Abstract
Liver X receptor (LXR) activation exerts an anti-tumor effect. However, whether the tumor LXR expression has prognostic significance in hepatocellular carcinoma (HCC) patient has not been addressed yet. Primary HCC and the adjacent non-tumor tissues were obtained from 169 patients who underwent routine curative surgical treatment. All patients were followed for prognosis analyses. Tumor LXR was detected by immunohistochemical analysis. In in vitro study, several HCC cell lines were cultured for cellular protein detection of LXR and other cytokines, including nuclear factor kappa (NFκB), Matrix metalloproteinases 2 and 9 (MMP-2 and -9). Meanwhile, the invasion ability of cultured HCC cell lines was performed. We found that LXR expression status in tumor samples is associated with the clinical characteristics, such as tumor stage and metastasis, of HCC patients. Prognosis analysis shows that tumor LXR expression status is closely related to the post-operative outcome in HCC patients who underwent surgical treatment. Patients with low LXR expression have a significantly lower mean 5-year overall survival rate and mean overall survival period than those with high LXR level. Our in vitro data reveal that HCC cell lines had increased NF-κB, MMP2, MMP9 and invasive ability than normal cell line, which are suppressed by LXR activation via NFκB pathway. Our data suggest that LXR could be used as a biomarker for HCC prognosis. Further study is warranted to explore the molecular mechanism under which LXR regulates tumor behaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies with poor prognosis [1] [2] [3]. Many HCC patients developed early recurrence or metastasis after surgical resection. The traditional serum marker for HCC, such as alpha-fetoprotein (AFP), does not meet the clinical need due to its low sensitivity and specificity. Therefore, the identification of new markers with higher sensitivity and specificity remains important.
Liver X receptor (LXR) belongs to the nuclear receptor family of ligand-dependent transcription factors and regulates cholesterol, glucose, fatty acid metabolism, and inflammatory responses in mammal cells [4]. Previous studies reported that LXR activation exerts an anti-tumor effect on breast, prostate, ovarian, lung, skin, and colorectal cancer cells, suggesting that LXR is a potential target in cancer prevention and treatment [5] [6] [7] [8]. A recent study revealed that LXR mediates hepatitis B virus X protein induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma; and there is a significant increase in the expression of LXR in HCC in comparison with adjacent non-tumorous nodules in human HBV associated HCC specimens [9] . However, whether the tumor LXR expression has prognostic significance in HCC patient has not been addressed yet.
Methods
Tissue Samples
Primary HCC and the adjacent non-tumor tissues were obtained from 169 patients who underwent routine curative surgical treatment at our hospital from May 2009 to May 2014. HCC was diagnosed by ultrasonography, computed tomography, and confirmed by liver biopsy. Among these patients, 114 patients were infected with HBV and 55 patients with HCV. Patients who had received radiotherapy or chemotherapy prior to surgery were excluded. Patient’s information including gender, age, tumor stage, size, cirrhosis and metastasis status were collected from medical charts. These patients were followed up after discharger from hospital. Overall survival (OS) was defined as the interval between treatment and death or last observation of surviving patients.
The study protocol was approved by the institute ethics committee, and informed consent was acquired from all patients and donors before the study commenced.
Immunohistochemical Analysis
Immunohistochemical (IHC) staining was performed using Dako Envision system (Dako, Carpinteria, CA) according to the manufacturer’s instructions. Briefly, 4 μm formalin-fixed paraffin-embedded sections were deparaffinized, followed by antigen retrieval by heating sections in a microwave oven. The sections were then incubated with rabbit polyclonal anti-LXRa antibody (1:100; Millpore Inc. USA) overnight at 4 °C. After sequential incubation with biotinylated anti-rabbit secondary antibody and streptavidin-horse-radish peroxidase, reaction product was developed with Diaminobenzidine (DAB). Negative control was composed of mixture with no primary antibody but normal goat serum instead. Two pathologists unaware of study protocol were invited to score the slides independently.
