Abstract
ALX-0081 is a novel nano-antibody inhibiting von Willebrand factor (vWF). We evaluated whether direct inhibition of vWF by ALX-0081 improves endothelial function. Stable patients (pts, n = 55) with single vessel disease undergoing percutaneous coronary intervention (PCI) were randomized to ALX-0081 (n = 38) or placebo (n = 17). vWF inhibition was assessed by vWF antigen level (vWF:Ag) and activity by ristocetin test (vWF:RiCo). Endothelial function was assessed before (BL), 6 h and 24 h after PCI by: (a) endothelial peripheral arterial tonometry (Endoscore); (b) endothelial microparticles (EMPs) by flow cytometry. vWF:Ag and vWF:RiCo decreased within 1 h from ALX-0081. In the placebo group, no significant Endoscore changes occurred from BL to 24 h. In ALX-0081 group, Endoscore increased from BL to 24 h (p = 0.014). A decrease in EMPs was observed after ALX-0081 (p < 0.01), while no changes occurred in placebo pts. An inhibition of vWF with ALX-0081 significantly improves peripheral endothelial function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Risk factors for cardiovascular disease have been associated with a chronic inflammatory process leading to loss of vasodilatory and antithrombotic properties of the vascular endothelium [1–4]. Impaired endothelial function is one of the initial steps in the atherosclerotic plaque formation and its presence represents one of the most powerful predictor of cardiovascular events [5, 6]. Improving endothelial function or preventing its worsening with different therapeutic strategies is associated with a reduction in cardiovascular events [7–10].
von Willebrand factor (vWF) is a marker of endothelial dysfunction [11, 12]. Improvement of endothelial function with aggressive medical therapy is associated with a significant reduction in vWF levels [13]. ALX-0081 (Ablynx, Belgium) is a novel nano-antibody directly inhibiting vWF. More specifically, ALX-0081 inhibits the interaction between vWF with the platelet glycoprotein GPIb receptor complex, which is the first step in platelet adhesion to the vessel wall [14]. The aim of the present study is to investigate whether direct inhibition of vWF by ALX-0081 is associated with an improvement in endothelial function.
Methods
The present investigation represents a sub-study of the phase Ib, double-blind, randomized, placebo-controlled study of ALX-0081 multiple dose administrations conducted in stable angina patients undergoing percutaneous coronary intervention [EUDRACT n. 2007–007263–24] [14].
Patient Population
Fifty-five stable angina patients aged ≥18 years old with single de novo vessel disease undergoing elective percutaneous coronary intervention were consecutively and prospectively included in the study. Exclusion criteria were as follows: previous by-pass surgery; history of disseminated intravascular coagulation, thrombotic microangiopathy, coagulopathy; severe hemorrhage ≤3 months requiring blood transfusions; stroke, transient ischemic attack, myocardial infarction within 3 months prior to screening; chronic congestive heart failure; major organ dysfunction; known hypersensitivity to human/humanized antibodies; infections; pregnant or lactating women; allergy; use of vitamin K antagonists and/or Factor X inhibitors within 2 weeks prior to inclusion.
Study Protocol
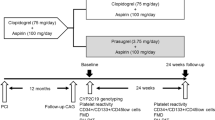
The study protocol is shown in Table 1 and Fig. 1. Briefly, patients were randomly assigned (3:1) to receive doses of either the von Willebrand factor inhibitor ALX-0081 or placebo.
The study was performed in three stages:
-
Stage A: a single dose (2, 4, 6 or 9 mg, 3 patients for each dose) of either ALX-0081 or placebo (0.9 % NaCl) was administrated as IV infusion at the time of PCI.
-
Stage B: a single dose of either ALX-0081 (6 mg) or placebo (0.9 % NaCl) was administrated as IV infusion at the time of PCI, followed by three boluses IV injection of ALX-0081 (4 mg each) or placebo (0.9 % NaCl) every 6 h.
