Abstract
The worldwide epidemic of diabetes and metabolic syndrome in the last few decades cannot be fully accounted for only by changes in the lifestyle factors, such as sedentary lifestyle and overeating. Besides genetic factors, there must be other causes to explain this rapid change. They could not be infectious in nature and induce insulin resistance as key biochemical abnormality. Mitochondrial dysfunction could be underlying mechanism behind the insulin resistance, thus metabolic syndrome. Then there have been increasing number of reports suggesting that chronic exposure to and accumulation of endocrine disrupting chemicals (EDCs), especially so-called the persistent organic pollutants (POPs) within the body might be associated with metabolic syndrome. Combining two concepts, we developed new “EDCs-induced mitochondrial dysfunction hypothesis of metabolic syndrome”. In this review we suggest that classifying those chemicals into 5 groups might be clinically useful considering their removal or avoidance; POPs, non-persistent organic pollutants, heavy metals, air pollutants and drugs. We will also discuss briefly how those insights could be applied to clinical medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
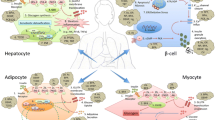
Multiple cardiovascular disease risk factors, such as obesity, type 2 diabetes mellitus (T2DM), dyslipidemia, and hypertension are often present in one person [1]. This clustering of risk factors and its association with insulin resistance led investigators to propose a pathophysiological condition called insulin resistance syndrome or metabolic syndrome [1, 2]. While the definition of metabolic syndrome emphasizes its clinical aspect, insulin resistance syndrome is focused on as its underlying pathophysiological abnormality. T2DM is a consequence of this state; it develops when insulin is not secreted enough to overcome the insulin resistance (Fig. 1).
Conceptual framework outline of mitochondria-based model. Genetic factors, early life nutritional environment and exposure to endocrine-disrupting chemicals are causes of various disease states (shown in bold), such as T2DM, metabolic syndrome, and atherosclerosis. Mitochondrial dysfunction changes insulin sensitivity, and chemical and physical composition of the body, and eventually leads to organ failure. (Reprinted with permission from Lee HK and Shim EB:J Diab Investig 2013;4:19–33)
Currently there are five hypotheses explaining the cause of metabolic syndrome or insulin resistance syndrome; mitochondrial dysfunction, thrifty phenotype, (epi-) genetic (thrifty genotype), excessive fat accumulation due to overfeeding and less exercise (mediated by adiponectin), and environmental pollutants. We could not review this huge subject, but briefly review mitochondrial dysfunction hypothesis, in which mitochondrial dysfunction is a central pathophysiology of insulin resistance and the environmental pollutants are causes. For the details of this hypothesis, readers are referred to our previous reviews [3, 4]. We fully acknowledge that this hypothesis is not well established. Furthermore, concepts such as insulin resistance, metabolic syndrome, endocrine disrupting chemicals (EDCs) and persistent organic pollutants (POPs) are not well defined and evolving. Meyer et al. took a similar view on insulin resistance that the environmental pollutants are causes [5]. Lee et al. also appreciated mitochondrial dysfunction in the pathogenesis of metabolic syndrome, but emphasized environmental pollutants, particularly (POPs) as of major importance [6]. In this review we take “bottom up approach” and classify those chemicals into 5 groups, considering their removal or avoidance as a clinical treatment; POPs, non-persistent organic pollutants, heavy metals, air pollutants and drugs.
2 Hypotheses for metabolic syndrome
2.1 EDC-induced mitochondrial dysfunction as a cause of metabolic syndrome
Mitochondrion is a powerhouse of a cell which generates chemical form of energy, ATP, needed to sustain life. It has its own DNA from its evolutionary symbiotic origin. As an organelle of the cell, it shows partly independent characters. It moves inside of cell and across the cells, if it is needed.
We developed mitochondrial dysfunction or mitochondrion-based model of insulin resistance when we observed the decrease of mitochondrial DNA (mtDNA) density in the peripheral blood cells preceded the development of T2DM in a population-based prospective cohort study [7]. In this cohort, mtDNA density correlated with parameters of metabolic syndrome. Association between mitochondrial dysfunction and insulin resistance is firmly established by works of Yale group [8, 9]. Using sophisticated nuclear magnetic resonance spectroscopy and glucose clamp studies in insulin resistant subjects, they established mitochondrial dysfunction exists in most of insulin resistant subjects.
Consistent with the hypothesis, the amount of mitochondria in any given tissue was known as a controlling determinant for the total oxygen utilization of the body since 1950s [10]. The maximal oxygen consumption rate (VO2 max) and work performance of the quadriceps muscles shows a strong correlation with the functional parameters of mitochondrial unit (i.e., mitochondrial protein, cytochrome aa3, citrate synthase, and respiratory activities), suggesting specific mitochondrial function at tissue levels seems to be quantitatively related to whole body insulin sensitivity [11, 12].
