Abstract
MicroRNAs (miRNAs) are small, non-coding RNAs of endogenous origin. Accumulating studies have shown aberrant miRNA expression plays an important role in many tumor types. miR-192 and -215, which have the same “seed region”, have not been comprehensively investigated using a large number of cases in gastric cancer. The total RNA was extracted from 118 gastric cancer tissues and three gastric cancer cell lines as well as matched non-tumor adjacent tissues (NATs). After polyadenylation and reverse transcription, expression levels of miR-192 and -215 were determined by real-time PCR and calculation using the 2-∆∆CT method for evaluation of the association between miR-192, and -215 expression levels and clinicopathological characteristics. There were no significant differences in miR-192 and -215 expression levels between gastric cancer tissues and non-tumor counterparts (both p > 0.05, paired t-test). Interestingly, miR-192 and -215 were down-regulated in MGC-803 cells, BGC-823 cells and SGC-7901 cells (all p < 0.01, paired t-test). Also, the down-regulation of miR-192 and -215 was demonstrated to be associated with increased tumor sizes (both p = 0.003, Mann–Whitney U test) and advanced Borrmann type tumors (p = 0.015 and p = 0.044, respectively, Kruskal-Wallis H test). Moreover, the expression of miR-192 was significantly lower in the pT4 stage of gastric cancer than in pT1, pT2 and pT3 stages (p = 0.026). Furthermore, there was a strong correlation between miR-192 and -215 in gastric cancer tissues (p < 0.001, Pearson regressions). miR-192 and -215 might be related to the proliferation and invasion of gastric cancer. Potentially, they could become important biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
miRNAs are an abundant class of endogenous small single-strand non-coding RNAs of 19–22 nucleotides with a primary role of regulating the translation of many genes in plants, animals and humans [1]. It is now well established that miRNAs may have causal roles in many normal/tumor cellular processes, such as development, differentiation, proliferation and apoptosis [2, 3]. Moreover, accumulating studies have shown that miRNAs have been classified as oncogenes and tumor-suppressor genes [4, 5]. More than 50% of miRNA genes are located in cancer-associated genomic regions or in fragile sites, suggesting that aberrant miRNA expression plays an important role in cancer pathogenesis [6]. Over the past decade, a growing number of studies have suggested that miRNA expression is dysregulated in many tumor types including lung cancer [7], esophageal cancer [8], liver cancer [9], pancreatic cancer [10], colorectal cancer [11] and ovarian cancer [12].
In recent studies, several miRNAs have been found to be correlated with gastric cancer. Tsukamoto et al. [13] investigated a total of 470 human miRNA profiles in gastric carcinomas by a miRNA microarray method. In this study, 33 miRNAs were significantly up-regulated and the other six miRNAs were down-regulated, of which miR-375 was the most down-regulated and was considered a candidate tumor suppressor miRNA in gastric carcinoma. Our previous studies showed similar results in that miR-203 was significantly down-regulated in gastric cancer cell lines by real-time polymerase chain reaction (real-time PCR). Moreover, over-expression of miR-203 inhibited cell proliferation of gastric cancer and miR-203 might be an important factor as a tumor suppressor [11]. Research on the expression of miR-148a and -152 in a large number of cases with gastric cancer also found similar results in that down-regulation of miR-148a and -152 was significantly related to tumor size and depth of invasion [14]. Furthermore, the investigation revealed that miR-148b had the same “seed sequence” as miR-148a and -152, which was down-regulated in 106 gastric cancer tissues compared with NATs. Further analysis showed that down-regulation of miR-148b was associated with tumor size and its over-expression inhibited cell proliferation of gastric cancer [15]. Moreover, Xiong et al. also indicated that down-regulated miRNA-214 Induces a cell cycle G1 arrest in gastric cancer cells [16].
To define miR-192 and -215 expression profiles in gastric cancer, we performed real-time PCR on a large number of cases and compared expression levels in gastric cancer tissues and their non-tumor counterparts. Also, we detected expression levels of miR-192 and -215 in three gastric cancer cell lines compared to normal gastric tissues. Further investigation revealed a strong correlation between miR-192 and -215 expression levels and clinicopathological characteristics in gastric cancer. Thus, miR-192 and -215 might play a role in the invasion and progression of gastric cancer and our findings will likely help to elucidate the functions of miRNAs.