-
Stage C: a single dose of either ALX-0081 (6 mg) or placebo (0.9 % NaCl) was administrated as IV bolus (instead of the infusion) at the time of PCI, always followed by three boluses IV injection of ALX-0081 (4 mg each) or placebo (0.9 % NaCl) every 6 h.
Of note, we included in the present sub-study all 46 patients (38 actively treated with vWF inhibitor, and 8 receiving placebo) recruited in the randomized trial, plus additional 9 placebo patients to comply with sample size analysis calculation.
All vasoactive medications were withheld >24 h before the endothelial function measurement. All patients were on chronic aspirin and clopidogrel. After a 12-h fasting period, peripheral endothelial function and blood withdrawal were performed (baseline). Patients were then randomized into ALX-0081 group (n = 38) or placebo group (n = 17). PCI was performed according to current practice [15], and peripheral endothelial function measurements and blood sampling were performed again 6 h post-PCI for all patients (stage A, stage B and C) and 24 h post-PCI for patients of stage B and C only (ALX-0081 group, n = 26; placebo group, n = 9).
Measurements of Peripheral Endothelial Function
Peripheral endothelial function was measured by digital pulse amplitude with the Endothelial Peripheral Arterial Tonometry (Endo-PAT2000, Itamar Medical, Caesarea, Israel), as previously described [12]. In brief, the device measures the distal finger blood volume changes that accompany pulse waves. A peripheral arterial tonometry finger probe was placed at the tip of each index finger, and a blood pressure cuff was placed at the level of the study arm. After a 5-min resting period (baseline), the blood pressure cuff was inflated to 20 mmHg greater than the systolic pressure for 5 min (occlusion). Next, the blood pressure cuff was deflated, and the peripheral arterial tonometry recording was continued for an additional 5 min. The endothelial responses were assessed using the recently validated Framingham Reactive Hyperemia Index (Endoscore), as previously described [12, 16].
Inhibition of von Willebrand Factor and Platelet P2Y12 Pathway
Since treatment with biologics can affect circulating target levels, to monitor the effect of ALX-0081 in the inhibition of von Willebrand factor, we measured: (a) the levels of von Willebrand factor antigen (vWF:Ag, %) and factor VIII:chromogene (FVIII:C, %); (b) the inhibition of ristocetin co-factor activity (vWF:RICO, % max aggregation). The vWF:RICO explores the interaction of vWF with the platelet receptor glycoprotein Ib and subendothelial collagen. It is based on the property of the antibiotic ristocetin to agglutinate formalin-fixed normal platelets in the presence of plasma vWF. vWF:RICO was measured using the vWF ristocetin co-factor assay (Trinity Biotech, Bray, Ireland).
The degree of clopidogrel inhibition of P2Y12 pathway was assessed in a blood sample collected in a 2-mL tube containing 3.2 % sodium citrate. The point-of-care VerifyNow assay (Accumetrics, San Diego, California) was used to assess the platelet response to clopidogrel, as previously described [17, 18].
Endothelial Microparticles
Endothelial microparticles (EMPs) are sub-microscopic membrane vesicles shed from endothelial cells during activation and/or apoptosis and can be measured as a marker for endothelial injury. The levels of EMPs before and after ALX-0081 treatment were analyzed by flow cytometry [19]. Blood samples were drawn into Monovette® collection tubes containing К2-EDTA (Sarstedt). Plasma was obtained by centrifugation for 10 min at 2,500×g. Further centrifugation for 25 min at 13,500×g was performed to obtain platelet free plasma (PFP). The PFP was stored at -80 °C and thawed once before analysis. A volume of 50 μl of PFP was incubated with 5 μl of CD42a-FITC, CD31-PE and CD45-PerCP (3-D) for 30 min, respectively, in the dark at room temperature after which 2 ml of filtered (22 μm) FACSFlowTM solution (Becton Dickinson) was added. Finally, in each sample, 50 μl of SPHEROTM AccuCount particles (Spherotech Inc.) was added as reference particles with known number of particles per milliliter used to calculate the number of microparticles per microliter detected in the samples. Microparticles were analyzed on a BD FACSCantoTM and were defined as particles <1 μm on a fluorescence/forward light scatter plot with the use of 1 μm diameter precision particles (microparticles GmbH, Germany). The EMP population was characterized as CD31+/CD42a− microparticles.