Immunoreactivity was scored for the extent and intensity of the nuclear staining. Extent of staining was scored as follows: 0, no positive cells; 1, < 25% positive cells; 2, 25–50% positive cells; 3, 50–75% positive cells and 4, > 75% positive cells. Intensity was scored as follows: 0, no positive staining; 1, weak staining; 2, moderate staining and 3, strong staining. Multiplying extent by intensity gave the LXR staining scores from low expression (0–6) to high expression (> 6) accordingly.
Cell Culture and Treatment
Several HCC cell lines, including HepG2, SMMC-7721, Bel-7404, and human hepatocyte cell line HL-7702, were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum. Cells were treated with LXR activator GW3965 (20 μM for 6 h; Invitrogen, Carlsbad, USA) or NFκB inhibitor, BAY 11–7082 (5 μM,6 h).
Protein Extraction and Western-Blotting Analysis
After siRNA transfection, cells were harvested in RIPA lysis buffer (Thermal Scientific inc. USA). Proteins (10 μg) were separated by SDS-PAGE, transferred to Hybond-C Extra membranes (Amersham, UK), and immunoblotted using antibodies against human LXRa, NFκB, Matrix metalloproteinases 2 and 9 (MMP-2 and -9), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (all from Santa Cruz Biotechnology, Santa Cruz, CA).
Cell Invasion Assay
Cells were incubated in the upper chambers of 24-well transwell plates (Corning Incorporated, New York, NY, USA), which were coated with 50% Matrigel (BD Biosciences, USA). After 18 h incubation, invaded cells were stained with 0.5% crystal violet, examined by bright field microscopy (OLYMPUS, Japan), and photographed. Invasion rate was quantified by counting the invaded cells in five random fields per chamber under the fluorescence microscope.
Statistical Analysis
Statistical analyses were performed using a statistical software package (SPSS19.0, Chicago, IL). The chi-square test was used to analyze the relationship between tumor LXRa expression and clinical characteristics. Survival times were evaluated using the Kaplan-Meier survival curves, and compared by the log-rank test. The significance of various variables for survival was analyzed by multivariate survival analysis using Cox’s regression model. P value less than or equal to 0.05 was considered to be statistically significant.
Results
LXRa Expression in HCC Tissues
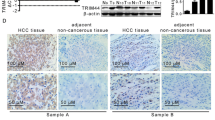
Our immunohistochemical analysis show that LXRa is predominantly expressed in nuclei. LXRa expression level in HCC patient tissues are high in normal noncancerous tissues, but significantly low in HCC HCC samples (Fig. 1). Representative images of LXRa staining in HCC patient tissues and adjacent noncancerous tissues are shown in Fig. 1. Of the 169 HCC patients, 104 patients had staining scores lower than 6, while 65 had staining scores over or equal to 6. The LXRa expression levels was not related to age, sex, tumor size and cirrhosis, but correlated to tumor stage and metastasis (Table 1).
Typical immunohistochemical analysis for LXRa. LXR was predominantly expressed in nuclei detected by LXR Immunohistochemical analysis. LXR expression is high in normal noncancerous tissues (Fig. 1a.),but is much lower in in HCC sample (Fig. 1b. Typical images for high LXR expression in HCC sample; and Fig. 1c. for low LXR expression in HCC sample)
Correlation of LXRa Expression with HCC Overall Survival Rate
The HCC patients were followed for up to 60 months. The 5-year overall survival rates for patients with low and high LXRa expression were 32.4% and 45.7%, respectively (P = 0.001, Fig. 2). The overall survival period of the patients with low LXRa expression was significantly lower than that of high LXR expression group (19.5 ± 3.5 vs. 25.7 ± 3.6, month, P < 0.001 by log rank analysis).
Prognosis analyses in HCC patients by using Kaplan-Miere curves and log rank tests. The 5-year overall survival rates for patients with low and high LXR expression were 32.4% and 45.7%, respectively (P = 0.001, Fig. 2 ). The overall survival period of the patients with low LXR expression was significantly lower than that of high LXR expression group(19.5 ± 3.5 vs. 25.7 ± 3.6, P < 0.001 by log rank analysis)
We next employed the Cox proportional hazards mode to determine the effects of the independent factors, including tumor LXRa expression status, gender, age, tumor size, serum AFP, tumor size, liver cirrhosis, stage, tumor metastasis, and tumor differentiation. Univariate Cox proportional hazards mode screened that LXR expression, serum AFP, tumor size, tumor metastasis and TNM stage as possible prognostic factors of survival (Table 2). In the multivariate Cox proportional hazards mode, LXRa and tumor metastasis were identified to have significant correlation with clinical outcome of HCC patients.
