Abstract
Emerging evidence indicates that CXCL12/CXCR4 signaling is involved in chronic pain. However, few studies have systemically assessed its role in direct nerve injury-induced neuropathic pain and the underlying mechanism. Here, we determined that spared nerve injury (SNI) increased the expression of CXCL12 and its cognate receptor CXCR4 in lumbar 5 dorsal root ganglia (DRG) neurons and satellite glial cells. SNI also induced long-lasting upregulation of CXCL12 and CXCR4 in the ipsilateral L4–5 spinal cord dorsal horn, characterized by CXCL12 expression in neurons and microglia, and CXCR4 expression in neurons and astrocytes. Moreover, SNI-induced a sustained increase in TNF-α expression in the DRG and spinal cord. Intraperitoneal injection (i.p.) of the TNF-α synthesis inhibitor thalidomide reduced the SNI-induced mechanical hypersensitivity and inhibited the expression of CXCL12 in the DRG and spinal cord. Intrathecal injection (i.t.) of the CXCR4 antagonist AMD3100, both 30 min before and 7 days after SNI, reduced the behavioral signs of allodynia. Rats given an i.t. or i.p. bolus of AMD3100 on day 8 of SNI exhibited attenuated abnormal pain behaviors. The neuropathic pain established following SNI was also impaired by i.t. administration of a CXCL12-neutralizing antibody. Moreover, repetitive i.t. AMD3100 administration prevented the activation of ERK in the spinal cord. The mechanical hypersensitivity induced in naïve rats by i.t. CXCL12 was alleviated by pretreatment with the MEK inhibitor PD98059. Collectively, our results revealed that TNF-α might mediate the upregulation of CXCL12 in the DRG and spinal cord following SNI, and that CXCL12/CXCR4 signaling via ERK activation contributes to the development and maintenance of neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve injury and inflammation often result in neuropathic pain, which has been described as ‘the most terrible of all tortures’ [1]. However, pharmacotherapy for this problem has achieved limited success, and commonly-used pain-reducing drugs have resulted in little or no response. Consequently, there is a considerable need to explore novel treatment modalities [1]. Emerging evidence suggests that the peripheral and central neuroinflammation associated with the chemokine–cytokine network following nerve damage plays pivotal roles in the pathogenesis of neuropathic pain [2, 3]. Thus, further investigation of the functions of the chemokine–cytokine network-mediated regulation of neuroinflammation might lead to novel therapeutic strategies [4].
Chemokines are a family of small (8–14 kD), functionally-related, secreted molecules. The term “chemokine” was derived from their leukocyte chemoattractant and cytokine-like activities. Chemokines have been classified into CXC, CC, C, and CX3C subfamilies based on the arrangement of the two cysteine residues near the N-terminus [5]. In the last decade, numerous studies have demonstrated that, in addition to their role in coordinating the immune response, chemokines have important functions in the nervous system [6]. For example, excitatory chemokine signaling has been implicated in the pathogenesis of chronic pain in several animal models [7]. Stromal cell-derived factor-1α (SDF-1α), now also named CXCL12, belongs to the CXC subfamily and is a ligand for the G-protein-coupled receptor CXCR4. The binding of CXCL12 to CXCR4 activates multiple signaling pathways, including the extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase-Akt, cAMP/cAMP-dependent protein kinase, and phospholipase C pathways, and results in increased intracellular calcium levels and the release of cytokines by glial cells [8–10]. Increasing evidence suggests that CXCL12/CXCR4 play important roles in nociceptive signal processing [11–14]. They are widely distributed and constitutively expressed in small amounts in small- and medium-sized dorsal root ganglia (DRG) neurons and glia (satellite cells) and in the spinal cord [15, 16]. The administration of antiretroviral drugs or unilateral chronic constriction injury of the sciatic nerve leads to the development of neuropathic pain, which is correlated with the upregulation of both CXCL12 and CXCR4 in the DRG [17, 18]. Intrathecal (i.t.) injection of AMD3100, a specific antagonist of CXCR4, or CXCL12-neutralizing antibodies ameliorates established bone cancer pain and prevents its development [12]. Furthermore, sustained mechanical allodynia occurs after a single intraplantar or i.t. injection of CXCL12 in naïve rats [15]. These findings imply that CXCL12 functions as a neuromodulator of both physiological and pathological pain.
Although previous studies have revealed a role of CXCL12/CXCR4 signaling in the genesis of chronic pain, the time-course, the cell types involved, their expression in the DRG and spinal cord, and the contribution of this signaling to the development and maintenance of neuropathic pain following direct peripheral nerve injury remain largely unknown. It is known that cytokines such as tumor necrosis factor-alpha (TNF-α) regulate chemokine release from glial cells in the spinal cord following lumbar 5 spinal nerve ligation (L5 SNL) [19] and that chemokines such as CXCL12 promote TNF-α secretion from cultured astrocytes [9]. Thus, in the present study, the time-course of CXCL12/CXCR4 expression, its cellular localization in the DRG and spinal cord, and the role of TNF-α in the regulation of CXCL12 expression were examined following spared nerve injury (SNI) in rats. The potential roles of CXCL12/CXCR4 signaling in the development and maintenance of neuropathic pain were also evaluated via i.t. and systemic administration of AMD3100 and CXCL12-neutralizing antibodies. The possible role of the CXCL12/CXCR4 signaling pathway in neuropathic pain was further investigated by examining spinal ERK activation.
Materials and Methods
Animal Preparation
Male Sprague–Dawley rats weighing 200–300 g were housed in separate cages with free access to food and water. The room temperature was maintained at 23 ± 2 °C under the natural light–dark cycle. All experimental procedures were approved by the Animal Care and Use Committee of Zhengzhou University and were carried out in accordance with the National Institutes of Health guidelines on animal care and the ethical guidelines for the experimental investigation of pain in conscious animals [20].
