Abstract
We used resting-state fMRI to evaluate longitudinal alterations in local spontaneous brain activity in Parkinson’s disease (PD) over a 2-year period. Data were acquired from 23 PD patients at baseline and follow-up, and 27 age- and sex-matched normal controls. Regional homogeneity (ReHo) and voxel-based-morphometry (VBM) were used to identify differences in local spontaneous brain activity and grey matter volume. With disease progression, we observed a progressive decrease in ReHo in the sensorimotor cortex, default-mode network, and left cerebellum, but increased ReHo in the supplementary motor area, bilateral temporal gyrus, and hippocampus. Moreover, there was a significant positive correlation between the rates of ReHo change in the left cerebellum and the rates of change in the Unified Parkinson’s Disease Rating Scale-III scores. VBM revealed no significant differences in the grey matter volume among the three sets of acquisitions. We conclude that ReHo may be a suitable non-invasive marker of progression in PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by tremor, rigidity, bradykinesia, and gait disturbance [1]. These motor impairments result from a typical pathophysiology of degeneration of the substantia nigra [2], with other neurons in the subcortical nuclei and cortex affected during the progression of the disease [3]. This pathology may lead to alterations in brain function [4]. However, the progression of pathological deterioration and the complementary changes in brain function occurring in PD are currently not well understood.
A non-invasive imaging method, functional magnetic resonance imaging (fMRI), has been demonstrated to be a potentially useful tool to detect alterations in brain function in PD [5]. Results from cross-sectional fMRI studies suggest that motor impairment in PD correlates with deactivation of the primary motor cortex (M1) [6], the supplementary motor area (SMA) [7], and the premotor area [8]. Resting-state fMRI studies on PD patients have reported lower functional connectivity in the striatum [9], the inferior parietal lobule [10], and the dorsolateral prefrontal cortex [11], while increased activation of the cerebellum [12] has been regarded as a compensatory modulatory mechanism. Concurrently, researchers have also demonstrated functional disruption of the default mode network (DMN) in PD patients, this being noted before the impairment of cognition [13]. However, with such cross-sectional studies, it is difficult to longitudinally assess changes in brain function during the course of the disease, and the current evidence is far from satisfactory in explaining the underlying brain abnormalities in PD.
To this day, longitudinal studies systematically assessing disease progression in terms of the resting brain activity remain inadequate, although a few attempts using fMRI to explore longitudinal changes in PD have been made. One study [14] on five PD patients obtained longitudinal fMRI acquisitions 2 years apart, and concluded that the cerebello-thalamo-cortical circuits are statistically different over time when a sequential finger movement task is performed. Another study using task-based fMRI found a decline in functional activity in the putamen and M1 of PD patients over time compared to multiple system atrophy and progressive supranuclear palsy [15]. Nevertheless, these task-based functional changes are to a large extent dependent on patient cooperation, and patients with PD have difficulty performing motor tasks [16]. Fortunately, resting-state fMRI overcomes this problem. A resting-state fMRI study [17] showed continuously decreased functional connectivity in PD patients over a 3-year period, and the decreases occurred prominently in the posterior parts of the brain. This study focused on whole-brain functional connectivity, whereas local spontaneous activity is also known to be a basic characteristic of the human brain [18]. However, it remains unknown how local spontaneous brain activity in PD patients varies throughout the course of the disease. Exploring this question will advance our understanding of PD progression.
Regional homogeneity (ReHo) is an advanced method of resting-state fMRI that evaluates the similarity between the time series of a given voxel and its nearest neighbors, and such similarity can be changed or modulated by specific conditions [18]. Thus, ReHo can be used to quantify the status of local spontaneous brain activity in different diseases. In the present study, we longitudinally evaluated the local spontaneous activity occurring throughout the whole brain in PD patients, and explored the potential relationships between this activity and clinical symptoms.