Material and Methods
Tissues Samples
In the current study, 118 pairs of gastric cancer tissues and non-tumor counterparts (as controls) were obtained from patients that underwent radical resection between 2007 and 2010 at the first hospital of China Medical University (Shenyang, China). Matching non-tumor mucosa specimens were obtained from a part of the resected specimen at the farthest distance from the tumor. The samples were snap-frozen in liquid nitrogen immediately after surgery before RNA extraction. No previous local or systemic treatment had been conducted on these patients before surgery.
Gastric cancer was subsequently diagnosed based on histopathological evaluation. One section of each sample was stained with hematoxylin-eosin (H&E). The histological grade of cancer was classified using the TNM staging system of the American Joint Committee on Cancer (AJCC; 2010) and the International Union Against Cancer (UICC) according to the standard of the World Health Organization. The study was approved by the Research Ethics Committee of China Medical University (Shenyang, China) and informed consent was obtained from all patients.
Cell Lines
Human gastric cancer cell lines (MGC-803, BGC-823 and SGC-7901) were obtained from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China). All of the cell lines were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere of 5% CO2. Media were supplied with 10% fetal bovine serum (FBS).
RNA Extraction
According to the manufacturer’s instructions, we isolated the total RNA from the specimens using a mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA). The concentration and purity of RNA were controlled by UV spectrophotometry using a NanoPhotometer UV/Vis spectrophotometer (Implen, Schatzbogen, München, Germany). Using a Poly(A) Tailing Kit, poly(A) tail was added to RNA in a 37°C water bath for 30 min by Escherichia coli poly(A) polymerase (E-PAP) according to the manufacturer’s instructions (Ambion, Austin, TX, USA) [17]. RNAs were dissolved in diethyl pyrocarbonate (DEPC)-treated water after purification by phenol-chloroform and ethanol. The first-strand cDNA was synthesized with SuperScript® III First-Strand Synthesis System using a reverse transcriptase-polymerase chain reaction kit (Invitrogen, Carlsbad, CA, USA). To generate cDNA of miRNA, a 10 μL reverse transcriptase reaction mixture containing 1 μg of the RNA sample, 1 μL RT-primer (Table 1), 1 μL 10 mM deoxyribonucleotide triphosphate (dNTP) mix and DEPC-treated water at 65°C was incubated for 5 min. Then, a 10 μL mixture containing 2 μL 10 × RT buffer, 4 μL 25 mM MgCl2, 2 μL 0.1 M DTT, 1 μL RNaseOUT (40 U/μL) and 1 μL SuperScript III RT (200 U/μL) was added. The total reaction mixture was incubated in a 96-well plate of GeneAmp PCR 9700 Thermocycler (Applied Biosystems, Hayward, CA, USA) at 50°C for 50 min, 85°C for 5 min, and 37°C for 20 min after adding 1 μl RNase H to the mixture and then held at 4°C.
Real-Time PCR
Using the SYBR Premix Ex Taq™ IIKit (TaKaRa, Bio, Kyoto, Japan), real-time PCR was performed with a Rotor-gene 6000 system (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions [17]. The 25 μl mixture of PCR consisted of 12.5 μl SYBR Green supermix, 8.5 μl RNase-free water, 1 μl forward primers, 1 μl reverse primers and 2 μl reverse transcribed product. We determined threshold cycle data by setting a default threshold and the reactive condition was 45 amplification cycles of 95°C for 5 s, 58°C for 20 s and 72°C for 30 s in a 36-well optical plate using a Rotor-gene 6000 system. The U6 RNA was selected as an endogenous reference to evaluate the relative expression levels of miR-192 and -215 in cancerous samples compared to non-tumor counterparts using the 2-∆∆Ct method [18]. All samples were performed in triplicate and repeated three times. The products were confirmed by TA cloning and a sequencing assay. The primers for miR-192, -215 and the endogenous control U6 are shown in Table 1.
Statistical Analysis
The threshold cycle of fluorescence (Ct) for each sample was determined by real-time PCR to evaluate the association between the cancer group and control group by the 2-∆∆Ct method. ∆∆Ct was the difference in the ∆Ct value between two types of groups (∆∆Ct = ∆Ct cancer−∆Ct control) and ∆Ct was the difference in the Ct value between the target and U6 (∆Ct = Ct target−Ct U6). The 2-∆∆Ct value (fold value) was calculated and distinguished as 1-fold (20 equals one) and less than 1-fold was defined as low expression [11]. Differences in miRNA expression levels were measured by comparing the values of ΔCt cancer and ΔCt control. The statistical analysis was determined using a paired t-test in cancer tissues and cancer cell lines relative to non-tumor counterparts. Moreover, the association between miRNA expression levels and clinicopathological parameters was analyzed by a non-parametric test (Mann–Whitney U test between two groups and Kruskal-Wallis H test for three or more groups). P < 0.05 was considered significant. Statistical analysis were performed using the Statistical Program for Social Sciences (SPSS) software 16.0 (SPSS Incorporated, Chicago, IL, USA).