Spared Nerve Injury
SNI followed the procedures described by Decosterd and Woolf [21]. The rats were anesthetized with 2%–3% ethyl ether. An incision was made on the lateral surface of the mid-thigh and the biceps femoris muscle was blunt-dissected to expose the left sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerves. The common peroneal and the tibial nerves were ligated with 5-0 silk, transected distal to the ligation, and a 2–3 mm length of each nerve was removed. Great care was taken to avoid any contact with or stretching of the intact sural nerve. In the sham-operated group, all procedures were identical to those in the experimental group, except that the nerves were not ligated and transected. The wound was closed by suturing the muscle and skin layers with 4-0 silk. This model developed relatively stable behavioral signs of mechanical hypersensitivity.
Drugs and Delivery
The CXCR4-specific antagonist AMD3100 was from Sigma (St. Louis, MO) and dissolved in normal sterile saline daily prior to use. Anti-CXCL12 neutralizing antibody and anti-IgG antibody (for control) were from Abcam (Cambridge, MA) and diluted in sterile artificial cerebrospinal fluid (ACSF) containing (in mmol/L) 126.6 NaCl, 2.5 KCl, 2.0 MgCl2, and 1.3 CaCl2. Thalidomide (an inhibitor of TNF-α synthesis) and PD98059 (a specific MEK (ERK kinase) inhibitor) were from Sigma, and both were dissolved in sterile saline containing 10% DMSO. CXCL12 was from R&D Systems Inc. (Minneapolis, MN) and was diluted in ACSF. The concentrations of AMD3100, anti-CXCL12 neutralizing antibody, CXCL12 [12, 15], thalidomide [22], and PD98059 [23] used were based on previous studies.
Drugs were delivered i.t. or intraperitoneally (i.p.). Implantation of the i.t. catheter followed the procedures described by Storkson et al. [24] with modifications. After the animal was anesthetized with ethyl ether, a midline skin incision was made in the lumbar region and the intervertebral membrane between L5 and S1 was exposed. A needle was then used to puncture the membrane, and a polyethylene (PE-10) tube was inserted rostrally into the subarachnoid space to reach the lumbar enlargement. The tube was fixed to muscle, and the other end was tunneled rostrally underneath the skin to exit in the occipital region. The incisions were then closed in layers using 4-0 silk. The rats were allowed to recover for 7 days before behavioral testing or i.t. drug injection. The position of the catheter was checked postmortem. Animals that displayed any abnormal neurological signs were excluded from experiments.
Behavioral Test for Allodynia
Animals were habituated to the testing environment daily for at least two days before baseline testing. Before starting the test, animals were placed in boxes on an elevated metal mesh floor and allowed 30 min for habituation. The room temperature and humidity remained stable in all experiments. Mechanical allodynia was assessed with von Frey hairs using the up-and-down method [25] following the procedure described by Chaplan et al. [26]. Briefly, a series of von Frey hairs with logarithmically incremental stiffness (0.41, 0.70, 1.20, 2.04, 3.63, 5.50, 8.51, and 15.14 g) were applied to the plantar surface of both hind paws. The 2.04 g stimulus was applied first. In the event of no paw withdrawal, the next stronger stimulus was used. If the paw was withdrawn, a weaker stimulus was applied. By repeating this procedure, the stimulus strength that corresponded to a 50% response rate was determined by interpolation. All behavioral tests were performed by an investigator who was blind to the experimental group.
Immunohistochemistry
Immunohistochemistry was done following a method described previously [27]. Briefly, after defined survival times, control and nerve-injured rats were deeply anesthetized with ethyl ether and perfused through the ascending aorta with normal saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. After perfusion, the L4–5 spinal segments and L5 DRGs were removed and postfixed for 3 h in the same fixative, and then replaced by 30% sucrose in PBS over two nights. Transverse spinal sections (25 μm) and DRG sections (20 μm) were cut on a cryostat and prepared for immunofluorescence staining. Sections were randomly selected and placed into different wells of 24-well plates. After washing with PBS, the sections were blocked with 5% goat serum in 0.3% Triton X-100 for 1 h at 37 °C, and incubated with primary antibody overnight at 4 °C. For double immunofluorescence staining, all the sections (except for IB4-treated DRG sections, which were only incubated with Cy3-conjugated secondary antibody) were incubated with a mixture of goat anti-mouse FITC- (1: 200, Jackson ImmunoResearch, Amish, PA) and goat anti-rabbit Cy3-conjugated secondary antibody (1:400, Jackson ImmunoResearch) for 1 h at 37 °C. The stained sections were mounted on slides, examined under a fluorescence microscope (Olympus IX71, Olympus Optical, Tokyo, Japan), and images were captured with a CCD spot camera. The following primary antibodies were used: rabbit anti-CXCL12 (1:100; Abcam ab25117, Cambridge, MA), rabbit anti-CXCL12 (1:100; Santa Cruz, Dallas, TX), and rabbit anti-CXCR4 (1:50, Santa Cruz). The following cell-specific markers were used: neurofilament-200 (NF-200, a marker for myelinated A-fibers, 1:200, Chemicon, Billerica, MA), FITC-conjugated isolectin B4 (IB4, a marker for unmyelinated non-peptidergic C-fibers, 20 μg/mL, Sigma, St. Louis, MO), substance P (a marker for unmyelinated peptidergic C-fibers, 1:100; Santa Cruz), glial fibrillary acidic protein (GFAP, a marker for astrocytes, 1:200; Chemicon), monoclonal neuronal-specific nuclear protein (NeuN, a neuronal marker, 1:500; Chemicon), and OX42 (CD11b, a microglial marker, 1:200; Chemicon).