Our in vitro data show that all HCC cell lines had a dramatically lower LXRa expression level compared to normal liver cell line HL-7702 (Fig. 3a), which is consistent with what we observed in HCC patients.
a LXR expression in HCC cells and normal cells by western blot assay. Compared to normal cell line HL-7702, all three HCC cell lines had significantly lower LXR expressions. b Western blot assays show that LXR activation induced NF-κB, MMP2 and MMP9 expressions, which was abolished by NFκB inhibitor. c Invasiveness assays showed LXR activation by GW3965 treatment significantly reduced the invasive ability of HCC cell lines, while NFκB inhibitor abrogated this effect
Contrast to the reduced LXR expression, our western blot assay show that HCC cell lines had considerable increases in cytokines related to tumor invasiveness, including NF-κB, MMP2 and MMP9, than normal cell line. We noted that LXR activation by GW3965 significantly decreased NF-κB, MMP2 and MMP9 levels (Fig. 3b). When these cells received co-incubation of GW3965 and NFκB inhibitor, we observed the NFκB inhibitor abrogate effect of GW3965 on MMP2 and MMP9, suggesting NFκB is involved in the pro-invasive effect of LXR activation.
Tumor cell invasion assays showed that the numbers of cells passing through Matrigel in HepG2, SMMC-7721 and Bel-7404 was significantly higher than that in the HL-7702 (Fig. 3b). LXR activation by GW3965 treatment significantly reduced the invasive ability of HCC cell lines. However, Notably, the addition of NFκB inhibitor abolished the effect of GW3965 on the invasive ability of HCC cell lines (Fig. 3c).
Discussion
In this study, we found that tumor LXRa expression status is associated with the clinical characteristics, such as tumor stage and metastasis, of HCC patients. More importantly, tumor LXRa level is closely related to the post-operative outcome in HCC patients. Patients with low LXR expression have a significantly lower mean 5-year overall survival rate and mean overall survival period than those with high LXR level, suggesting tumor LXRa can be used as a marker for HCC prognosis. Our in vitro data reveal that HCC cell lines had increased NF-κB, MMP2, MMP9 and invasive ability than normal cell line, which are suppressed by LXR activation via NFκB pathwa. This finding provides a novel insights into the molecular mechanisms for the anti-tumor effect of LXR.
Accumulating evidences suggest that LXR plays a critical role in cell proliferation and tumor development [10] . Previous reports show that LXR reduces proliferation of human colorectal cancer cells. LXR-deficient mice display increased proliferation in the colonic epithelial, whereas activation of LXR inhibits epithelial proliferation [11]. In human breast cancer cell lines, activation of LXR could significantly inhibit proliferation via PI3K/AKT pathway [5]. It is possible that the altered levels of LXR in different HCC stages differentially impact cancer cell proliferation and thus tumor development.
Despite the anti-tumor activity of LXR has been reported in many studies, the underlying mechanisms and signaling pathways remain elusive. It has been reported that LXR and its ligands could regulate metabolic genes, cell cycle regulatory proteins, hormone signaling, and apoptosis in cells [8]. We demonstrated in this study that LXR activation suppressed the expression of MMP-2 and MMP-9 in HCC cell lines, along with antagonism of the NFκB, a protein complex that controls transcription of DNA, cell proliferation and cell survival. Treatment with NFκB inhibitor abolished the LXR-dependent suppression of MMP-2 and -9 expressions. These results suggest LXR may act against cancer at least in part by regulating of MMPs through NFκB signaling pathway.