Participants and Methods
Participants
A total of 106 patients diagnosed with idiopathic PD and 31 normal controls (NCs) were scanned with 3D T1-weighted structural imaging and resting-state functional MRI at baseline. Finally 23 PD patients and 27 age- and sex-matched NCs were included in the longitudinal evaluation after 2 years (Fig. 1). All participants were recruited from Beijing Hospital between December 12, 2012 and January 1, 2016, and all were right-handed. PD was diagnosed by an experienced neurologist according to the UK Parkinson’s Disease Brain Bank. The PD patients were asked to withdraw from all antiparkinsonian medications ~12 h prior to the MRI scans and clinical assessments. All participants were assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS), the Hoehn and Yahr disability scale (HY), and the Mini-Mental State Examination (MMSE). The exclusion criteria included: (1) neurological disease or disorders that impair the function of the central nervous system (stroke, brain tumor, severe cerebral atrophy, epilepsy, kidney disease, liver disease, drug/alcohol/nicotine abuse), (2) head trauma with loss of consciousness or other complications, (3) psychiatric conditions (e.g. schizophrenia, delirium), (4) metal dentures and other metal implants. This study was approved by the Research Ethics Committee of Beijing Hospital, and written informed consent was given by all participants.
MRI Data Acquisition
The PD patients were scanned twice, at an interval of 24.9 months. The NCs were scanned on a single morning session. The MRI examinations were acquired at Beijing Hospital on a 3.0-T scanner (Achieva, Philips Medical Systems, Best, Netherlands) with a birdcage-type standard quadrature head coil. Headphones and foam padding were used to reduce scanner noise and limit head motion. Participants were instructed to relax, keep their eyes closed, and stay awake for the entire scan. We acquired high-resolution T1-weighted sagittal images (3D turbo field echo, repetition time (TR) = 7.4 ms, echo time (TE) = 3.0 ms, field of view (FOV) = 240 × 240 mm, matrix size = 256 × 256, voxel dimensions = 0.94 × 0.94 × 1.20 mm, slice thickness = 1.2 mm, 140 slices) and axial echo-planar imaging (EPI) resting-state fMRI images (TR = 3000 ms, TE = 35 ms, flip angle = 90°, FOV = 240 × 240 mm, matrix size = 64 × 64, voxel dimensions 3.75 × 3.75 × 4.00 mm, slice thickness = 4 mm, slices = 33, time points = 210, acquisition time = 15 min).
fMRI Data Preprocessing
fMRI data preprocessing and ReHo analysis were conducted using Data Processing Assistant for Resting-State fMRI V2.1 (http://www.restfmri.net). The first 10 volumes of the functional time series were discarded to allow for participants’ acclimatization to the scanning noise and for the signal to reach equilibrium. The remaining 200 fMRI images were corrected for within-volume slice-acquisition time differences, and were then realigned to correct for inter-scan head motion. The images from two patients and three NCs were excluded because their head motion exceeded 2 mm in displacement or 2° in rotation. The head motion of the remaining participants [19] was compared between the groups and revealed no statistically significant difference (P = 0.81). The functional images were then normalized to the Montreal Neurological Institute (MNI) EPI template, and resampled to a 3-mm isotropic resolution. This was followed by temporal filtering (0.01 Hz < f < 0.08 Hz) to remove the effects of high-frequency physiological and low-frequency drift noise, and the linear trend was then removed.
ReHo Analysis
The Kendall’s coefficient concordance between the time series of a given voxel and those of its nearest 26 neighboring voxels was calculated in a voxel-wise method. ReHo maps were then transformed to MNI coordinates. Spatial smoothing was applied to each ReHo map (full width at half maximum [FWHM] = 8 mm). Finally, the ReHo map of each participant was divided by its own mean ReHo for standardization purposes. The rates of ReHo change were calculated as: (ReHofollow-up − ReHoBaseline)/ReHoBaseline [20], similar to the method used for calculating the change in rates of UPDRS-III scores.