To test the specificity of the anti-CXCL12 and anti-CXCR4 antibodies, we used a pre-incubation method [28, 29]. The anti-CXCL12 antibody was pre-incubated with 10 μg/mL CXCL12 (5-fold that of the anti-CXCL12 antibody) at 4 °C for 1 h, and then the pre-incubated antibody was applied to spinal cord sections to detect the immunoreactivity (IR) of CXCL12 by immunohistochemistry. The results revealed that CXCL12-IR in the sections in which the pre-incubated antibody was used was significantly lower than that in the sections in which no pre-incubation was used. To test the specificity of the anti-CXCR4 antibody, spinal sections that had been blocked in 5% goat serum were pre-incubated with CXCL12 for 1 h at room temperature. The anti-CXCR4 antibody was then directly loaded onto the sections and immunohistochemistry was used to assess CXCR4-IR. The results revealed that the CXCR4-IR in sections pre-incubated with CXCL12 was lower than that in the sections without pre-incubation (data not shown). Besides the anti-CXCL12 antibody supplied by Santa Cruz, another antibody to CXCL12 from Abcam (ab25117) was also used to further confirm the specificity of CXCL12.
To quantify the immunofluorescence staining in the DRG, the numbers of CXCL12-IR- and CXCR4-IR-positive cells per section were counted. In each rat, 4–6 sections of the L5 DRG at each time-point were selected randomly. The average percentages of CXCL12-IR- and CXCR4-IR-postive cells relative to the total number of cells were obtained for each animal across the different sections, and are presented as mean ± SEM [27, 29].
Western Blotting
Western blotting was performed according to our published procedures [30, 31]. Briefly, the animals were sacrificed by decapitation and the L4–5 spinal dorsal horns were harvested and placed temporarily in liquid nitrogen. Then the samples were homogenized in ice-cold lysis buffer (10 mmol/L Tris, 5 mmol/L EGTA, 0.5% Triton X-100, 2 mmol/L benzamidine, 0.1 mmol/L PMSF, 40 µmol/L leupeptin, 150 mmol/L NaCl, 1% phosphatase inhibitor cocktail 2 and 3). The crude homogenates were centrifuged at 4 °C for 15 min at 3 000 rpm, and the supernatants were collected. After the protein concentrations were determined, the samples were heated for 5 min at 99 °C, and 30–60 μg protein was loaded onto 12.5% SDS–polyacrylamide gels, then electrophoretically transferred onto PVDF membranes. The membranes were blocked with 3% non-fat milk for 1 h and incubated overnight at 4 °C with primary antibody. The following primary antibodies were used: rabbit anti-CXCL12 (1:500; Abcam ab25117), rabbit anti-CXCR4 (1:200; Santa Cruz), rabbit anti-p-ERK1/2 (1:1000; Cell Signaling, Danvers, MA), and mouse anti-β-actin (1:10 000; Sigma). The proteins were detected with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Bio-Rad, 1:3000, Philadephia, PA), visualized using the chemiluminescence reagents provided with the ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ), and exposed to film. The intensities of the blots were quantified by densitometry. The blot density of the control rats was set at 100%. The relative density values of the other groups were determined by dividing the optical density values of these groups by that of the control rats.
Enzyme-Linked Immunoassay (ELISA)
The rat TNF-α kit was from R&D Systems, Inc. (Minneapolis, MN). Protein sample preparation was as for western blotting. Protein concentrations were measured by the Bradford method, and proteins were loaded into 96-well plates (100 μg/well). ELISA was performed according to the manufacturer’s protocol. The standard curve was included in each experiment.
Statistical Analyses
Statistical analyses were performed with SPSS 10.0 (SPSS Inc., Chicago, IL) and SigmaStat (Systat, San Jose, CA). All data are presented as mean ± SEM. Differences over time were tested using one-way ANOVA followed by individual post hoc comparisons (Tukey’s post hoc test) or Student’s t-test if only two groups were used. For behavioral tests, nonparametric tests were used for comparisons between testing days and surgical groups, as variations existed between different rats. The data between testing days were analyzed with Friedman’s ANOVA for repeated measures, followed by the Wilcoxon matched pairs test when appropriate. The data between groups on a given testing day were analyzed with the Mann–Whitney U-test. P < 0.05 was considered to be statistically significant.
Results
SNI Increases CXCL12 and CXCR4 in the DRG
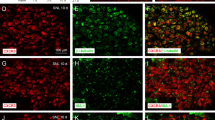
SNI induced a substantial increase in CXCL12-IR in the ipsilateral L5 DRG compared to the sham group (Fig. 1A–C). This increase was detectable on day 1 after surgery and reached a peak on day 3. This change remained significant for at least 14 days and returned to baseline by day 21 (Fig. 1C). Double immunofluorescence staining with NF-200 (A-type neurons), IB4 (C-type non-peptidergic neurons), substance P (C-type peptidergic neurons), and GFAP (satellite glial cells) revealed that the increase in CXCL12-IR occurred in neurons (A-type and C-type) and satellite glial cells (Fig. 1D–O).
Spared nerve injury increases CXCL12 immunoreactivity in the DRG. A, B Representative images showing changes in the CXCL12-IR in the ipsilateral L5 DRG on day 3 after SNI and sham-operation. C Quantification of CXCL12-positive neurons in the L5 DRG (*P < 0.05; **P < 0.01; ***P < 0.001 vs sham group, one-way ANOVA followed by Tukey’s post hoc test). D–O Double immunofluorescence staining of ipsilateral L5 DRGs for CXCL12 (red; E, H, K, and N) and NF-200, a marker of A-type neurons (green, D); IB4, a marker of C-type non-peptidergic neurons (green, G); substance P (SP), a marker of C-type peptidergic neurons (green, J); and GFAP, a marker of satellite glial cells (green, M). The pairs of images are merged in F, I, L, and O. The results indicated co-localization of CXCL12 with NF-200, IB4, SP, and GFAP in L5 DRGs 3 days after SNI. Scale bars for A and B, 100 μm; for D–O, 50 μm.
In addition, significant increases in the percentages of CXCR4-IR cells were found in the L5 DRG on days 1, 3, 7, and 14 after SNI (Fig. 2A–C). Double immunofluorescence staining for CXCR4 and NF-200, IB4, substance P, or GFAP revealed that CXCR4 was expressed in DRG neurons and satellite glial cells (Fig. 2D–O).