The metabolic rate of any organism is known to be proportional to body mass raised to the power of three-quarters [13]. This relation is known as metabolic scale law. West et al. showed this metabolic relationship exists across 27 orders of body size magnitude, encompassing elephants, humans, mice, even the size of mitochondria and electron transfer chain enzymes [14]. They suggested that the metabolic scaling relationship is the consequence of building a nutrient supply and heat dissipating channel system to keep core body temperature optimal. If the heat production of each cell in the body remains unchanged with increasing body size, the core temperature will eventually increase. In relation to this notion, we demonstrated in our previous paper that if the unit cellular metabolic rate is decreased, adaptation can occur by increasing body mass to restore core temperature [3]. Data published by Ravussin et al. support this notion [15]. Body temperature is a key constraint of metabolism; Gibbs free energy of endothermic animal enzymes is lowest at 37 ºC, as enzyme system of endotherms are adaptively evolved to this temperature [16].
Therefore we consider the mitochondria-based model of metabolic syndrome is a special case of metabolic scale law, which extend to mitochondrion itself and its electron transfer system [3, 4]. Then, it must be stressed that this law is inevitable consequence of bioenergetics in large scale. Other aspect of mitochondrial dysfunction hypothesis could be found in excellent reviews by Patti and Corveraand and Szendroedi et al. [17, 18].
2.2 Genetic cause (s) of metabolic syndrome
There is a large number of literature showing genetic base of metabolic syndrome and T2DM, which will be discussed by others of this issue. We want to emphasize the genetic studies were consistent with mitochondrial dysfunction hypothesis of metabolic syndrome [3]. For an example, conplastic animal strains with different mitochondrial genomes with the same nuclear genetic background, clearly showed that the different mitochondrial genomes make systemic metabolic disturbances relevant to various risk factors of T2DM [19].
In humans, common mtDNA haplogroups have been shown to modulate the susceptibility to T2DM; haplogroup N9a is protective against T2DM, and haplogroups D5 and F are susceptible to T2DM in Korean and Japanese population; nucleotide variation in the control region of mtDNA (16,189 T > C) was associated with insulin resistance and T2DM among Asians [20, 21]. A recent survey on the mitochondrial genetic variants among Europeans revealed significant association with BMI, but not with T2DM [22].
2.3 Thrifty phenotype hypothesis
This hypothesis developed by Hales and Barker emphasizes that the fetal and early life physical development by the nutritional environment in uterus and in early postnatal period determines insulin sensitivity in adult life [23]. Many epidemiologic and experimental observations fit with this hypothesis, suggesting epigenetic changes are important determinants of metabolic syndrome. We will not discuss the details of this hypothesis, but simply accept and incorporate it into the mitochondrial hypothesis as a part. Thrifty genotype hypothesis, a corollary of thrifty phenotype hypothesis developed to explain very high prevalence of T2DM, could be seen as a part of genetic cause [24].
2.4 Overeating and less exercise due to sedentary lifestyle
It is widely accepted that overeating and less exercise due to sedentary lifestyle, along with the industrialization is the cause of insulin resistance and obesity. Increased serum free fatty acids and alterations in cytokines such as adiponectin are regarded as mediators causing poor insulin action. We appreciate that there are numerous reports showing apparent associations between the obesity and overfeeding or lack of exercise.
However, simple nutritional overload could not cause obesity. Usually alterations in body weight are always compensated by adequate alterations in food intake and thermogenesis [25]. Adipostat hypothesis is almost identical to the mitochondrial hypothesis, but this hypothesis emphasizes a hypothetical adipostat in hypothalamus, which control body weight through a large number of hormones and neural mechanisms [26].
We agree with Lee et al. that overeating and inactivity could be secondary to accumulation of EDCs like POPs [6]. We postulated in our previous paper that if the unit cellular metabolic rate is decreased due to accumulation of EDCs like POPs, adaptation would occur to restore core temperature, resulting in increased body mass [4]. This view is consistent with the view of Cannon and Nedergaard who emphasized the importance of thermogenesis [25].
2.5 EDCs as a cause of mitochondrial dysfunction
2.5.1 Hormones control body metabolisms
Most hormones are mitochondrial stimulants. We want to remind the readers that many natural chemicals including nutrients also stimulate mitochondrial function. They change behavior of cells by binding to their specific receptors, go inside the cell itself (sometimes into mitochondrion) or send signal inside, and activate mechanisms which result in synthesis of certain proteins, which require energy, mostly from mitochondrion. Thus appropriate response in mitochondrial function is essential for a proper hormone action. When there is a chemical interfering with a specific hormonal action (i.e., EDCs), tissues will show resistance to that hormone action.
Insulin is a strong stimulant of mitochondrial function, sending anabolic signal particularly to liver and muscles [27]. If mitochondria of liver or muscles could not function properly, pancreas would secrete more insulin to counteract results of that defect. This mechanism might explain why resistance to insulin action was found as a key biochemical change in type 2 diabetes in early days of research.
Insulin like growth factor 1 (IGF-1) is also known to stimulate mitochondrial function, but send its signal with different pathways. IGF-1 and other factors, such as neuregulin act synergistically to increase mitochondrial biogenesis and mitochondrial DNA replication, resulting in increased mitochondrial density in neuronal cells. Furthermore, IGF-1 showed synergy with constitutive oncogenic Ras signaling, resulting in a further increase in mitochondrial density. This synergistic effect required both the extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K) signaling pathways and is mediated by the transcription factor estrogen-related receptor alpha (ERRalpha) [28]. Catecholamines are also strong stimulants of mitochondrion in the circumstances of cold exposure. They act through different pathways.
