhances the migration of vascular smooth muscles cells via suppression of Akt phosphorylation. Biochimica et Biophysica Acta 1800: 275–281.
Beischlag, T.V., J. Luis Morales, B.D. Hollingshead, and G.H. Perdew. 2008. The aryl hydrocarbon receptor complex and the control of gene expression. Critical Reviews in Eukaryotic Gene Expression 18: 207–250.
Beischlag, T.V., S. Wang, D.W. Rose, J. Torchia, S. Reisz-Porszasz, K. Muhammad, W.E. Nelson, M.R. Probst, M.G. Rosenfeld, and O. Hankinson. 2002. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Molecular and Cellular Biology 22: 4319–4333.
Camacho, I.A., N. Singh, V.L. Hegde, M. Nagarkatti, and P.S. Nagarkatti. 2005. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. Journal of Immunology 175: 90–103.
Chen, Q., N.N. Huang, J.T. Huang, S. Chen, J. Fan, C. Li, and F.K. Xie. 2009. Sodium benzoate exposure downregulates the expression of tyrosine hydroxylase and dopamine transporter in dopaminergic neurons in developing zebrafish. Birth Defects Research Part B, Developmental and Reproductive Toxicology 86: 85–91.
Chen, S.C., T.L. Liao, Y.H. Wei, C.R. Tzeng, and S.H. Kao. 2010. Endocrine disruptor, dioxin (TCDD)-induced mitochondrial dysfunction and apoptosis in human trophoblast-like JAR cells. Molecular Human Reproduction 16: 361–372.
Choi, Y.S., J.H. Jeong, H.K. Min, H.J. Jung, D. Hwang, S.W. Lee, and Y.K. Pak. 2011. Shot-gun proteomic analysis of mitochondrial D-loop DNA binding proteins: Identification of mitochondrial histones. Molecular BioSystems 7: 1523–1536.
Denison, M.S., A. Pandini, S.R. Nagy, E.P. Baldwin, and L. Bonati. 2002. Ligand binding and activation of the Ah receptor. Chemico-Biological Interactions 141: 3–24.
Denison, M.S., A.A. Soshilov, G. He, D.E. Degroot, and B. Zhao. 2011. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological Sciences 124: 1–22.
Dimauro, S., and E.A. Schon. 2003. Mitochondrial respiratory-chain diseases. New England Journal of Medicine 348: 2656–2668.
Evans, B.R., S.I. Karchner, L.L. Allan, R.S. Pollenz, R.L. Tanguay, M.J. Jenny, D.H. Sherr, and M.E. Hahn. 2008. Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: role of DNA binding and competition for AHR nuclear translocator. Molecular Pharmacology 73: 387–398.
Forgacs, A.L., L.D. Burgoon, S.G. Lynn, J.J. Lapres, and T. Zacharewski. 2010. Effects of TCDD on the expression of nuclear encoded mitochondrial genes. Toxicology and Applied Pharmacology 246: 58–65.
Hong, N.S., K.S. Kim, I.K. Lee, P.M. Lind, L. Lind, D.R. Jacobs, and D.H. Lee. 2012. The association between obesity and mortality in the elderly differs by serum concentrations of persistent organic pollutants: A possible explanation for the obesity paradox. International Journal of Obesity (Lond) 36: 1170–1175.
Jonietz, E. 2012. Pathology: Cause and effect. Nature 485: S10–S11.
Kiss, E.A., C. Vonarbourg, S. Kopfmann, E. Hobeika, D. Finke, C. Esser, and A. Diefenbach. 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334: 1561–1565.
Lane, N. 2006. Mitochondrial disease: Powerhouse of disease. Nature 440: 600–602.
Larsen, N.B., M. Rasmussen, and L.J. Rasmussen. 2005. Nuclear and mitochondrial DNA repair: Similar pathways? Mitochondrion 5: 89–108.
Lee, C.S., H.H. Ko, J.H. Song, and E.S. Han. 2002. Effect of R-(−)-deprenyl and harmaline on dopamine- and peroxynitrite-induced membrane permeability transition in brain mitochondria. Neurochemical Research 27: 215–224.
Lee, D.H., I.K. Lee, K. Song, M. Steffes, W. Toscano, B.A. Baker, and D.R. Jacobs Jr. 2006. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care 29: 1638–1644.