Results
Expression of miR-192 and -215 in Gastric Cancer Tissues
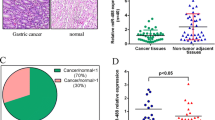
Expression levels of miR-192 and -215 were detected in all 118 pairs of gastric cancer tissues and corresponding NATs by real-time PCR. The values of ∆Ct (mean ± SD) were 3.161 ± 3.051 and 4.556 ± 3.031 in gastric cancer tissues as well as 2.702 ± 2.592 and 4.288 ± 2.541 in non-tumor counterparts (p = 0.070 and p = 0.309, respectively, paired t-test, Fig. 1a–d). There were no statistically significant differences in miR-192 and -215 expression levels between gastric cancer tissues and matched NATs. However, 53.4% (63/118) and 50.9% (60/118) of the cases showed a >50% reduction in miR-192 and -215 expression levels in gastric cancer tissues compared with matched NATs.
Expression of miR-192 and -215 in 118 patients with gastric cancer. (a) (c) Quantification of miRNAs was measured by SYBR Premix Ex Taq™ II. Each sample was analyzed in triplicate and repeated three times. Data were presented as log2 of fold-change of gastric cancer tissues relative to matched non-tumor adjacent tissues (NATs). (b) (d) miR-192 and -215 were differentially expressed between gastric cancer tissues and NATs. The miRNAs were normalized by U6RNA. ΔCt = Ct miRNAs-Ct U6RNA. However, the ΔCt of miRNAs were not significant in gastric cancer tissues compared to NATs (p > 0.05, paired t-test)
miR-192 and -215 Were Significantly Down-Regulated in Gastric Cell Lines
Next, real-time PCR analysis were performed on three gastric cancer cell lines to evaluate the expression levels of miR-192 and -215. It is noteworthy that miR-192 and -215 were significantly down-regulated in MGC-803 cells (p < 0.001 and p = 0.003, respectively, paired t-test), BGC-823 cells (p = 0.001 and p < 0.001, respectively) and SGC-7901 cells (all p < 0.001) relative to normal gastric tissues (randomly selected 3 NATs from previous 118 cases as controls, Fig. 2) [19].
Expression of miR-192 and -215 in three gastric cancer cell lines (MGC-803, BGC-823 and SGC-7901). Quantification of miRNAs was measured by SYBR Premix Ex Taq™ II. Data were presented in gastric cancer cell lines relative to normal gastric tissues (randomly selected from previous 118 cases as controls, *p < 0.01, **p < 0.001)
Association between miR-192 and -215 Expression Levels and Clinicopathological Characteristics in Gastric Cancer
The down-regulated miR-192 and -215 tended to be correlated with increased tumor sizes (both p = 0.003, Mann–Whitney U test) and advanced Borrmann type tumors (p = 0.015 and p = 0.044, respectively, Kruskal-Wallis H test). Moreover, expression levels of miR-192 were significantly lower in the pT4 stage of gastric cancer than in pT1, pT2 and pT3 stages (p = 0.026). This means that patients with invasion of the visceral peritoneum or adjacent structures may have significantly lower expression levels of miR-192 in gastric cancer. However, there was no significant difference between miR-192 and -215 expression levels and other clinicopathological characteristics, such as sex, age, tumor location, histologic grade, lauren grade, pN stage, TNM stage or lymphatic vessel invasion (Table 2).
Strong Correlations between miR-192 and -215
There was a strong correlation between the expression levels of miR-192 and -215 in gastric cancer tissues by Pearson regressions (p < 0.001, Fig. 3). The correlation coefficient was 0.906.
Discussion
In recent years, more evidence has shown miRNAs may be a novel class of oncogenes/tumor suppressors and the correlation between miRNAs and cancers has become a focus for the diagnosis and treatment of cancer [20]. miRNAs can be quantified using many methods, including a microarray assay, bead-based flow cytometric assay and real-time PCR assay. Yanaihara et al. [21] evaluated expression levels of 43 miRNAs among lung cancer tissues versus corresponding noncancerous lung tissues with a miRNA microarray method, of which miR-192 was up-regulated in lung cancer. Moreover, the up-regulated miR-192 was also found in 170 patients with esophageal cancer compared to non-tumor counterparts using a miRNA microarray method [22]. Furthermore, Gui et al. indicated that miR-215 in hepatocellular carcinoma was up-regulated by real-time PCR [23]. However, down-regulated miR-192 and -215 were also found in colorectal cancer tissues and cell lines using a microarray analysis [24, 25]. Also, a dramatic reduction of miR-192 and -215 expression levels was observed in rat colon adenocarcinoma with real-time PCR [26]. Taken together, we think the differential expression of miR-192 and -215 may be the result of tissue-specific differences. Just as previous study suggested, different levels of miRNA expression were found in different organs of origin and it was found that the expression of miRNAs were markedly tissue-specific [27].