2.5.2 Endocrine disrupting chemicals (EDCs)
In 2002, the International Programme on Chemical Safety (IPCS), a joint program of the World Health Organization (WHO), the United Nations Environment Program (UNEP) and the International Labor Organization (ILO), published a document entitled “Global Assessment of the State-of-the-Science of Endocrine Disruptors” [29]. It defined EDC as “an exogenous substance or mixture that alters function (s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations”.
2.5.3 Merits of mitochondrial hypothesis
Many EDCs, if not all, could cause mitochondrial dysfunction, because they interrupt hormonal function. As discussed above, this view is consistent with those of Meyer et al., Lee et al. and Cannon and Nedergaard [5, 6, 25]. Thus mitochondria function test might be used in the identification of EDCs. In other words, those chemicals harmful to mitochondrion might be regarded as a cause of metabolic syndrome. At the same token, it could be used in identifying genes and epigenetic changes responsible to metabolic syndrome. This model also suggests agents that stimulate mitochondrial function might be beneficial to the subjects with metabolic syndrome.
We suggested previously that various degree of metabolic adaptation observed along with the weight change might be explained by metabolic adaptation; weight gain occurs when energy intake exceeds energy expenditure [3]. Adaptive thermogenesis or the auto-regulated production of heat in response to cold temperature or food intake is recognized as an important factor in determining weight maintenance. In this sense, mitochondria (dysfunction) hypothesis is a special case of the metabolic scaling relation occurring in humans [4].
3 Classification of EDCs
In IPCS document, almost 800 chemicals are listed as known or suspected chemicals capable of interfering with hormone receptors, hormone synthesis or hormone conversion, and only a small fraction of these chemicals have been investigated with tests capable of identifying overt endocrine effects in intact organisms [29]. Furthermore it says that the vast majority of chemicals in current commercial use have not been tested at all. As stated above, we suggest testing those chemicals for mitochondrial toxicity might be useful.
In 2013 National Toxicology Program organized a workshop to review the chemicals that have been linked to diabetes and obesity. POPs, arsenic, bisphenol A (BPA), phthlatates, organotins, nonpersistent pesticides were found associated with at least in some populations studied [30]. In this review, we will classify these EDCs into 5 groups from clinical point of view; persistent organic pollutants, nonpersistent pesticides (those organic EDCs not persisting in our body including bisphenol A, phthalates and others), heavy metals (arsenic), air pollutants and drugs used in medicine (Table 1). We made air pollution a separate class, although the components of air pollutants are mixture of EDCs [31].
3.1 Persistent organic pollutants (POPs)
POPs are organic chemicals that are persistent and widely distributed in the environment, have bio-accumulative properties and are toxic to humans and wildlife. POPs were identified by an international environmental treaty; Stockholm Convention on Persistent Organic Pollutants. This treaty aims to eliminate or restrict the production and use of POPs. There are currently 23 classes of chemicals listed as POPs, 1) organochlorine (OC) pesticides including of dichloro-diphenyl-trichloroethane (DDT), lindane, chlordane, and hexachlorobenzene (HCB), and industrial chemicals or byproducts, such as polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDD), poly-chlorinated dibenzofurans (PCDF), and polybrominated diphenyl ethers (PBDEs). A short summary of POPs is described in Supplementary Table 1. These chemicals are dangerous because they persist in the environment very long, bioaccumulate when they go through food chain, thus appear inside of our bodies many years after they are banned. And some POPs, such as DDT, are still in use in several countries.
Lee et al. reported for the first time that there is quantitative association between serum concentrations of (sum of six) POPs and prevalence of T2DM using the data from the National Health and Nutrition Examination Survey 1999–2002 [32]. In the following years, they reported that the some subclasses of POPs and OC pesticides were strongly and consistently associated with metabolic syndrome [6]. Dioxin-like PCBs also showed a positive association, whereas non-dioxin-like PCBs showed an inverted U-shaped association. Interestingly, the levels of OC pesticides were significantly associated with all components of metabolic syndrome, but not with high blood pressure.
Another POPs, PCBs, were significantly associated with waist circumference, triacylglycerol, and impaired fasting glucose. This finding suggested that they are more closely associated with metabolic syndrome and possible difference between the pathogenesis of T2DM and hypertension. PCDDs and PCDFs showed small but significant associations only with high blood pressure.
These findings suggest that the phenotypic components of metabolic syndrome are related to background exposure to a mixture of POPs. For the further details, readers are referred to a National Toxicology workshop report and an excellent review published recently by Lee et al. [6, 31].
Although POPs are well-known EDCs, it remains largely unknown what specific kinds of endocrine disrupting properties of POPs may have in the pathogenesis of T2DM. Lee et al. list two mechanisms; traditional endocrine disrupting-related mechanisms mediated through estrogen receptor and other receptors, and mitochondrial dysfunction-related mechanisms, which might be mediated by arylhydrocarbon receptor (AhR), a non-traditional hormonal receptor [6, 33].
Effect of an organophosphorus compound (OPC) on the mitochondrial function was reported as early as 1986 [34]. They showed the gills of guppies showed histopathological abnormalities in mitochondria after exposure to 1.2–3.0 ppb of DDT. The changes did not resemble the typical nonspecific pattern of pesticide poisoning, and concluded that they were probably specific to DDT. Shertzer et al. reported that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), better known as dioxin, inhibited mitochondrial function when injected into mice just once. TCDD-treated mice had approximately 60 % of the normal rate of ATP production in mitochondria [35].