Statistical Analysis
The primary endpoint of the study was the Endoscore improvement with vWF inhibition. In previous preclinical studies, at least an 80 % vWF:RICO reduction (reflecting the degree of vWF inhibition) was reported with the administration of ALX-0081 [20]. In the present study, we hypothesized that a 25 % improvement in Endoscore can be expected in patients receiving ALX-0081 as compared with patients receiving placebo. With this assumption, we calculated that at least 17 patients per group are needed for an 80 % power and two-sided p value of 0.05.
Statistical analysis was performed using the GraphPad Prism software, version 5. Continuous data are summarized as mean ± standard deviation or as median (interquartile ranges). Categorical variables are reported as frequencies and percentages. Two-tailed Student’s t test was used to compare continuous variables. Fisher’s exact or Mantel–Haenszel chi-square tests were used to assess the differences in categorical variables between groups. One-way ANOVA analysis of variance was used to compare changes in Endoscore and EMPs before and after ALX-0081 administration. A p value of <0.05 was considered statistically significant.
Results
Patients’ characteristics are shown in Table 2. There were no differences in baseline clinical characteristics, with the exception of higher rate of hyperlipidemia in the placebo group. Likewise, no difference was observed in the rate of statin between ALX-0081 and placebo group (n = 29 [76 %] vs. N = 14 [82 %], p = 0.73, respectively).
von Willebrand Factor and Factor VIII
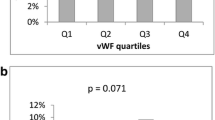
At baseline, no differences were observed in vWF:Ag (106 ± 5 vs. 121 ± 7 %, p = 0.39, respectively), FVIII:C (126 ± 14 vs. 143 ± 7 %, p = 0.35, respectively) and vWF:RICO (96 ± 15 vs. 120 ± 6 %, p = 0.13, respectively) between placebo and ALX-0081 groups (Fig. 2a and b). At 6 h, compared with placebo, a significant decrease in vWF:Ag (107 ± 9 vs. 75 ± 4 %, p < 0.01, respectively), FVIII:C (135 ± 18 vs. 103 ± 6 %, p = 0.067, respectively) and vWF:RICO (113 ± 13 vs. 12 ± 1 %, p < 0.001, respectively) was observed in the ALX-0081 group. This decrease was transient and levels of vWF:Ag and FVIII:C returned to baseline in all patients at 48 h, while the time to normalization for vWF:RICO was different according to duration of treatment as per protocol (data not shown).
Panel a Levels of von Willebrand factor antigen (vWF:Ag [%]) and factor VIII:chromogene (FVIII:C [%]) at baseline and 6 h post-PCI in both placebo- (white bars) and ALX-0081- (black bars) treated patients. Panel b Inhibition of ristocetin co-factor activity (vWF:RICO, % max aggregation) at baseline and 6 h post-PCI in both placebo- (white bars) and ALX-0081- (black bars) treated patients
Peripheral Endothelial Function
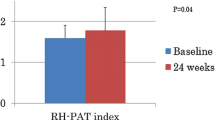
The average Endoscore for placebo group at baseline, 6 and 24 h were 0.40 ± 0.04, 0.38 ± 0.07 and 0.41 ± 0.09, respectively. The average Endoscore for ALX-0081 group at baseline, 6 and 24 h were 0.35 ± 0.06, 0.35 ± 0.07 and 0.47 ± 0.05. Changes in Endoscore along the study protocol are shown in Fig. 3. In the placebo group, there were no significant changes in Endoscore from baseline to 24 h (ΔEndoscore −0.05 ± 0.14 at 6 h and −0.39 ± 0.17 at 24 h; ANOVA p = 0.09). In the ALX-0081 group, significant changes in Endoscore were observed from baseline to 24 h (ΔEndoscore 8.51 ± 3.77 at 6 h; 11.11 ± 4.16 at 24 h; ANOVA p = 0.014). With respect to placebo, ALX-0081 is associated with a significant increase in ΔEndoscore at 6 h (p < 0.05), and at 24 h (p < 0.05) from the first administration.