MMPs are Zn2+-containing endopeptidases that degrade extracellular matrix proteins during normal and pathogenic tissue turnover. Activation of MMPs has been implicated in development, invasion and metastasis of cancer, including HCC [12] [13] [14] [15] .In murine peritoneal macrophages, LXR has been shown to regulate MMP-9 expression via antagonism of the NFκB signaling pathway [16]. Moreover, treatment with LXR agonists GW3965 and TO901317 suppressed the levels of MMP-9 in brains after experimental stroke and lead to neuroprotection and reduced neuroinflammation (Morales 2008, circulation). In line with these previous findings, our data confirmed the effect of LXR activation on MMPs repression via NFκB in HCC cell lines. Future study is required to confirm these results in human.
Several limitations should be addressed. Firstly, the sample size is relative small and enrolled only Chinese patients, thus the conclusion of this study need to be validated with further study with larger sample size. Secondly, we found that LXR mediate MMP2 and MMP9 expression via NFκB. Further in votro study is warranted to explore the molecular mechanism mediating the interaction between LXR and NFκB.
Conclusion
Our data reveal a correlation of LXR expression levels with HCC stage and metastasis and suggest the potential role of LXR as a prognostic marker for HCC patients. Further more, we have linked activation of LXR to NFκB-dependent suppression of MMPs in HCC cell lines, providing important novel insights into the molecular mechanisms for LXR to inhibit cancer development.
References
Kim JU, Shariff MI, Crossey MM, Gomez-Romero M, Holmes E et al (2016) Hepatocellular carcinoma: review of disease and tumor biomarkers. World J Hepatol 8:471–484
Chen KW, Ou TM, Hsu CW, Horng CT, Lee CC et al (2015) Current systemic treatment of hepatocellular carcinoma: a review of the literature. World J Hepatol 7:1412–1420
Karaman B, Battal B, Sari S, Verim S (2014) Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 20:18059–18060
Baranowski M (2008) Biological role of liver X receptors. J Physiol Pharmacol 59(Suppl 7):31–55
El Roz A, Bard JM, Huvelin JM, Nazih H (2012) LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res 32:3007–3013
Lo Sasso G, Bovenga F, Murzilli S, Salvatore L, Di Tullio G, et al. (2013) Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology 144: 1497-1507, 1507 e1491-1413.
Mehrotra A, Kaul D, Joshi K (2011) LXR-alpha selectively reprogrammes cancer cells to enter into apoptosis. Mol Cell Biochem 349:41–55
Chuu CP, Lin HP (2010) Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res 30:3643–3648
Na TY, Shin YK, Roh KJ, Kang SA, Hong I et al (2009) Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology 49:1122–1131
Russo V (2011) Metabolism, LXR/LXR ligands, and tumor immune escape. J Leukoc Biol 90:673–679
McFadden JW, Corl BA (2010) Activation of liver X receptor (LXR) enhances de novo fatty acid synthesis in bovine mammary epithelial cells. J Dairy Sci 93:4651–4658
Zhao G, Zhang H, Huang Z, Lv L, Yan F (2016) Cortactin and Exo70 mediated invasion of hepatoma carcinoma cells by MMP-9 secretion. Mol Biol Rep 43:407–414
Lempinen M, Lyytinen I, Nordin A, Tervahartiala T, Makisalo H et al (2013) Prognostic value of serum MMP-8, −9 and TIMP-1 in patients with hepatocellular carcinoma. Ann Med 45:482–487
Sakamoto Y, Mafune K, Mori M, Shiraishi T, Imamura H et al (2000) Overexpression of MMP-9 correlates with growth of small hepatocellular carcinoma. Int J Oncol 17:237–243
Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M et al (2002) Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer 97:425–431
Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA et al (2004) LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119:299–309
Acknowledgements
This study was supported by a grant from Shanxi Medical University.
Author information
Authors and Affiliations
Contributions
HL and QH conceive and design this study. HL, XG and SQ enrolled patients and collected data. QH analyze and interpretation of data, and drafting the article.
GUARANTOR Qingxing Huang.
Corresponding author
Ethics declarations
Competing Interest
None.
Rights and permissions
About this article
Cite this article
Long, H., Guo, X., Qiao, S. et al. Tumor LXR Expression is a Prognostic Marker for Patients with Hepatocellular Carcinoma. Pathol. Oncol. Res. 24, 339–344 (2018). https://doi.org/10.1007/s12253-017-0249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0249-8