Brominated phenolic substances are becoming widely used as flame retardant and other industrial purposes and have been reported to be associated with T2DM and other disease conditions. One of those substances, tetrabromo-bisphenol A (TBBPA) and its analogues were reported to cause concentration− and time-dependent cell death accompanied by the loss of cellular ATP, suggesting it induces mitochondrial dysfunction [36]. In a comparative study on the effects of BPA, TBBPA, and tetrachloro-bisphenolA (TCBPA) on cell viability, mitochondrial membrane potential, and some toxic parameters, BPA appeared less toxic than the others, but still showed ill effects.
Perfluoroalkyl substances (PFAS) are a group of chemicals used in a broad spectrum of products and processes, and were recently added to POPs. Due to their unique properties such as extreme stability and surface activity, they are ideal for coatings of paper, textiles, upholstery as well as reaction additives in various processes. At the same time, these properties have lead to the global distribution in the environment including humans. Circulating levels of PFAS was found in association with prevalent diabetes in the Swedish elderly [37]. There were also a significant non-linear relationship between perfluorononanoic acid (PFNA) and diabetes, even after adjusting for multiple confounders. Perfluorooctanoic acid (PFOA) also showed such a relationship. PFOA was related to the proinsulin/insulin ratio (a marker of insulin secretion), but none of the PFAS was related to the homeostasis model assessment of insulin resistance (HOMA-IR) index after the adjustment for multiple confounders.
In general there are apparent associations between POPs exposure, mitochondrial dysfunction and development of diabetes mellitus, but further studies are definitely needed to clarify the associations.
3.2 Non-persisting EDCs
Many herbicides, insecticides, rodenticides, industrial products, and industrial toxic wastes were reported in association with T2DM and obesity. These chemicals are not listed as POPs, but among EDCs. For a striking example, human intoxication with a rodenticide vacor [N-3-pyridyl-methyl-N’-p-nitrophenyl urea or 1-(4-nitrophenyl)-3-(3-pyridyl-methyl) urea] induces acute insulin-dependent diabetes, which was explained by its inhibitory effect on mitochondrial complex I in the pancreatic beta cells [38].
OPCs are cholinesterase inhibiting chemicals, mainly used as pesticides. Inhibition of acetylcholinesterase and the subsequent accumulation of the neurotransmitter acetylcholine at nerve synapses is the suggested mechanism for OPC toxicity in non-target species [39]. Acetylcholine normally interacts with muscarinic M3 receptors, increase insulin secretion by raising intracellular Ca2+ levels or through affecting the efficacy of Ca2+ in stimulating exocytosis. Therefore, malathion, an OPC, could damage downstream signaling pathway, cause a functional loss of Ca2+/calmodulin-dependent protein kinase, a protein associated with insulin secretory granules, thereby decreasing Ca2 + -induced insulin secretion. Malathion was found to reduce the activity of mitochondrial complexes and muscular glycogen with an elevation of lactate in association with impairment of cellular respiration when applied to the cultured muscle cells. The reduction in mitochondrial proapoptotic stimuli is indicative of autophagic process inducing cytoprotective effects in the early stage of stress [40]. OPCs are also known to interfere with estrogen action and thyroid hormones by specifically increasing the expression of estrogen responsive genes and preventing thyroid hormone receptor binding, respectively [41, 42].
Herbicide atrazine and related triazine herbicides have been extensively used in the USA since the early 1960s, a time period that corresponds to the beginning of the present obesity epidemic. They are routinely found as a contaminant in many surface and ground waters. Atrazine usage and obesity maps of USA show striking overlaps, suggesting that its heavy usage may be associated with the risk of obesity. Atrazine binds irreversibly to the plastoquinone-binding sites of the photosystem II complex on thylakoid membranes in chloroplasts, thereby inhibiting electron transport and it also inhibits mitochondrial function and induces insulin resistance in cultured cells and in rats [43, 44].
Newly introduced insecticides, fipronil and imidacloprid, suspected as a cause of colony collapse disorder, were tested for their effects on mitochondrial activity using Africanized honeybees, and were found indeed they are inhibitors of mitochondrial bioenergetics, resulting in depleted ATP [45].
Another subclass of non-persisting environmental pollutants, phthalates, is suspected to cause obesity. They are commonly used as plasticizers and vehicles for cosmetic ingredients. They do not bio-accumulate in our body, but the urinary excretion rate of their metabolites has been reported consistently associated with obesity [46]. Some phthalate monoesters, such as mono-ethylhexyl-phthalate (MEHP), di (2-ethylhexyl) phthalate (DEHP), mono-iso-nonyl phthalates, and mono-isodecyl phthalate were shown to act as proliferator-activated receptor gamma (PPARγ) agonists, similar to thiazolidinediones, through which they promote adipose tissue differentiation and lipid accumulation [47–49].