Spared nerve injury increases CXCR4 immunoreactivity in the DRG. A, B Representative images showing the change in CXCR4-IR in the ipsilateral L5 DRG on day 7 after SNI and sham-operation. C Quantification of CXCR4-positive neurons in the L5 DRG (*P < 0.05; **P < 0.01 vs sham group, one-way ANOVA followed by Tukey’s post hoc test). Scale bar for A and B, 100 μm. D–O Double immunofluorescence staining in ipsilateral L5 DRGs between CXCR4 (red; E, H, K, and N), and NF-200, A-type neuronal marker (green, D); IB4, C-type non-peptidergic neuronal marker (green, G); substance P (SP), C-type peptidergic neuronal marker (green, J); and GFAP, satellite glial cell marker (green, M), 7 days after SNI. The pairs of images are merged in F, I, L, and O. The results showed that CXCR4 co-localized with neurons (F, I, and L) and satellite glial cells (O). Scale bar for D–O, 50 μm.
CXCL12 and CXCR4 in the contralateral L5 DRG were not affected by SNI (data not shown).
SNI Increases CXCL12 and CXCR4 Expression in the Spinal Dorsal Horn
As the location of the second-order neurons of pain-related message transmission, the spinal cord plays a pivotal role in the generation and maintenance of neuropathic pain. Thus, the expression of CXCL12 and CXCR4 in the dorsal horn was determined following SNI in rats. Our results revealed that SNI induced a clear increase in CXCL12-IR (Fig. 3A, B) particularly in the ipsilateral dorsal horn (Fig. 3A). Western blotting revealed that, compared to the sham group, the CXCL12 protein level increased on day 1, peaked on day 3, and remained significantly elevated until day 14 after SNI (Fig. 3B). Double immunofluorescence staining revealed that the upregulated CXCL12 primarily co-localized with the neuronal marker NeuN (Fig. 3C–E) and the microglial marker OX42 (Fig. 3I–K), but not with the astrocytic marker GFAP (Fig. 3F–H).
Upregulation of CXCL12 immunoreactivity in ipsilateral dorsal horn after SNI. A Representative image showing the difference between CXCL12-IR in the ipsilateral and contralateral spinal cord at L5 on day 7 after SNI in rat. B Histogram of the mean ± SEM of CXCL12 in the ipsilateral dorsal horn from western blots at different time points after SNI and sham surgery (*P < 0.05, **P < 0.01, ***P < 0.001 vs sham group, one-way ANOVA followed by Tukey’s post hoc test). Scale bar, 200 μm. C–K Images of double immunofluorescence staining in L5 ipsilateral dorsal horn between CXCL12 (red; D, G, and J) and NeuN, a neuronal marker (green, C); GFAP, an astrocytic marker (green, F); and OX42, a microglial marker (green, I). The pairs of images are merged in E, H, and K. The results showed that CXCL12 co-localized with neurons (E) and microglia (K) 7 days after SNI. Scale bar, 50 μm.
Similarly, SNI significantly increased the CXCR4 level in the ipsilateral dorsal horn (Fig. 4A). Western blotting revealed that, compared to the sham group, CXCR4-IR was increased on day 3, peaked on days 7 and 14, and remained significantly elevated until day 21 after SNI. Double immunofluorescence staining for CXCR4 and NeuN, GFAP, or OX42 revealed that the upregulated CXCR4-IR was primarily localized to neurons and astrocytes but not microglia (Fig. 4C–K).
Upregulation of CXCR4 immunoreactivity in the ipsilateral dorsal horn after SNI. A Representative image of the distribution of CXCR4-IR in the spinal cord on day 7 after SNI. B Western blot data for CXCR4 in the ipsilateral L4–5 dorsal horn at different time points after SNI and sham surgery (*P < 0.05, **P < 0.01, ***P < 0.001 vs sham group, one-way ANOVA followed by Tukey’s post hoc test). Scale bar, 200 μm. C–K Images of double immunofluorescence staining of the ipsilateral L4–5 dorsal horn for CXCR4 (red; D, G, and J) with NeuN, a neuronal marker (green, C); GFAP, an astrocytic marker (green, F); and OX42, a microglial marker (green, I). The pairs of images are merged in E, H, and K. The results revealed that CXCR4 co-localized with neurons (E) and astrocytes (H) 7 days after SNI. Scale bar, 50 μm.
Role of TNF-α in Upregulation of CXCL12 in the Lumbar DRG and Spinal Cord During the Development of Neuropathic Pain Following SNI
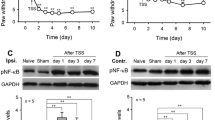
Recent studies have shown that TNF-α regulates CXCL1 release in the spinal cord following SNL at L5 [19]. Here, we found that SNI induced an increase in the expression of TNF-α in the DRG and spinal cord compared to the sham group. The increases began on day 1, peaked on day 3, and persisted for more than 2 weeks after surgery. Pretreatment with the inhibitor of TNF-α synthesis thalidomide (50 mg/kg, i.p.) and once daily for 7 days prevented the development of SNI-induced neuropathic pain. Compared to the SNI + vehicle group, thalidomide prevented the reduction in paw withdrawal threshold (PWT) from days 1 to 10 (Fig. 5B) after SNI. Western blot data revealed that thalidomide treatment for 7 days inhibited the expression of CXCL12 in the DRG and the spinal cord at L4–5 following SNI (Fig. 5C).
Role of TNF-α in upregulation of CXCL12 in DRG and spinal cord during the development of SNI-induced neuropathic pain. A TNF-α expression in the DRG and spinal cord at L4-5 following SNI (*P < 0.05, **P < 0.01, ***P < 0.001, # P < 0.05, ## P < 0.01 vs corresponding sham, one-way ANOVA followed by Tukey’s post hoc test). B Intraperitoneal injection of thalidomide (50 mg/kg) 2 h prior to SNI and once daily thereafter until day 7 after surgery attenuated the mechanical allodynia (*P < 0.05 vs SNI + vehicle group at corresponding time points; # P < 0.05; ## P < 0.01 vs preoperative baseline or corresponding time point of the sham group, Friedman’s ANOVA followed by Wilcoxon matched pairs test). C Intraperitoneal injection of thalidomide (50 mg/kg) 2 h before SNI and once daily thereafter until day 7 after surgery inhibited the expression of CXCL12 in the DRG and spinal cord at L4–5 (**P < 0.01, ***P < 0.001 vs SNI + vehicle group, Student’s t-test). Veh, vehicle (saline containing 10% DMSO); Thali, thalidomide; SC, spinal cord.