Structural Image Analysis
It has been reported that brain atrophy may induce a partial volume effect in functional imaging techniques [21]. Therefore, to explore the potential effects of any brain atrophy, a VBM analysis of the structural images was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) with default parameters, and incorporating the DARTEL toolbox from SPM 12 software (http://www.fil.ion.ucl.ac.uk/spm). Individual structural images were first co-registered using a linear transformation. The transformed images were then segmented into grey matter (GM), white matter, and cerebrospinal fluid using a unified segmentation algorithm. The warped GM volume and density maps were affine-transformed into MNI space with intensity modulation to compensate for the local compression and stretching that occur as a consequence of the warping and affine transformations. Finally, the resultant GM volume and density maps were smoothed with a Gaussian kernel of 8 mm FWHM.
Statistical Analysis
Group differences in demographic and clinical data were analyzed using paired t-tests and two-sample t-tests respectively, using SPSS 19 software (IBM Corporation, New York). The significance level was set at P < 0.05. Two-sample t-tests were performed on the smoothed GM intensity maps from the PD baseline and NC acquisitions, using age and gender as covariates. Paired t-tests were used to analyze differences between PD baseline and PD follow-up, using the time interval between the two scans as a covariate. The resultant images were used to identify the brain regions with GM loss. Statistical analyses of the ReHo procedures were implemented in DPABI (http://www.restfmri.net). Group maps of the ReHo differences between the NC and PD baseline acquisitions were analyzed using two-sample t-tests with sex, age, and GM volume as covariates (P < 0.001, cluster level False Discovery Rate [FDR]-corrected). There were no significant FDR-corrected differences in ReHo between the NC and PD baseline acquisitions. An exploratory test in which the uncorrected statistical threshold was set to P < 0.005 and a cluster size of ≥10 voxels, as reported by Ekman et al. [22], was then performed. Paired t-tests were performed to create a group map of ReHo differences between the follow-up and baseline PD maps, using the VBM images as covariates (P < 0.001, cluster level FDR-corrected). The statistical threshold for the VBM analysis was set to P < 0.001 (cluster level FDR-corrected). Correlations between the clinical scores and the ReHo of each voxel of the significantly different brain regions were evaluated using Spearman correlation with a significance level of P < 0.05.
Results
Demographic and Clinical Information
The demographic and clinical data of all participants are summarized in Table 1. There were significant differences in the UPDRS-III score (P = 0.001) and the H-Y stage (P = 0.017) between the PD baseline and PD follow-up sessions. The PD follow-up data showed improvement of motor manifestations (decreased UPDRS-III scores) compared with the PD baseline, following the administration of anti-parkinson pharmaceuticals over 2 years. There were no differences in age, sex, or MMSE scores between NC and baseline PD sessions.
Cross-Sectional Results
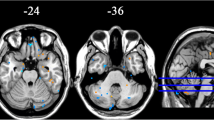
Our exploratory findings showed that, compared with NCs, the PD baseline acquisitions had decreased ReHo in the SMA, the bilateral precentral gyrus, and the bilateral postcentral gyrus [sensorimotor cortex (SMC)], and increased ReHo in the right superior temporal gyrus and right hippocampus (Fig. 2; Table 2). The VBM analysis did not reveal any differences in GM volume and density between the NC and baseline PD acquisitions.
Longitudinal Results
Compared with the PD baseline results, the PD follow-up images showed significantly decreased ReHo in the SMC, DMN (bilateral precuneus, bilateral angular gyrus, bilateral parietal lobule, bilateral middle frontal lobule, and anterior cingulate cortex), and left cerebellum, and increased ReHo in the SMA, bilateral temporal gyrus, and bilateral hippocampus (Fig. 3A, B; Table 2). A similar pattern was also found when the PD baseline images were compared with those from the NC group. Moreover, there was a correlation between the rate of ReHo change in the left cerebellum and the rate of UPDRS-III score change (R = 0.41; P < 0.05; Fig. 4) over the 2 years. The VBM analysis revealed no significant differences in GM volume and density between the follow-up and baseline sessions.