We could not find direct evidence that phthalates inhibit mitochondrial function, but found Ishido et al. developed an in-vitro neurosphere assay using rat mesencephalic neural stem cells, and reported all phthalates tested inhibited cell migration with a linear or non-linear range of concentrations when comparing migration distance to the logarithm of the phthalate concentrations [50]. They reported also that phthalates do not induce apoptosis, at least in a tested level of 100 μM. These findings could not be explained solely by their PPARγ agonistic activity, but inhibition of cell function, possibly mitochondrial dysfunction. Although further studies are needed, a recent review concluded that there are not enough evidences to classify them as an obesogen clinically [51]. Then there are always possibility of interactions with other well defined EDCs and phthalates.
Another non-persisting EDC regarded as a cause of insulin resistance or T2DM is BPA [52]. In a comprehensive review, Alonso-Magdalena et al. concluded that evidence already exists to consider exposure to BPA as a risk factor in the etiology of type 2 diabetes mellitus and other diseases related to insulin resistance [53]. BPA is used primarily to make plastics. Although BPA is poorly soluble in water, but leaches out from plastics slowly and contaminates drinking water and surroundings. Our group and others had confirmed it induces oxidative stress and mitochondrial dysfunction in appropriate cells and tissues [53–54].
3.3 Heavy metals
Several metals, such as cadmium, mercury and the metalloid arsenic, have been epidemiologically linked to the incidence of T2DM and arsenic was established as a cause of metabolic syndrome as well [56, 57]. Interestingly, arsenic was also found toxic to mitochondrial function [58].
3.4 Air pollution
Many epidemiological studies have demonstrated a positive association between particulate matter or traffic-related air pollutants and T2DM. We could not review this rapidly emerging subject and readers are referred to an excellent review by Liu et al. for details [59]. One of the most convincing might be the result of a study on the development of new T2DM in relation to air pollutants evaluated in a prospective manner. The Danish Diet Cancer and Health Cohort study showed a strong support for the relationship between air-pollution levels and diabetes with NO2 levels as a proxy for air-pollution exposure [60]. There is another interesting result from the Black Women’s pollution levels, which has shown an association between new onset DM and long-term exposure to NO2, a metric of traffic-related pollution [61]. This relationship was found among African American women living within the greater Los Angeles area.
Although epidemiological findings strongly support the association between air pollution, in particular traffic-related sources, and DM, Liu et al.conclude that not all aspects of this relationship have been consistently reported nor are they fully elucidated at this time [59]. The varying associations noted between studies may relate to numerous differences. They list the population characteristics, their associated risk factors, individual genetic susceptibilities, robustness of the cohort data and the absolute prevalence/incidence rates of DM and so on. Technical aspects of the exposure assessment methodologies, pollution types/sources, and the degree and duration of air-pollution exposures are also listed.
In general, air pollution due to particle is known as “particulate matters (PM).” They are a complex mixture of extremely small particles and liquid droplets. Particle pollution is made up of a number of components, including acids (such as nitrates and sulfates), organic chemicals, metals, and soil or dust particles. The size of particles is directly linked to their potential for causing health problems. Particles 10 micrometers in diameter or smaller generally pass through the throat and nose and enter the lungs, and can affect the heart and lungs and cause serious health effects. US EPA (http://www.epa.gov/pm/) divides particle pollution into two categories:“Inhalable coarse particles,” such as those found near roadways and dusty industries, are larger than 2.5 micrometers and smaller than 10 micrometers in diameter (PM2.5-10). “Fine particles (PM2.5),” such as those found in smoke and haze, are 2.5 micrometers in diameter and smaller. These particles can come from forest fires, power plants, industries and automobiles.
PM is considered to represent a prototypical inflammatory trigger for cellular responses from innate and adaptive immune cells, possibly with synergistic effects from other bronchial epithelial cells. PM2.5-mediated elevation in blood glucose level has been shown in mice fed with same normal diet.
Recently Liu et al. reported that PM2.5 exposure influenced energy metabolism including O2 consumption, CO2 production, respiratory exchange ratio and thermogenesis [62]. These changes were accompanied by worsened insulin resistance, visceral adiposity and inflammation in spleen and visceral adipose depots. Plasma adiponectin level was decreased in response to PM2.5 exposure while leptin level increased. PM2.5 exposure resulted in a significant increase in the expression of inflammatory genes and decreased uncoupling protein 1 (UCP1) expression in brown adipose tissue and activated p38 and ERK pathways in the liver of the KK mice, which is a genetically susceptible diabetic model. These results suggest air pollutants make mitochondrial dysfunction and provide insights into the mechanisms surrounding EDCs acting on the genetic susceptibility, other than that provided by mitochondrial genome itself.
For the inhalable larger PM10–2.5 particles, there is a suggestive evidence of increased morbidity and mortality in relation to higher short-term PM10–2.5 concentrations, with stronger relationships for respiratory than cardiovascular endpoints. Reported associations were highly heterogeneous. Additional research is required to better understand sources of heterogeneity of associations between PM10–2.5 and adverse health outcomes [63].
3.5 Drugs
Insulin resistance and altered fat distribution (loss of subcutaneous fat and a relative increase in abdominal fat) are common in the patients receiving the highly active antiretroviral therapy (HAART). Mitochondrial dysfunction and impaired oxidative capacity (especially in skeletal muscles) caused by nucleoside-analogue reverse transcriptase inhibitors are well-known side effects of anti-retroviral therapy. These agents inhibit DNA polymerase gamma, which plays a key role in mtDNA replication [64]. Furthermore, mitochondrial function, morphology and the metabolic derangement improves after discontinuing these treatments [65].