Effects of AMD3100 on SNI-Induced Neuropathic Pain
To evaluate the role of upregulation of CXCL12 and CXCR4 in the DRG and spinal cord in the development of neuropathic pain following SNI in rats, AMD3100, a specific antagonist of CXCR4, was injected i.t. at different doses, beginning 0.5 h before SNI and daily thereafter until day 7 after surgery. SNI significantly decreased the PWT in the ipsilateral but not the contralateral hind paw on day 1 after SNI, and this persisted for more than 3 weeks after surgery (Fig. 6A, B). Compared to the SNI + i.t. saline group, AMD3100 partially prevented the SNI-induced reduction in PWT in a dose- and time-dependent manner (Fig. 6A). The significant differences between the SNI + AMD3100 and the SNI + saline groups were maintained until day 12, i.e., 5 days after cessation of AMD3100 administration (Fig. 6A). Injection of AMD3100 at a dose of 20 μg/day i.t. for 7 days had no effect on the basal PWT of naïve rats (data not shown).
AMD3100, a specific antagonist of CXCR4, reduces the mechanical allodynia induced by SNI. A Pretreatment with intrathecal injections (i.t.) of AMD3100 30 min before SNI and once daily thereafter until day 7 after surgery prevented the reduction in paw-withdrawal threshold in the ipsilateral hind paw in a dose- and time-dependent manner. *P < 0.05, **P < 0.01 vs SNI + saline group at corresponding time points (Mann–Whitney U-test); ## P < 0.01, ### P < 0.001 vs preoperative baseline (Friedman’s ANOVA for repeated measures, followed by Wilcoxon matched pairs test) or the sham group at corresponding time points (Mann–Whitney U-test). B Administration of AMD3100 i.t. beginning on day 7 after SNI and continuing once daily thereafter for 5 days partially ameliorated the established abnormal pain. *P < 0.05, **P < 0.01 vs SNI + saline group at corresponding time points (Mann–Whitney U-test); ## P < 0.01, ### P < 0.001 vs preoperative baseline (Friedman’s ANOVA for repeated measures, followed by Wilcoxon matched pairs test) or the sham group at corresponding time points (Mann–Whitney U-test).
To investigate the role of the SNI-induced upregulation of CXCL12 and CXCR4 in established neuropathic pain, the rats received daily AMD3100 (20 μg/day i.t.) for 5 days, beginning on day 7 after SNI, at which time the SNI-induced mechanical allodynia had fully developed. The behavioral results revealed that AMD3100 partially reversed the PWT reduction in SNI rats. Compared to the SNI + saline group, AMD3100 significantly increased the PWT throughout the treatment period (Fig. 6B). SNI treatment did not affect the PWT of the contralateral hind paw (data not shown).
To further confirm the above results, a single i.t. injection of CXCL12-neutralizing antibody or AMD3100 was administered to the SNI rats on day 8. Compared to the rats that received control i.t. IgG injection, the bolus of CXCL12-neutralizing antibody increased the PWT in a dose- and time-dependent manner (Fig. 7A). The increase began 1 h after injection, reached a peak at 2 h, and returned to the control level 4 h after treatment (Fig. 7A). Similar results were obtained when the rats received a single dose of AMD3100 (20 μg i.t.) on day 8 after SNI. Compared to the rats given i.t. saline, those receiving AMD3100 displayed an increased PWT at 1 h after treatment. This effect peaked at 4 h, and persisted until 6 h after the i.t. administration (Fig. 7B). Western blots showed that i.t. injection of AMD3100 (20 μg) on day 8 after SNI significantly inhibited the activation of ERK in the dorsal horn at 2 h after treatment, compared to the group receiving i.t. saline (Fig. 7D).
Single dose of AMD3100 or CXCL12-neutralizing antibody transiently and partially ameliorates SNI-induced mechanical allodynia. A An i.t. bolus of CXCL12-neutralizing antibody on day 8 alleviated the SNI-induced allodynia behavior. **P < 0.01, ***P < 0.001 vs control i.t. IgG group, Mann–Whitney U-test; ## P < 0.01 vs preoperative baseline (BL), Mann–Whitney U-test. B An i.t. bolus of AMD3100 on day 8 attenuated the SNI-induced mechanical allodynia. *P < 0.05; **P < 0.01 vs i.t. saline group, Mann–Whitney U-test; ## P < 0.01 vs preoperative baseline, Mann–Whitney U-test. C A single intraperitoneal (i.p.) injection of AMD3100 (5 mg/kg) on day 8 inhibited the SNI-induced reduction of PWT. *P < 0.05; **P < 0.01 vs i.p. saline group, Mann–Whitney U-test; ## P < 0.01 vs preoperative baseline, Mann–Whitney U-test. D An i.t. bolus of AMD3100 on day 8 after SNI inhibited the activation of ERK in spinal cord (*P < 0.05 vs SNI + i.t. saline group, Student’s t-test). Sa: saline.
These results indicated that i.t. injection of either AMD3100 or the CXCL12-neutralizing antibody partially ameliorated the SNI-induced reduction in PWT. To further determine the effects of systemic AMD3100 on established neuropathic pain following SNI, 5 rats received a single i.p. dose of AMD3100 (5 mg/kg) on day 8 after SNI, while control rats received an i.p. injection of saline. Behavioral tests revealed that the AMD3100 significantly increased the PWT at 1 h after administration and this effect peaked at 2 h. A significant difference between the AMD3100 and saline groups was maintained until 6 h after treatment (Fig. 7C).