Result of correlation analysis. Association between the rate of ReHo change in the left cerebellum and the rate of the UPDRS-III score change (R = 0.41; P < 0.05). Rate of ReHo change was calculated as: (ReHofollow-up − ReHoBaseline)/ReHoBaseline. The rate of UPDRS-III score change was calculated in the same way.
Discussion
As far as we are aware, this is the first longitudinal research using ReHo to explore changes in local spontaneous brain activity in PD patients. We demonstrated that compared with NCs, the PD baseline images showed a tendency for decreased ReHo in the SMC and increased ReHo in the SMA, right superior temporal gyrus, and right hippocampus. However, the follow-up analysis of the PD patients demonstrated significantly decreased ReHo in the SMC, DMN, and left cerebellum, while ReHo in the SMA, bilateral temporal gyrus, and hippocampus significantly increased. Moreover, the correlation analysis showed that the rates of ReHo change in the left cerebellum were significantly correlated with the rates of change in the UPDRS-III scores.
The SMC showed a trend for decreased ReHo in the PD baseline acquisitions in comparison with the NCs. After an average period of 2 years, significantly decreased ReHo was found in the follow-up acquisitions from the PD patients. These decreases in ReHo may represent desynchronized blood flow within brain regions [23], which could be regarded as a disorder. This would imply that the homogeneity of brain functions in such regions progressively deteriorated during the course of the disease. Our results are supported by previous fMRI studies on PD patients showing decreased functional activity and deactivation in the SMC [6, 17, 24], and single-photon emission computed tomography and positron emission tomography studies showing diminished regional cerebral blood flow in the SMC [25, 26]. Recently, Choe et al. [23] also investigated decreased ReHo in the primary motor cortex and primary sensory cortex of PD patients, making comparisons with controls.
Our findings support the basal ganglia-thalamo-cortical (BgT) circuitry model [24], which conjectures that dopamine deficiency leads to reduced excitatory thalamic outflow to the SMC. As the thalamic output to the SMC diminishes, it is difficult to prepare and execute motor commands [27]. Considering this, we hold that the decreased ReHo in the SMC may be a result of disrupted motor-execution in PD patients. We expand on the previous studies by conjecturing that the SMC may represent the core pathological cortical injury, and that it progressively deteriorates with PD progression.
We discovered that changes in the SMA were contrary to the trend described above, with PD patients exhibiting increased ReHo in the SMA over time. The clinical motor manifestations of the PD patients improved (decrease in UPDRS-III) after 2 years of follow-up. Currently, the SMA is believed to impact the parkinsonian motor system through the BgT circuitry [28]. Diminished thalamic output to the SMC impairs the initiation of movement [27], and for the successive fine-tuning of movement, the SMA receives more input from the basal ganglia. An independent component analysis indicated that increased activity in the SMA is a topographic characteristic of a PD-related pattern, and is correlated with UPDRS ratings [29]. Caproni et al. [30] found that the SMA exhibits over-activation during multi-tasking, and interpreted this as cortical compensatory re-organization in PD patients. Moreover, an electroencephalogram study [31] found that increased connectivity between the SMA and basal ganglia is correlated with reduced dopamine levels, as the basal ganglia produce a high-frequency drive back to the SMA. Our results seem to be in contrast with a previous study [32] describing lower ReHo in the SMA in PD patients than in NCs. This divergence may be due to the fact that this other study used participants who were younger and had a shorter disease duration than ours, and this may have affected the role of the SMA in PD. Therefore, we suspect that the SMA in PD patients may play a compensatory role in motor impairment, and that the improved clinical motor manifestation may result from compensatory regulation by the SMA.