Many, but not all, drugs with organ toxicity have a mitochondrial liability. In 2007 Boelsterli and Lim reported screen of over 550 drugs revealed 34 % of them have mitochondrial liabilities. Depending on potency, if a drug has a mitochondrial liability, it will have deleterious consequences [66]. Detection of drug-associated mitochondrial toxicity has now become a routine procedure in pharmaceutical industry [67].
4 Biologic tests for EDCs
Understanding the pathogenesis of metabolic syndrome in the context of our mitochondrion unit-based model explicitly suggests that the preventive tactics should be based on avoidance of mitochondrial toxins and protecting mitochondria from further damage. It also provides important implications for the diagnosis and treatment of T2DM, metabolic syndrome, and related diseases such as atherosclerosis. Many compounds act by binding through receptors [estrogenic compounds to estrogen receptors (ER), ERs, and ERa, dioxin-like compounds to the arylhydrocarbon receptor (AhR), etc], which may lead to additive or synergistic effects of the individual compounds.
In a recent review on the POPs exposure and development of T2DM, Hectors et al. pointed out that current research predominantly takes “top-down” approach in identification of insulin resistance inducing metabolic disruptors [68].
Environment-Wide Association Study (EWAS), in which epidemiological data are comprehensively and systematically interpreted in a manner analogous to a Genome Wide Association Study (GWAS) might be such “top-down” approach. This method was employed by Patel et al., who applied logistic regression models (adjusted for age, sex, BMI, ethnicity, and an estimate of socioeconomic status (SES), to data available from Centers for Disease Control (CDC) National Health and Nutrition Examination Survey (NHANES) cohorts study from years 1999 to 2006, and discovered significant associations for the pesticide-derivative heptachlor epoxide and the vitamin gamma-tocopherol with DM [74]. Higher concentrations of PCBs such as PCB170 were also found. There were protective factors associated with T2DM, including beta-carotenes. Although the results are consistent with EDCs-induced T2D model, this kind of approach is not useful in identifying individual EDC for its toxicity.
Hectors et al. emphasized the needs for the development of dedicated in vitro or ex vivo methods to allow and time− and cost effective “bottom up” screening [68]. This approach is similar one advocated by Meyer et al., who calls for testing those chemicals suspected as EDCs for their interaction with AhR or ER, and inhibition of mitochondrial function [5].
We took such “bottom up” approach and tested if subjects with T2DM or metabolic syndrome have higher serum AhR transactivation activity [69]. We developed novel cell-based AhR ligand activity (CALA) assay without solvent extraction process and analyzed whether low-dose circulating AhR ligands in human serum are associated with parameters of metabolic syndrome and mitochondrial function. Serum AhR ligand activities were measured as serum TCDD equivalent (TCDDeq) in 97 Korean participants (47 with glucose intolerance and 50 matched controls). Serum TCDDeq were higher in participants with glucose intolerance than normal controls and were positively associated with obesity, blood pressure, serum triglyceride, and fasting glucose. Body mass index was in a positive linear relationship with serum AhR ligands in healthy participants. When myoblast cells were incubated with human sera, ATP generating power of mitochondria became impaired in an AhR ligand concentration-dependent manner. Our results suggested that circulating AhR ligands may directly reduce mitochondrial function in tissues, leading to glucose intolerance and metabolic syndrome. We found inhibitory effect of serum on ATP generating power of mitochondria was mediated by AhR dependent and non-AhR dependent pathways, probably through ER, pregnane X receptor (PXR), and constitutive active/androstane receptor (CAR) [70–72]. We are currently testing if these assays for EDCs (AhR ligand assay) and inhibitory activity of mitochondrial function are predictive of development of T2DM and other diseases with sera obtained from community based cohorts.
There are other tests proposed. Ishido et al. developed an in vitro neurosphere assay using rat mesencephalic neural stem cells, and Pereira-Fernandes et al. tested a screening system for obesogenic EDCs with peroxisome PPARγ dependency using the 3T3-L1 cell line, a well-characterized adipogenesis model [50, 73].
5 Summary and clinical considerations with EDCs-induced mitochondrial dysfunction as a cause of metabolic syndrome
In this review we took a “bottom-up” approach in that the decreased function of the mitochondrion (total ATP generating capacity) caused by EDCs is the basic abnormality of insulin resistance or metabolic syndrome. This approach with mitochondria-based model predicts that the mitochondrial toxins in the environment will predispose humans to the development of metabolic syndrome. This approach is supported by several groups including Meyer et al., Lee et al. and Hectors et al., and will be useful identifying those EDCs causing metabolic syndrome, T2DM and other related disease [5, 6, 68].
In our hands, a new cell-based AhR ligand activity assay and serum inhibitory activity on mitochondrial function (as measured by cell ATP level) was found as a good biomarker for metabolic syndrome. Those findings suggest they could be employed as a diagnostic marker of metabolic syndrome and T2DM. In fact, patients with complications from DM had higher serum AhR ligands levels, suggesting they might contribute to the development of diabetic complications as well [75].
Classifying EDCs into 5 subclasses might be helpful, when the EDCs-induced mitochondrial dysfunction model is applied to clinical medicine. Nature of those chemicals will be of prime importance in avoiding or eliminating them from patients. This concept is summarized in Table 1.