Endothelial Microparticles
A significant decrease in EMPs was observed after ALX-0081 administration (ANOVA p = 0.0002): from baseline to 6 h (129 [114–258] to 122 [80–243] /μl, p < 0.001), then after 24 h (113 [87–225], p < 0.01 vs. baseline) (Fig. 4). No significant differences were observed in EMPs along the study protocol in the placebo group (ANOVA p = 0.204): from baseline to 6 h (131 [74–171] to 165 [131–211]), then after 24 h (164 [118–215]).
Discussion
In this prospective study conducted in patients with stable angina undergoing elective PCI, we observed that a direct inhibition of vWF with ALX-0081 nanobody was associated with a significant improvement in peripheral endothelial function as assessed by endothelial pulse amplitude tonometry and endothelial microparticles.
Endothelium modulates the interaction between the circulating blood, the vascular wall, and the surrounding tissue [21]. With dysfunctional endothelium this interaction is altered, triggering a cascade of events leading to atherosclerotic plaque formation and increased thrombogenicity [22]. von Willebrand factor plays a primary role in these events. In fact, a close correlation between platelet reactivity, vWF activity and endothelial function was previously reported [12]. The release of vWF in humans is enhanced by blockade of endothelial nitric oxide [23, 24]. In addition, the cyclic flow variations observed in severe coronary artery stenoses, potentially leading to the thrombotic occlusion of the vessel [25–27], are prevented by inhibiting the interaction between platelet glycoprotein Ib receptor and surface-bound von Willebrand factor [28].
The present study shows for the first time that a decrease in vWF serum level and activity is associated with an improvement of peripheral endothelial function. Endothelial function was assessed by using (1) endothelial pulse amplitude tonometry (Endo-PAT), and by measuring (2) endothelial microparticles, biomarkers related to endothelium turnover. Endo-PAT is a noninvasive technique to assess peripheral microvascular endothelial function by measuring amplitude of digital pulse volume during a shear stress induced by reactive hyperemia (RH) [29]. Assessment of pulse-wave amplitude response during RH has been shown to be correlated with gold standard flow-mediated dilation [30], and has been validated in different population [31–33]. In a Framingham cohort of patients, the Framingham Reactive Hyperemia Index, equivalent to the Endoscore index used in the present study, was related with traditional cardiovascular risk factor [16], and was found to predict cardiovascular events in an intermediate risk patient population [34]. In our study, we found no significant changes of Endoscore at either time point after percutaneous coronary intervention in the placebo group. In contrast, in patients receiving ALX-0081, we observed an early increase in Endoscore at 6 h that was preserved at 24 h post-PCI in parallel with the marked reduction in vWF levels and activity. The increase was also associated with a significant decrease in circulating endothelial microparticles, biomarker of endothelial cell damage and endothelium turnover [19, 35, 36]. Thus, based on these data as well as on our previous study showing correlation between vWF activity and endothelial function [12], we hypothesize that improved endothelial function is secondary to the direct inhibition of the vWF pathway. Yet, it remains unclear whether the improvement of the endothelial function extends beyond the immediate post-PCI period treatment. This should be addressed in larger clinical studies.
Our results might be clinically relevant if considering that transient endothelial impairment has been reported at the time of percutaneous coronary intervention [37]. The administration of ALX-0081 before PCI by directly modulating vWF levels could contribute to prevent this impairment or even improve endothelial function.