The DMN plays a crucial role in cognitive processing, both in neurodegenerative disorders [33] and normal aging [34]. Several fMRI studies have indicated that the DMN is injured in PD patients before cognition is impaired [11, 13, 35]. In accord with these previous studies, we found that PD patients with normal cognition showed a decreased ReHo in the DMN over time. These findings extend the previous evidence that the DMN is affected before cognitive impairment, and that it continuously deteriorates with disease progression. In addition, we also found that PD patients showed an increased ReHo in the bilateral temporal gyrus and bilateral hippocampus. This finding has not been reported. The temporal gyrus has been reported to exhibit higher ReHo values in early-onset PD [36], and decreased alpha1- and alpha2-band connectivity over a 4-year period [37]. These may be associated with maintaining normal cognition; however, further information is required to interpret and discuss this issue in depth.
A significant positive correlation between the rate of ReHo change in the left cerebellum and the rate of change in UPDRS-III scores was found during the disease course in our PD patients. These findings indicate that PD patients with more severe motor symptoms exhibit a higher ReHo in the cerebellum. Therefore, our results further confirm the complementary role of the cerebellum in PD, as supported by previous studies [30, 38, 39], implying an important role of the cerebellum in PD [40]. However, we did find a decrease in ReHo in this region in the follow-up sessions, while there were no significant differences between baseline and normal controls. It is probable that the management with antiparkinsonian drugs contributed to reductions in the complementary functions of the cerebellum [41]; this was also demonstrated by an improvement in clinical symptoms at the end of the 2-year follow-up.
This study is subject to several limitations that deserve mention. First, the relatively small sample sizes may somewhat limit the validity of the results. Future studies with larger samples are necessary to establish the reliability of the results and to validate the current findings. Second, we failed to obtain data on the levodopa equivalent daily dose, which could explain the improved motor manifestations after 2 years.
In conclusion, we showed that both compensatory mechanisms (the SMA, bilateral temporal gyrus, and bilateral hippocampus) and loss of efficiency (the SMC and the DMN) occurred with the progression of PD. In particular, the left cerebellum exhibited decreased regional homogeneity with disease progression, the changes being associated with clinical characteristics. These findings represent longitudinal alterations in local spontaneous brain activity in PD.
References
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008, 79: 368–376.
Guan X, Xu X, Zhang M. Region-specific iron measured by MRI as a biomarker for Parkinson’s disease. Neurosci Bull 2017. doi:10.1007/s12264-017-0138-x.
Jellinger KA. Post mortem studies in Parkinson’s disease–is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 1999, 56: 1–29.
Olde Dubbelink KT, Hillebrand A, Stoffers D, Deijen JB, Twisk JW, Stam CJ, et al. Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain 2014, 137: 197–207.
Stoessl AJ. Functional imaging studies of non-motoric manifestations of Parkinson’s Disease. Parkinsonism Relat Disord 2009, 15 Suppl 3: S13–16.
Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI–cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 2003, 126: 451–461.
Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 2001, 124: 558–570.
Rowe J, Stephan KE, Friston KJ, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson’s disease - Impaired effective connectivity among frontal cortical regions. Brain 2002, 125: 276–289.
Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain 2012, 135: 3699–3711.
Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, et al. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp 2011, 32: 1443–1457.
Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2012, 79: 2226–2232.
Kishore A, Meunier S, Popa T. Cerebellar influence on motor cortex plasticity: behavioral implications for Parkinson’s disease. Front Neurol 2014, 5: 68.
Sandrone S, Catani M. Journal Club. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2013, 81: e172–175.
Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X. Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson’s disease. Neuroscience 2010, 166: 712–719.
Burciu RG, Chung JW, Shukla P, Ofori E, Li H, McFarland NR, et al. Functional MRI of disease progression in Parkinson disease and atypical parkinsonian syndromes. Neurology 2016, 87: 709–717.
Tessitore A, Giordano A, De Micco R, Russo A, Tedeschi G. Sensorimotor connectivity in Parkinson’s disease: the role of functional neuroimaging. Front Neurol 2014, 5: 180.
Olde Dubbelink KT, Schoonheim MM, Deijen JB, Twisk JW, Barkhof F, Berendse HW. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology 2014, 83: 2046–2053.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004, 22: 394–400.
Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A 2014, 111: 6058–6062.
Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Brain atrophy rates in Parkinson’s disease with and without dementia using serial magnetic resonance imaging. Mov Disord 2005, 20: 1571–1576.
Bokde AL, Pietrini P, Ibanez V, Furey ML, Alexander GE, Graff-Radford NR, et al. The effect of brain atrophy on cerebral hypometabolism in the visual variant of Alzheimer disease. Arch Neurol 2001, 58: 480–486.
Ekman U, Eriksson J, Forsgren L, Domellöf ME, Elgh E, Lundquist A, et al. Longitudinal changes in task-evoked brain responses in Parkinson’s disease patients with and without mild cognitive impairment. Front Neurosci 2014, 8: 207.
Choe IH, Yeo S, Chung KC, Kim SH, Lim S. Decreased and increased cerebral regional homogeneity in early Parkinson’s disease. Brain Res 2013, 1527: 230–237.
Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 1990, 85: 119–146.
Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, et al. Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol 1992, 49: 144–148.
Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol 1992, 32: 151–161.
Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2001, 124: 2131–2146.
Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain 2003, 126: 199–212.
Vo A, Sako W, Fujita K, Peng S, Mattis PJ, Skidmore FM, et al. Parkinson’s disease-related network topographies characterized with resting state functional MRI. Hum Brain Mapp 2017, 38: 617–630.
Caproni S, Muti M, Principi M, Ottaviano P, Frondizi D, Capocchi G, et al. Complexity of motor sequences and cortical reorganization in Parkinson’s disease: a functional MRI study. PLoS One 2013, 8: e66834.
Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain 2002, 125: 1558–1569.
Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, et al. Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp 2009, 30: 1502–1510.
Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol Aging 2012, 33: 1564–1578.
Tedeschi G, Trojsi F, Tessitore A, Corbo D, Sagnelli A, Paccone A, et al. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol Aging 2012, 33: 886–898.
Koshimori Y, Cho SS, Criaud M, Christopher L, Jacobs M, Ghadery C, et al. Disrupted nodal and hub organization account for brain network abnormalities in Parkinson’s disease. Front Aging Neurosci 2016, 8: 259.
Sheng K, Fang W, Zhu Y, Shuai G, Zou D, Su M, et al. Different alterations of cerebral regional homogeneity in early-onset and late-onset Parkinson’s disease. Front Aging Neurosci 2016, 8: 165.
Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Hillebrand A, et al. Resting-state functional connectivity as a marker of disease progression in Parkinson’s disease: A longitudinal MEG study. Neuroimage Clin 2013, 2: 612–619.
Pan P, Zhan H, Xia M, Zhang Y, Guan D, Xu Y. Aberrant regional homogeneity in Parkinson’s disease: A voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neurosci Biobehav Rev 2017, 72: 223–231.
Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 2007, 35: 222–233.
Zeng LL, Xie L, Shen H, Luo Z, Fang P, Hou Y, et al. Differentiating patients with Parkinson’s disease from normal controls using gray matter in the cerebellum. Cerebellum 2017, 16: 151–157.
Duchesne C, Gheysen F, Bore A, Albouy G, Nadeau A, Robillard ME, et al. Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals. Neuroimage Clin 2016, 12: 559–569.
Acknowledgements
This work was supported by the 13th Five-year Plan for National Key Research and Development Program of China (2016YFC1306600), the 12th Five-year Plan for National Science and Technology Supporting Program of China (2012BAI10B04), and the National Natural Science Foundation of China (81571654, 81371519, and 81301190). We acknowledge the Department of Radiology, Beijing Hospital, for sharing their clinical database for evaluation for this study. We thank all healthy controls and patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, Q., Guan, X., Law Yan Lun, J.C.F. et al. Longitudinal Alterations of Local Spontaneous Brain Activity in Parkinson’s Disease. Neurosci. Bull. 33, 501–509 (2017). https://doi.org/10.1007/s12264-017-0171-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0171-9