CXCL12/CXCR4 Signaling-Mediated Neuropathic Pain Depends on ERK Activation in the Spinal Cord
Previous studies have shown that the binding of CXCL12 to CXCR4 activates multiple intracellular signaling pathways, including the ERK pathway [32], so we assessed ERK activation in the spinal cord following SNI. The level of p-ERK increased on day 1, peaked on days 3 and 7, and lasted for 3 weeks after SNI (Fig. 8A). Treatment with AMD3100 (20 μg/day i.t., once daily for 7 days) prevented this ERK activation. Compared to the SNI + saline group, rats that received i.t. AMD3100 displayed reduced levels of p-ERK (Fig. 8B) following SNI. AMD3100 (i.t.) alone had no effect on the basal level of p-ERK in naïve rats (Fig. 8B). In another experiment, the rats received a single dose of CXCL12 (1 μg, i.t.). This procedure resulted in allodynia in the hind paws, beginning at 0.5 h after treatment and lasted > 24 h (Fig. 8C). Pretreatment with PD98059 (10 μg i.t.), which is a potent inhibitor of MEK, followed by CXCL12 (1 μg i.t., 30 min after the PD98059 treatment) prevented the CXCL12-induced reduction in PWT. Compared to the vehicle group, the significant increase in the PWT of PD98059-pretreated rats began 2 h after the treatment and continued more than 6 h (Fig. 8C).
CXCL12/CXCR4 signaling-mediated SNI-induced neuropathic pain depends on ERK activation. A SNI increased the phosphorylated-ERK (p-ERK) level in the ipsilateral dorsal horn from days 1 to 21 after surgery (*P < 0.05, **P < 0.01, ***P < 0.001 vs sham group, one-way ANOVA followed by Tukey’s post hoc test). B AMD3100 injected 30 min before surgery and once daily thereafter for 7 days prevented the SNI-induced activation of ERK in the spinal cord (***P < 0.001 vs sham group, ## P < 0.01 vs SNI + saline group, one-way ANOVA followed by Tukey’s post hoc test). C Intrathecal (i.t.) injection of the MEK inhibitor PD98059 alleviated the mechanical allodynia induced by i.t. CXCL12 in naïve rats (*P < 0.05, **P < 0.01 vs vehicle + CXCL12 group at corresponding time points, Mann–Whitney U-test; # P < 0.05, ## P < 0.01 vs preoperative baseline, Friedman’s ANOVA for repeated measures, followed by Wilcoxon matched pairs test). Veh: vehicle (saline containing 10% DMSO).
Discussion
Here, we demonstrated the critical role of the upregulation of CXCL12/CXCR4 in peripheral nerve injury-induced neuropathic pain. Our results revealed that the SNI model had increased expression of CXCL12 and CXCR4 in the L4–5 DRG and dorsal horn. SNI increased the expression of TNF-α in the same areas. Inhibition of TNF-α synthesis reduced the expression of CXCL12 in the DRG and spinal cord. AMD3100 (i.t.) significantly reduced the mechanical allodynia. The established neuropathic pain following SNI was also attenuated by i.t. administration of a CXCL12-neutralizing antibody or i.p. injection of AMD3100. Further, AMD3100 prevented the activation of ERK in the spinal cord, and the mechanical allodynia induced by i.t. CXCL12 was alleviated by pretreatment with the specific inhibitor of MEK, PD98059. These results indicated that TNF-α mediates the upregulation of CXCL12 in the DRG and spinal cord following SNI, and that the CXCL12/CXCR4 signaling contributes to the SNI-induced neuropathic pain mediated at least partially by the activation of spinal ERK signaling.
SNI Upregulates CXCL12 and Its Cognate Receptor CXCR4 in the DRG and Spinal Cord
The CXCL12/CXCR4 pair has attracted much attention regarding its involvement in nociceptive signal processing. A recent study showed that CXCL12 and its cognate receptor CXCR4 are constitutively expressed in the DRG and spinal cord of naïve rats [15, 33]. In the DRG, CXCL12 and CXCR4 are widely distributed in small amounts in small- and medium-sized neurons and glial cells (satellite cells). Using IR, CXCL12 and CXCR4 have been detected in spinal neurons and astrocytes. Recent studies have shown that CXCL12 and CXCR4 expression is upregulated in the L4–5 DRG by unilateral chronic constriction injury of the rat sciatic nerve [17] and i.p. administration of the antiviral agent 2′,3′-dideoxycytidine [34]. But systemic studies of the temporal profiles of CXCL12 and CXCR4 expression following nerve injury were lacking. In the present study, we demonstrated that the increases in CXCL12-IR and CXCR4-IR in both the DRG and the spinal cord began on day 1 and persisted for ~3 weeks following SNI in rats. This long-lasting expression of CXCL12 and CXCR4 may be involved in the initiation and maintenance of neuropathic pain following SNI. Double immunofluorescence staining further demonstrated that the increased CXCL12 and CXCR4 were both distributed in DRG neurons and satellite cells. Unexpectedly, in the spinal cord, the SNI-induced CXCL12 was localized in neurons and microglia, while CXCR4 was co-localized in neurons and astrocytes. The expression patterns of CXCL12 and CXCR4 in the DRG are consistent with previous reports. However, the cellular localization of CXCL12 in the spinal cord following SNI differs from that reported in a recent study, in which CXCL12 expression was found mainly in astrocytes in rats with cancer pain [12]. This discrepancy might be due to the different pain models used and the different time points for measurement. Our results imply that autoregulation of CXCL12 secretion might occur during the pathogenesis of neuropathic pain in DRG neurons and glial cells and that neuron-glia communication via the binding of CXCL12 released by spinal neurons to the CXCR4 expressed by astrocytes might occur following SNI.