References
Meigs JB. Invited commentary: insulin resistance syndrome? Syndrome X? Multiple metabolic syndrome? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am JEpidemiol. 2000;152:908–11.
Reaven GM. Banting lecture, Role of insulin resistance in human disease. Diabetes. 1998;37:1595–607.
Lee HK, Cho YM, Kwak SH, Lim S, Park KS, Shim EB. Mitochondrial dysfunction and metabolic syndrome-looking for environmental factors. Biochim Biophys Acta. 2010;1800(3):282–9.
Lee HK, Shim EB. Extension of Mitochondria Dysfunction Hypothesis of Metabolic Syndrome to Atherosclerosis with Emphasis on the Endocrine Disrupting Chemicals and Biophysical Laws. J Diabetes Investig. 2013;4(1):19–33.
Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, et al. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134(1):1–17.
Lee DH, Porta M, Jacobs Jr DR, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35(4):557–601.
Lee HK, Song JH, Shin CS, Park DJ, Park KS, Lee KU, et al. Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998;42:161–7.
Jucker BM, Dufour S, Ren J, Cao X, Previs SF, Underhill B, et al. Shulman GI.Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc Natl Acad Sci U S A. 2000;97:6880–4.
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2.
Smith RE. Quantitative relations between liver mitochondria metabolism and total body weight in mammals. Ann NY Acad Sci. 1956;62:403–22.
Rasmussen UF, Rasmussen HN, Krustrup P, Quistorff B, Saltin B, Bangsbo J. Aerobic metabolism of human quadriceps muscle: in vivo data parallel measurements on isolated mitochondria. Am J Physiol Endocrinol Metab. 2001;280:301–7.
Lee HK. Method of proof and evidences for the concept that mitochondrial genome is a thrifty genome. Diabetes Res Clin Pract. (Suppl. 2) 2001;54–63.
Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–53.
West GB, Woodruff WH, Brown JH. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci U S A. 2002;99 Suppl 1:2473–8.
Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for bodyweight gain. N Engl J Med. 1988;318:467–72.
Chun PW. Why does the human body maintain a constant 37-degree temperature?: thermodynamic switch controls chemical equilibrium in biological systems. Phys Scr. 2005;118:219–22.
Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31(3):364–95.
Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(2):92–103.
Pravenec M, Hyakukoku M, Houstek J, Zidek V, Landa V, Mlejnek P, et al. Direct linkage of mitochondrial genome variation to risk factors for type 2 diabetes in conplastic strains. Genome Res. 2007;17:1319–26.
Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, et al. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet. 2007;80:407–15.
Park KS, Chan JC, Chuang LM, Suzuki S, Araki E, Nanjo K, et al. A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia. 2008;51:602–8.
Flaquer A, Baumbach C, Kriebel J, Meitinger T, Peters A, Waldenberger M, et al. Mitochondrial Genetic Variants Identified to Be Associated with BMI in Adults. PLoS One. 2014;9(8):e105116.
Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601.
Knowler WC, Pettitt DJ, Bennett PH, Williams RC. Diabetes mellitus in the Pima Indians: genetic and evolutionary considerations. Am J Phys Anthropol. 1983;62:107–14.
Cannon B, Nedergaard J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc. 2009;68(4):401–7.
Koleva DI, Orbetzova MM, Atanassova PK. Adipose tissue hormones and appetite and body weight regulators in insulin resistance. Folia Med (Plovdiv). 2013;55(1):25–32.
Krebs HA, Eggleston LV. The effect of insulin on oxidations in isolated muscle tissue. Biochem J. 1938;32(5):913913.
Echave P1, Machado-da-Silva G, Arkell RS, Duchen MR, Jacobson J, Mitter R, et al. Extracellular growth factors and mitogens cooperate to drive mitochondrial biogenesis. J Cell Sci. 2009;122(24):4516–22.
Damstra T, Barlow S, Aake B, Kavlock R, Van Der Kraak G. International Programme on Chemical Safety: Global Assessment of the State-of-the-Science on Endocrine Disruptors. 2002. http://www.who.int/pcs/emerg_site/edc/global_edc_TOC.htm
Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–89.
Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol. 2013;41(2):361–73.
Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29(7):1638–44.
Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118(4):465–71.
Virtanen MT. Histopathological and ultrastructural changes in the gills of Poeciliareticulatus induced by an organochlorine pesticide. J Environ Pathol Toxicol Oncol. 1986;7:73–85.
Shertzer HG, Genter MB, Shen D, Nebert DW, Chen Y, Dalton TP. TCDD decreases ATP levels and increases reactive oxygen production through changes in mitochondrial F (0) F (1)-ATP synthase and ubiquinone. Toxicol Appl Pharmacol. 2006;217:363–74.
Nakagawa Y, Suzuki T, Ishii H, Ogata A. Biotransformation and cytotoxicity of a brominated flame retardant, tetrabromobisphenol A, and its analogues in rat hepatocytes. Xenobiotica. 2007;37:693–708.
Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia. 2014;57(3):473–9.
Esposti MD, Ngo A, Myers MA. Inhibition of mitochondrial complex I may account for IDDM induced by intoxication with the rodenticide Vacor. Diabetes. 1996;45:1531–4.