Conclusion
The inhibition of the von Willebrand factor pathway with the nanobody ALX-0081 is associated with an improvement in peripheral endothelial function. Our study provides an efficacy signal that should be further explored in a larger patient population with clinical end points.
References
Reriani, M. K., Lerman, L. O., & Lerman, A. (2010). Endothelial function as a functional expression of cardiovascular risk factors. Biomarkers in Medicine, 4(3), 351–360.
Lembo, G., Morisco, C., Lanni, F., Barbato, E., Vecchione, C., Fratta, L., & Trimarco, B. (1998). Systemic hypertension and coronary artery disease: the link. The American Journal of Cardiology, 82, 2H–7H.
Barbato, E., Bartunek, J., Wyffels, E., Wijns, W., Heyndrickx, G. R., & De Bruyne, B. (2003). Effects of intravenous dobutamine on coronary vasomotion in humans. Journal of the American College of Cardiology, 42, 1596–1601.
Barbato, E., Piscione, F., Bartunek, J., Galasso, G., Cirillo, P. L., De Luca, G., Iaccarino, G., De Bruyne, B., Chiariello, M., & Wijns, W. (2005). Role of beta2 adrenergic receptors in human atherosclerotic coronary arteries. Circulation, 111(3), 288–294.
Bonetti, P. O., Lerman, L. O., & Lerman, A. (2003). Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis, and Vascular Biology, 23(2), 168–175.
Brevetti, G., Piscione, F., Cirillo, P., Galasso, G., Schiano, V., Barbato, E., Scopacasa, F., & Chiariello, M. (2008). In concomitant coronary and peripheral arterial disease, inflammation of the affected limbs predicts coronary artery endothelial dysfunction. Atherosclerosis, 201(2), 440–446.
Treasure, C. B., Klein, J. L., Weintraub, W. S., Talley, J. D., Stillabower, M. E., Kosinski, A. S., Zhang, J., Boccuzzi, S. J., Cedarholm, J. C., & Alexander, R. W. (1995). Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. The New England Journal of Medicine, 332(8), 481–487.
Ridker, P. M., Danielson, E., Fonseca, F. A., Genest, J., Gotto, A. M., Jr., Kastelein, J. J., Koenig, W., Libby, P., Lorenzatti, A. J., MacFadyen, J. G., Nordestgaard, B. G., Shepherd, J., Willerson, J. T., & Glynn, R. J. (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England Journal of Medicine, 359(21), 2195–2207.
Hambrecht, R., Wolf, A., Gielen, S., Linke, A., Hofer, J., Erbs, S., Schoene, N., & Schuler, G. (2000). Effect of exercise on coronary endothelial function in patients with coronary artery disease. The New England Journal of Medicine, 342(7), 454–460.
Hamilos, M., Sarma, J., Ostojic, M., Cuisset, T., Sarno, G., Melikian, N., Ntalianis, A., Muller, O., Barbato, E., Beleslin, B., Sagic, D., De Bruyne, B., Bartunek, J., & Wijns, W. (2008). Interference of drug-eluting stents with endothelium-dependent coronary vasomotion: evidence for device-specific responses. Circulation Cardiovascular Interventions, 1(3), 193–200.
Vischer, U. M. (2006). von Willebrand factor, endothelial dysfunction, and cardiovascular disease. Journal of Thrombosis and Haemostasis, 4(6), 1186–1193.
Muller, O., Hamilos, M., Bartunek, J., Ulrichts, H., Mangiacapra, F., Holz, JB., et al. Relation of endothelial function to residual platelet reactivity after clopidogrel in patients with stable angina pectoris undergoing percutaneous coronary intervention. American Journal of Cardiology, 105(3), 333–338
Bickel, C., Rupprecht, H. J., Blankenberg, S., Espinola-Klein, C., Rippin, G., Hafner, G., Lotz, J., Prellwitz, W., & Meyer, J. (2002). Influence of HMG-CoA reductase inhibitors on markers of coagulation, systemic inflammation and soluble cell adhesion. International Journal of Cardiology, 82(1), 25–31.