TNF-α Might Mediate the Increased CXCL12 Expression in the DRG and Spinal Cord Following SNI
Our results, together with those of previous studies using different pain models, have revealed that the expression of CXCL12 in the DRG and spinal cord increases during the pathogenesis of chronic pain [12, 14]. However, the underlying mechanism of the regulation of CXCL12 expression in neuropathic pain remains largely unknown. It has been reported that SNI results in a quickly-initiated and long-lasting increase in the expression of TNF-α in the spinal cord [22, 35]. Inhibition of TNF-α synthesis [36] and treatment with a TNF-α neutralizing antibody both attenuate the neuropathic pain induced by peripheral nerve injury [37]. The prevailing evidence shows that TNF-α stimulates IL-1β and IL-6 release from DRGs and the spinal cord following nerve injury [38, 39]. Further, the spinal nerve ligation-induced increase in the expression of CXCL1 in the L5 spinal cord is regulated by TNF-α [19] and i.t. administration of TNF-α induces the expression of monocyte chemoattractant protein-1 by spinal astrocytes [39]. Here, we found that SNI increased the expression of TNF-α in the DRG and spinal cord in a pattern similar to that previously reported [22]. Systemic administration (i.p.) of thalidomide, an inhibitor of TNF-α synthesis, prevented the development of SNI-induced neuropathic pain, and inhibited the increased expression of CXCL12 in the DRG and spinal cord following SNI. These findings indicated that this upregulation of CXCL12 might be regulated by TNF-α. This is consistent with a very recent report by Huang et al. that the i.p. administration of 2′,3′-dideoxycytidine (a nucleoside reverse transcriptase inhibitor) upregulates CXCL12 and CXCR4 in the lumbar DRG and spinal cord, and both are regulated by TNF-α [18]. Our previous study demonstrated that peripheral nerve injury upregulates TNF-α in the DRG and spinal neurons and astrocytes, while increased TNFR1 (one of the receptors for TNF-α) is primarily localized to neurons [29]. In the present study, the SNI-induced increase in CXCL12 was localized to spinal neurons. These results together indicate that the TNF-α released from neurons or glial cells following SNI might stimulate the release of CXCL12 by binding to TNFR1-expressing neurons in the DRG and spinal cord, thus creating a cytokine-chemokine interaction based on glia-neuron communication.
Upregulation of CXCL12 and CXCR4 in the DRG and Spinal Cord is Involved in the Initiation and Maintenance of Neuropathic Pain Following SNI
A growing number of studies have reported the effects of CXCL12/CXCR4 signaling on the pathogenesis of chronic pain [10, 12, 34]. However, few experiments have systemically assessed their roles in direct peripheral nerve injury-induced neuropathic pain. Because the dural membrane in rats extends onto the capsule of the DRG so that the proximal face of the DRG is in direct continuity with the subarachnoid space, i.t. injection of molecules affects cells in the DRG as well as those in the spinal cord [40]. Here, pretreatment with i.t. AMD3100 (beginning 30 min before SNI and continuing once daily thereafter for 7 days) prevented the genesis of SNI-induced mechanical allodynia in a dose- and time-dependent manner. Furthermore, established abnormal pain was markedly attenuated by both repetitive and single-bolus i.t. injection of AMD3100 on days 7 and 8 after SNI. To exclude possible nonspecific effects of AMD3100, a single i.t. dose of a CXCL12-neutralizing antibody was given on day 8 after SNI. This procedure also alleviated the established mechanical hypersensitivity. As most drugs are administered systemically in the clinic, we conducted an additional experiment in which five rats were treated with i.p. AMD3100 on day 8 after SNI. The behavioral data revealed that the established neuropathic pain was partially ameliorated and that this effect persisted for >6 h. These behavioral experiments clearly demonstrated that the upregulation of CXCL12 and CXCR4 in the DRG and spinal cord plays a critical role in SNI-induced neuropathic pain. Very recently, Luo et al. found that a single i.t. dose of AMD3100 delays the development of neuropathic pain and ameliorates established neuropathic pain in mice, and the effect persists for >3 days [41]. Reaux-Le et al. reported that a single i.t. dose of CXCL12 (1 μg) induces >3 days of mechanical allodynia in rats and that this neuropathic pain-like behavior is attenuated by i.t. administration of CXCL12-neutralizing antibody [15]. Shen et al. also reported that a single i.t. dose of a CXCL12-neutralizing antibody (10 μg/10 μL) on day 10 after tumor cell implantation (TCI), transiently and dose-dependently reverses bone cancer pain. Moreover, repetitive i.t. administration of a CXCL12-neutralizing antibody (10 μg/10 μL, once daily from days 3 to 5 after TCI) significantly delays the onset of TCI-induced pain behaviors by nearly five days [12]. The results of our study are consistent with these findings.
Intracellular Signaling Pathway of CXCL12-Mediated Neuropathic Pain Following SNI
CXCL12 has two receptors, the G-protein-coupled transmembrane receptor CXCR4 and CXCR7 [42]. CXCR4 appears to play a neuromodulatory role in the central nervous system [43], and CXCR7 is involved in the regulation of interneuron migration [44]. The present work and numerous previous studies have demonstrated that the CXCL12/CXCR4 pair plays an important role in chronic pain, but little is known about the intracellular signaling pathway of CXCR4-mediated neuropathic pain. Previous studies have shown that the binding of CXCL12 to CXCR4 activates several signaling pathways, including the ERK pathway [5, 45]. It is well known that ERK activation plays a pivotal role in the initiation and maintenance of neuropathic pain [23, 46, 47]. In the present study, SNI induced early-onset and long-lasting (>3 weeks) ERK activation in the spinal cord. However, this increased activation was dramatically inhibited by repeated i.t. administration of AMD3100 over 7 days and a single i.t. bolus of AMD3100 on day 8 after SNI. Furthermore, the neuropathic pain-like allodynia induced in naïve rats by i.t. CXCL12 was also attenuated by pretreatment with an i.t. bolus of PD98059, a specific inhibitor of MEK. These findings indicate that ERK activation is involved in CXCL12/CXCR4 signaling-mediated neuropathic pain. In a primary astrocyte culture model, Han et al. showed that CXCL12 stimulation triggers the activation of NF-κB and ERK, and that both of these contribute to the secretion of TNF-α [9]. In cultured hippocampal neurons, CXCL12-induced GAD67 (a key enzyme in GABA synthesis) expression is mediated by the G-protein-coupled CXCR4 and activation of the ERK pathway [32]. These results imply that ERK might be an important signal in CXCR4-mediated neuromodulation, and our present results provide evidence that spinal ERK activation contributes to CXCL12/CXCR4-mediated neuropathic pain.