Lee, D.H., L. Lind, D.R. Jacobs Jr, S. Salihovic, B. Van Bavel, and P.M. Lind. 2012. Associations of persistent organic pollutants with abdominal obesity in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environment International 40: 170–178.
Lee, D.H., M.W. Steffes, A. Sjodin, R.S. Jones, L.L. Needham, and D.R. Jacobs Jr. 2011. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS ONE 6: e15977.
Li, Y., S. Innocentin, D.R. Withers, N.A. Roberts, A.R. Gallagher, E.F. Grigorieva, C. Wilhelm, and M. Veldhoen. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147: 629–640.
Lim, S., S.Y. Ahn, I.C. Song, M.H. Chung, H.C. Jang, K.S. Park, K.U. Lee, Y.K. Pak, and H.K. Lee. 2009. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS ONE 4: e5186.
Murphy, M.P., M.J. Krueger, S.O. Sablin, R.R. Ramsay, and T.P. Singer. 1995. Inhibition of complex I by hydrophobic analogues of N-methyl-4-phenylpyridinium (MPP+) and the use of an ion-selective electrode to measure their accumulation by mitochondria and electron-transport particles. The Biochemical Journal 306(Pt 2): 359–365.
Neel, B.A., and R.M. Sargis. 2011. The paradox of progress: Environmental disruption of metabolism and the diabetes epidemic. Diabetes 60: 1838–1848.
Neri, T., V. Merico, F. Fiordaliso, M. Salio, P. Rebuzzini, L. Sacchi, R. Bellazzi, C.A. Redi, M. Zuccotti, and S. Garagna. 2011. The differentiation of cardiomyocytes from mouse embryonic stem cells is altered by dioxin. Toxicology Letters 202: 226–236.
Nicklas, W.J., I. Vyas, and R.E. Heikkila. 1985. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sciences 36: 2503–2508.
Opitz, C.A., U.M. Litzenburger, F. Sahm, M. Ott, I. Tritschler, S. Trump, T. Schumacher, L. Jestaedt, D. Schrenk, M. Weller, M. Jugold, G.J. Guillemin, C.L. Miller, C. Lutz, B. Radlwimmer, I. Lehmann, A. Von Deimling, W. Wick, and M. Platten. 2011. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478: 197–203.
Pak, Y.K., and J.H. Jeong. 2010. Mitochondria: The secret chamber of therapeutic targets for age-associated degenerative diseases. Biomolecules & Therapeutics 18: 235–245.
Patel, C.J., J. Bhattacharya, and A.J. Butte. 2010. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS ONE 5: e10746.
Piao, Y., H.G. Kim, M.S. Oh, and Y.K. Pak. 2012. Overexpression of TFAM, NRF-1 and myr-AKT protects the MPP(+)-induced mitochondrial dysfunctions in neuronal cells. Biochimica et Biophysica Acta 1820: 577–585.
Piper, P.W. 1999. Yeast superoxide dismutase mutants reveal a pro-oxidant action of weak organic acid food preservatives. Free Radical Biology & Medicine 27: 1219–1227.
Ruzzin, J., R. Petersen, E. Meugnier, L. Madsen, E.J. Lock, H. Lillefosse, T. Ma, S. Pesenti, S.B. Sonne, T.T. Marstrand, M.K. Malde, Z.Y. Du, C. Chavey, L. Fajas, A.K. Lundebye, C.L. Brand, H. Vidal, K. Kristiansen, and L. Froyland. 2010. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environmental Health Perspectives 118: 465–471.
Sarioglu, H., S. Brandner, M. Haberger, C. Jacobsen, J. Lichtmannegger, M. Wormke, and U. Andrae. 2008. Analysis of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced proteome changes in 5L rat hepatoma cells reveals novel targets of dioxin action including the mitochondrial apoptosis regulator VDAC2. Molecular and Cellular Proteomics 7: 394–410.
Schlezinger, J.J., P.L. Bernard, A. Haas, P. Grandjean, P. Weihe, and D.H. Sherr. 2010. Direct assessment of cumulative aryl hydrocarbon receptor agonist activity in sera from experimentally exposed mice and environmentally exposed humans. Environmental Health Perspectives 118: 693–698.