Companion paper from Bartunek, J., & Holtz J. (2013). Novel antiplatelet agents: ALX-0081, a nanobody directed toward von Willebrand factor. Journal Cardiovascular Translational Research
Cuisset, T., Hamilos, M., Melikian, N., Wyffels, E., Sarma, J., Sarno, G., Barbato, E., Bartunek, J., Wijns, W., & De Bruyne, B. (2008). Direct stenting for stable angina pectoris is associated with reduced periprocedural microcirculatory injury compared with stenting after pre-dilation. Journal of the American College of Cardiology, 51(11), 1060–1065.
Hamburg, N. M., Keyes, M. J., Larson, M. G., Vasan, R. S., Schnabel, R., Pryde, M. M., Mitchell, G. F., Sheffy, J., Vita, J. A., & Benjamin, E. J. (2008). Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham heart study. Circulation, 117(19), 2467–2474.
Cuisset, T., Hamilos, M., Sarma, J., Sarno, G., Wyffels, E., Vanderheyden, M., Barbato, E., Bartunek, J., De Bruyne, B., & Wijns, W. (2008). Relation of low response to clopidogrel assessed with point-of-care assay to periprocedural myonecrosis in patients undergoing elective coronary stenting for stable angina pectoris. The American Journal of Cardiology, 101(12), 1700–1703.
Mangiacapra, F., De Bruyne, B., Muller, O., Trana, C., Ntalianis, A., Bartunek, J., Heyndrickx, G., Di Sciascio, G., Wijns, W., & Barbato, E. (2010). High residual platelet reactivity after clopidogrel: extent of coronary atherosclerosis and periprocedural myocardial infarction in patients with stable angina undergoing percutaneous coronary intervention. Journal American College of Cardiology Cardiovasc Intervention, 3(1), 35–40.
Hamilos, M., Muller, O., Ntalianis, A., Trana, C., Bartunek, J., Sarno, G., Mangiacapra, F., Dierickx, K., Meeus, P., Cuisset, T., De Bruyne, B., Wijns, W., & Barbato, E. (2011). Relationship between peripheral arterial reactive hyperemia and residual platelet reactivity after 600 mg clopidogrel. Journal of Thrombosis and Thrombolysis, 32(1), 64–71.
Ulrichts, H., Silence, K., Schoolmeester, A., de Jaegere, P., Rossenu, S., Roodt, J., Priem, S., Lauwereys, M., Casteels, P., Van Bockstaele, F., Verschueren, K., Stanssens, P., Baumeister, J., & Holz, J. B. (2011). Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood, 118(3), 757–765.
Deanfield, J. E., Halcox, J. P., & Rabelink, T. J. (2007). Endothelial function and dysfunction: testing and clinical relevance. Circulation, 115(10), 1285–1295.
Hansson, G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. The New England Journal of Medicine, 352(16), 1685–1695.
Jilma, B., Pernerstorfer, T., Dirnberger, E., Stohlawetz, P., Schmetterer, L., Singer, E. A., Grasseli, U., Eichler, H. G., & Kapiotis, S. (1998). Effects of histamine and nitric oxide synthase inhibition on plasma levels of von Willebrand factor antigen. The Journal of Laboratory and Clinical Medicine, 131(2), 151–156.
Pernerstorfer, T., Stohlawetz, P., Kapiotis, S., Eichler, H. G., & Jilma, B. (2000). Partial inhibition of nitric oxide synthase primes the stimulated pathway of vWF-secretion in man. Atherosclerosis, 148(1), 43–47.
Golino, P., Buja, L. M., Ashton, J. H., Kulkarni, P., Taylor, A., & Willerson, J. T. (1988). Effect of thromboxane and serotonin receptor antagonists on intracoronary platelet deposition in dogs with experimentally stenosed coronary arteries. Circulation, 78(3), 701–711.