In summary, we have demonstrated important roles of CXCL12/CXCR4 signaling in the development and maintenance of neuropathic pain. The increased expression of TNF-α following peripheral nerve injury might contribute to the upregulation of CXCL12 in the lumbar DRG and spinal cord, and CXCL12 might mediate neuron-glia communication in the spinal cord that results in central sensitization via activation of the ERK signaling pathway. Therefore, the CXCL12/CXCR4 signaling pathway might be a useful therapeutic target for the treatment of neuropathic pain.
References
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009, 32: 1–32.
Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014, 13: 533–548.
Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol 2012, 12: 55–61.
Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014, 14: 217–231.
Bonavia R, Bajetto A, Barbero S, Pirani P, Florio T, Schettini G. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol Lett 2003, 139: 181–189.
Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther 2010, 126: 56–68.
White FA, Wilson NM. Chemokines as pain mediators and modulators. Curr Opin Anaesthesiol 2008, 21: 580–585.
Floridi F, Trettel F, Di BS, Ciotti MT, Limatola C. Signalling pathways involved in the chemotactic activity of CXCL12 in cultured rat cerebellar neurons and CHP100 neuroepithelioma cells. J Neuroimmunol 2003, 135: 38–46.
Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest 2001, 108: 425–435.
Luo X, Tai WL, Sun L, Qiu Q, Xia Z, Chung SK, et al. Central administration of C-x-C chemokine receptor type 4 antagonist alleviates the development and maintenance of peripheral neuropathic pain in mice. PLoS One 2014, 9: e104860.
Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun 2007, 21: 581–591.
Shen W, Hu XM, Liu YN, Han Y, Chen LP, Wang CC, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014, 11: 75.
Wilson NM, Jung H, Ripsch MS, Miller RJ, White FA. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun 2011, 25: 565–573.
Menichella DM, Abdelhak B, Ren D, Shum A, Frietag C, Miller RJ. CXCR4 chemokine receptor signaling mediates pain in diabetic neuropathy. Mol Pain 2014, 10: 42.
Reaux-Le GA, Rivat C, Kitabgi P, Pohl M, Melik PS. Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci 2012, 36: 2619–2631.
Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol Pain 2009, 5: 48.
Dubovy P, Klusakova I, Svizenska I, Brazda V. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol 2010, 133: 323–337.
Huang W, Zheng W, Ouyang H, Yi H, Liu S, Zeng W, et al. Mechanical allodynia induced by nucleoside reverse transcriptase inhibitor is suppressed by p55TNFSR mediated by Herpes simplex virus vector through the SDF1 alpha/CXCR4 system in rats. Anesth Analg 2014, 118: 671–680.
Zhang ZJ, Cao DL, Zhang X, Ji RR, Gao YJ. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013, 154: 2185–2197.
Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand Suppl 1986, 554: 221–233.
Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000, 87: 149–158.
Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacol 2011, 36: 979–992.
Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114: 149–159.
Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods 1996, 65: 167–172.
Dixon WJ: Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 1991, 15: 47–50.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994, 53: 55–63.
Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36: 57–68.
Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 2003, 17: 791–804.
Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain 2006, 123: 306–321.
Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 2014, 124: 592–603.
Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, et al. Toll-like receptor 4-mediated nuclear factor-κB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 2014, 30: 936–948.
Luo Y, Lathia J, Mughal M, Mattson MP. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem 2008, 283: 24789–24800.
Knerlich-Lukoschus F, von dR-B, Lucius R, Mehdorn HM, Held-Feindt J. Spatiotemporal CCR1, CCL3(MIP-1alpha), CXCR4, CXCL12(SDF-1alpha) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J Neurosurg Spine 2011, 14: 583–597.
Huang W, Zheng W, Ouyang H, Yi H, Liu S, Zeng W, et al. Mechanical allodynia induced by nucleoside reverse transcriptase inhibitor is suppressed by p55TNFSR mediated by herpes simplex virus vector through the SDF1alpha/CXCR4 system in rats. Anesth Analg 2014, 118: 671–680.
Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 2002, 22: 3052–3060.
George A, Marziniak M, Schafers M, Toyka KV, Sommer C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain 2000, 88: 267–275.
Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest 2014, 124: 1173–1186.
Wei XH, Na XD, Liao GJ, Chen QY, Cui Y, Chen FY, et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp Neurol 2013, 241: 159–168.
Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 2010, 58: 1871–1880.
Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36: 57–68.
Luo X, Tai WL, Sun L, Qiu Q, Xia Z, Chung SK, et al. Central administration of C-x-C chemokine receptor type 4 antagonist alleviates the development and maintenance of peripheral neuropathic pain in mice. PLoS One 2014, 9: e104860.
Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 2005, 280: 35760–35766.
Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci 2007, 8: 895–903.
Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, et al. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron 2011, 69: 61–76.
Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, et al. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res 2003, 63: 1969–1974.
Cao DL, Zhang ZJ, Xie RG, Jiang BC, Ji RR, Gao YJ. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol 2014, 261C: 328–336.
Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev 2009, 60: 135–148.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31171070, 81171060, 81501070, and 81571079).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liying Bai and Xinru Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bai, L., Wang, X., Li, Z. et al. Upregulation of Chemokine CXCL12 in the Dorsal Root Ganglia and Spinal Cord Contributes to the Development and Maintenance of Neuropathic Pain Following Spared Nerve Injury in Rats. Neurosci. Bull. 32, 27–40 (2016). https://doi.org/10.1007/s12264-015-0007-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-015-0007-4