Senft, A.P., T.P. Dalton, D.W. Nebert, M.B. Genter, A. Puga, R.J. Hutchinson, J.K. Kerzee, S. Uno, and H.G. Shertzer. 2002. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radical Biology and Medicine 33: 1268–1278.
Shen, D., T.P. Dalton, D.W. Nebert, and H.G. Shertzer. 2005. Glutathione redox state regulates mitochondrial reactive oxygen production. Journal of Biological Chemistry 280: 25305–25312.
Shetty, P.V., B.Y. Bhagwat, and W.K. Chan. 2003. P23 enhances the formation of the aryl hydrocarbon receptor–DNA complex. Biochemical Pharmacology 65: 941–948.
Storch, A., Y.I. Hwang, D.A. Gearhart, J.W. Beach, E.J. Neafsey, M.A. Collins, and J. Schwarz. 2004. Dopamine transporter-mediated cytotoxicity of beta-carbolinium derivatives related to Parkinson’s disease: Relationship to transporter-dependent uptake. Journal of Neurochemistry 89: 685–694.
Tanaka, T., A. Morita, M. Kato, T. Hirai, T. Mizoue, Y. Terauchi, S. Watanabe, and M. Noda. 2011. Congener-specific polychlorinated biphenyls and the prevalence of diabetes in the Saku Control Obesity Program (SCOP). Endocrine Journal 58: 589–596.
Tappenden, D.M., S.G. Lynn, R.B. Crawford, K. Lee, A. Vengellur, N.E. Kaminski, R.S. Thomas, and J.J. Lapres. 2011. The aryl hydrocarbon receptor interacts with ATP5alpha1, a subunit of the ATP synthase complex, and modulates mitochondrial function. Toxicology and Applied Pharmacology 254: 299–310.
Tariq, M., M. Arshaduddin, N. Biary, K. Al Moutaery, and S. Al Deeb. 2002. 2-Deoxy-d-glucose attenuates harmaline induced tremors in rats. Brain Research 945: 212–218.
Tian, Y., A.B. Rabson, and M.A. Gallo. 2002. Ah receptor and NF-kappaB interactions: Mechanisms and physiological implications. Chemico-Biological Interactions 141: 97–115.
Tsay, H.J., Y.H. Wang, W.L. Chen, M.Y. Huang, and Y.H. Chen. 2007. Treatment with sodium benzoate leads to malformation of zebrafish larvae. Neurotoxicology and Teratology 29: 562–569.
Wagner, B.K., T.J. Gilbert, J. Hanai, S. Imamura, N.E. Bodycombe, R.S. Bon, H. Waldmann, P.A. Clemons, V.P. Sukhatme, and V.K. Mootha. 2011. A small-molecule screening strategy to identify suppressors of statin myopathy. ACS Chemical Biology 6: 900–904.
Wagner, B.K., T. Kitami, T.J. Gilbert, D. Peck, A. Ramanathan, S.L. Schreiber, T.R. Golub, and V.K. Mootha. 2008. Large-scale chemical dissection of mitochondrial function. Nature Biotechnology 26: 343–351.
Wild, S., G. Roglic, A. Green, R. Sicree, and H. King. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053.
Wincent, E., J. Bengtsson, A. Mohammadi Bardbori, T. Alsberg, S. Luecke, U. Rannug, and A. Rannug. 2012. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proceedings of the National Academy of Sciences of the Unites States of America 109: 4479–4484.
Zudaire, E., N. Cuesta, V. Murty, K. Woodson, L. Adams, N. Gonzalez, A. Martinez, G. Narayan, I. Kirsch, W. Franklin, F. Hirsch, M. Birrer, and F. Cuttitta. 2008. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. Journal of Clinical Investigation 118: 640–650.
Acknowledgment
This study was supported by NRF grant funded by the Korean Ministry of Education, Science, and Technology (20120001162) and by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare (B12002912010000100). The funding source was not involved in study design; collection, analysis and interpretation of data; the writing of the manuscript; the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, WH., Kang, YC., Piao, Y. et al. Causal effects of synthetic chemicals on mitochondrial deficits and diabetes pandemic. Arch. Pharm. Res. 36, 178–188 (2013). https://doi.org/10.1007/s12272-013-0022-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0022-9