Rahimi R, Abdollahi MA. review on the mechanism involved in hyperglycaemia induced by organophosphorus pesticides. Pestic Biochem Physiol. 2007;88:115–21.
Karami-Mohajeri S, Hadian MR, Fouladdel S, Azizi E, Ghahramani MH, Hosseini R, et al. Mechanisms of muscular electrophysiological and mitochondrial dysfunction following exposure tomalathion, an organophosphorus pesticide. Hum ExpToxicol. 2014;33(3):251–63.
McKinlay R, Plant JA, Bell JNB, Voulvoulis N. Endocrine disrupting pesticides: implications for risk assessment. Environ Int. 2008;34:168–83.
Jeong SH, Kim BY, Kang HG, Ku HK, Cho JH. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0− andF1-generations. Toxicology. 2006;220:189–202.
Soloman KR, Baker DB, Richards RP, Dixon KR, Klaine SJ. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76.
Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009;4(4):e5186.
Nicodemo D, Maioli MA, Medeiros HC, Guelfi M, Balieira KV, De Jong D, et al. Fipronil and imidacloprid reduce honeybee mitochondrial activity. Environ Toxicol Chem. 2014;33(9):2070–5.
Lind PM, Zethelius B. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care. 2012;35(7):1519–24.
Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308.
Bility MT, Thompson JT, McKee RH, David RM, Butala JH, VandenHeuvel JP. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82:170.182.
Posnack NG, Swift LM, Kay MW, Lee NH, Sarvazyan N. Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ Health Perspect. 2012;120(9):1243–51.
Ishido M, Suzuki J. Classification of phthalates based on an in vitro neurosphere assay using rat mesencephalic neural stem cells. J Toxicol Sci. 2014;39(1):25–32.
Goodman M, Lakind JS, Mattison DR. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit Rev Toxicol. 2014;44(2):151–75.
Polyzos SA, Kountouras J, Deretzi G, Zavos C, Mantzoros CS. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: a concept implicating nonalcoholic fatty liver disease. Curr Mol Med. 2012;12(1):68–82.
Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7(6):346–53.
Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012;27(6):644–552.
Kaur K, Chauhan V, Gu F, Chauhan A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic Biol Med. 2014;76C:25–33.
Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH. Heavy metals and islet function and diabetes development. Islets. 2009;1:169–76.
Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol. 2009;238:289–93.
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95–107.
Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol. 2013;41(2):361–73.
Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, et al. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35(1):92–8.
Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125(6):767–72.
Liu C, Bai Y, Xu X, Sun L, Wang A, Wang TY, et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part Fibre Toxicol. 2014; 11:27.
Adar SD, Filigrana PA, Clements N, Peel JL. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr Environ Health Rep. 2014;8(1):258–74.
Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003;2:812–22.
Gerschenson M, Kim C, Berzins B, Taiwo B, Libutti DE J, Choi J, et al. Mitochondrial function, morphology and metabolic parameters improve after switching from stavudine to a tenofovir-containing regimen. J Antimicrob Chemother. 2009;63:1244–50.
Boelsterli UA, Lim PL. Mitochondrial abnormalities-a link to idiosyncratic drug hepatotoxicity? Toxicol Appl Pharmacol. 2007;220:92–107.
Amacher DE. Drug-associated mitochondrial toxicity and its detection. Curr Med Chem. 2005;12(16):1829–39.
Hectors TL, Vanparys C, Van Gaal LF, Jorens PG, Covaci A, Blust R. Insulin resistance and environmental pollutants: experimental evidence and future perspectives. Environ Health Perspect. 2013;121(11–12):1273–81.
Park WH, Jun DW, Kim JT, Jeong JH, Park H, Chang YS, et al. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors. 2013;39(4):494–504.
Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24(1):6–19.
Shizu R, Benoki S, Numakura Y, Kodama S, Miyata M, Yamazoe Y, et al. Xenobiotic-induced hepatocyte proliferation associated with constitutive active/androstane receptor (CAR) or peroxisome proliferator-activated receptor α (PPARα) is enhanced by pregnane X receptor (PXR) activation in mice. PLoS One. 2013;8(4):e61802.
Gao J, Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol Sci. 2012;33(10):552–8.
Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TL, Jorens PG, Blust R, et al. Evaluation of screening system for obesogenic compounds: screening of endocrinedisrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One. 2013;8(10):e77481.
Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One. 2010;5(5):e10746.
Kim JT, Kim SS, Jun DW, Hwang YH, Park WH, Pak YK, et al. Serum arylhydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J Diabetes Investig. 2013;4(5):483–91.
Acknowledgments
This work was supported in part by MIC & IITA through the IT Leading R & D Support Project.
Conflict of interest statement
Disclosure: The authors do not have any financial relationship with organizations. Dr. HK Lee owns a part of patent (PCT/KR2011/006583) for the application of cell based arylhydrocarbon receptor ligands assay to the diagnosis of metabolic syndrome. The authors have full control of all primary data and that we agree to allow the journal to review our data if requested.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Kim, J.T., Lee, H.K. Metabolic syndrome and the environmental pollutants from mitochondrial perspectives. Rev Endocr Metab Disord 15, 253–262 (2014). https://doi.org/10.1007/s11154-014-9297-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-014-9297-5