Yao, S. K., Ober, J. C., McNatt, J., Benedict, C. R., Rosolowsky, M., Anderson, H. V., Cui, K., Maffrand, J. P., Campbell, W. B., & Buja, L. M. (1992). ADP plays an important role in mediating platelet aggregation and cyclic flow variations in vivo in stenosed and endothelium-injured canine coronary arteries. Circulation Research, 70(1), 39–48.
Bush, L. R., Campbell, W. B., Buja, L. M., Tilton, G. D., & Willerson, J. T. (1984). Effects of the selective thromboxane synthetase inhibitor dazoxiben on variations in cyclic blood flow in stenosed canine coronary arteries. Circulation, 69(6), 1161–1170.
McGhie, A. I., McNatt, J., Ezov, N., Cui, K., Mower, L. K., Hagay, Y., Buja, L. M., Garfinkel, L. I., Gorecki, M., & Willerson, J. T. (1994). Abolition of cyclic flow variations in stenosed, endothelium-injured coronary arteries in nonhuman primates with a peptide fragment (VCL) derived from human plasma von Willebrand factor-glycoprotein Ib binding domain. Circulation, 90(6), 2976–2981.
Bonetti, P. O., Barsness, G. W., Keelan, P. C., Schnell, T. I., Pumper, G. M., Kuvin, J. T., Schnall, R. P., Holmes, D. R., Higano, S. T., & Lerman, A. (2003). Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. Journal of the American College of Cardiology, 41(10), 1761–1768.
Kuvin, J. T., Patel, A. R., Sliney, K. A., Pandian, N. G., Sheffy, J., Schnall, R. P., Karas, R. H., & Udelson, J. E. (2003). Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. American Heart Journal, 146(1), 168–174.
Krantz, D. S., Santiago, H. T., Kop, W. J., Bairey Merz, C. N., Rozanski, A., & Gottdiener, J. S. (1999). Prognostic value of mental stress testing in coronary artery disease. American Journal of Cardiology, 84(11), 1292–1297.
Lavie, P., Shlitner, A., Sheffy, J., & Schnall, R. P. (2000). Peripheral arterial tonometry: a novel and sensitive non-invasive monitor of brief arousals during sleep. The Israel Medical Association Journal, 2(3), 246–247.
Bonetti, P. O., Pumper, G. M., Higano, S. T., Holmes, D. R., Jr., Kuvin, J. T., & Lerman, A. (2004). Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology, 44(11), 2137–2141.
Rubinshtein, R., Kuvin, J. T., Soffler, M., Lennon, R. J., Lavi, S., Nelson, R. E., Pumper, G. M., Lerman, L. O., & Lerman, A. (2010). Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European Heart Journal, 31(9), 1142–1148.
Woywodt, A., Bahlmann, F. H., De Groot, K., Haller, H., & Haubitz, M. (2002). Circulating endothelial cells: life, death, detachment and repair of the endothelial cell layer. Nephrology, Dialysis, Transplantation, 17(10), 1728–1730.
Mallat, Z., Benamer, H., Hugel, B., Benessiano, J., Steg, P. G., Freyssinet, J. M., & Tedgui, A. (2000). Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation, 101(8), 841–843.
Hamilos, H., Sarma, J., Ostojic, M., Cuisset, T., Sarno, G., Melikian, N., Ntalianis, A., Muller, O., Barbato, E., Belesin, B., Sagic, D., De Bruyne, B., Bartunek, J., & Wijns, W. (2008). Interference of drug-eluting stents with endothelium-dependant coronary vasomotion. Circulation: Cardiovascular Intervention, 1, 193–200.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muller, O., Bartunek, J., Hamilos, M. et al. von Willebrand Factor Inhibition Improves Endothelial Function in Patients with Stable Angina. J. of Cardiovasc. Trans. Res. 6, 364–370 (2013). https://doi.org/10.1007/s12265-012-9422-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-012-9422-3