Because the main advantage of real-time PCR is that it is more quantitative and sensitive than other methods, we performed real-time PCR on 118 samples with gastric cancer to assess both miR-192 and -215 expression levels in the current study. There was no significant difference in miR-192 or -215 expression levels between gastric cancer tissues and matched NATs. Interestingly, miR-192 and -215 were down-regulated in gastric cancer cells compared with the control group. However, Jin et al. [28] reported that both miR-192 and -215 were significantly over-expressed in 25 primary gastric cancer tissues versus 31 non-neoplastic gastric tissues (1.838 vs. 0.937, p = 0.016; 0.222 vs. 0.083, p = 0.017, respectively, obtained from Baltimore, MD, USA) and gastric cancer cell lines (NCI-N87) versus normal gastric epithelial cells (NFE145). Therefore, research on the expression levels of miR-192 and -215 have been controversial in gastric cancer to date. As in previous studies, Volinia et al. [29] indicated that miR-155 was down-regulated in 39 pancreatic cancer tissues relative to 12 normal pancreatic tissues (obtained from Italy). However, up-regulated miR-155 was also found in 28 pancreatic cancer tissues compared to 21 non-tumor tissues (obtained from Oklahoma and Ohio, USA) [30]. Possibly, the different miRNA expression levels may be due to different populations and different environments [31]. Also, small sample sizes may have contributed to this inconsistency. Considering these variations in results, we think the differential expression of miR-192 and -215 may be caused by various combinations of factors, such as tissue-specificity, different populations, different environments and small sample sizes. To confirm, further investigation is needed.
In the current study, our investigation showed that the down-regulated miR-192 and -215 were closely correlated with increased tumor size and a higher degree of tumor invasiveness in gastric cancer. The expression levels of miR-192 and -215 were significantly lower in Borrmann III and IV types of gastric cancer than in Borrmann I and II types. Moreover, lower expression of miR-192 was also observed in the pT4 stage of gastric cancer than other pT stages. That means that patients with invasion of the visceral peritoneum or adjacent structures may have significantly lower expression of miR-192 in gastric cancer. Kim et al. [32] indicated that increased tumor size in gastric carcinoma was an independent, statistically significant prognostic factor using Cox’s proportional hazard regression model. Also, more studies have demonstrated that both macroscopic appearance and depth of invasion were independent prognostic indicators in gastric carcinoma [33, 34]. Thus, expression levels of miR-192 and -215 might associate with a more aggressive course in gastric carcinoma. Furthermore, we found a strong correlation between miR-192 and -215 in gastric cancer tissues. As shown on the miRbase website, miR-192 and -215 have the same 8-mer seed sequence, and only two nucleotides differ between them, with both miRNAs belonging to the miR-192 family. We therefore suggest that miR-192 and -215 might contribute to carcinogenesis of gastric cancer in synergism. In future study, we will provide more information to explain the functions of miR-192 and -215 in gastric cancer.
The miRNA expression levels were affected by many factors, including transcriptional factors, mutations, deletions, and methylation. Pichiorri et al. reported that hypermethylation of the miR-194-2-192 cluster promoter in multiple myeloma (MM) cell lines suggested that epigenetic down-regulation of both miRNAs, which leads to an increase in murine double minute 2 (MDM2) mRNA and protein expression, decreases the ability of p53 to down-modulate MDM2 expression [35]. Moreover, miR-192 and -215 have the same “seed regions”. Thus, we speculate that hypermethylation might be the mechanism for the down-regulation of miR-192 and -215 in gastric cancer.
In conclusion, miR-192 and -215 were down-regulated in gastric cancer cell lines and their down-regulation in gastric cancer tissues tended to be associated with increased tumor sizes and advanced Borrmann types of tumors. Moreover, expression levels of miR-192 were significantly lower in the pT4 stage of gastric cancer than other pT stages. Target genes and functions of miR-192 and -215 will become a focus of our studies in gastric cancer. To confirm the significance of miR-192 and -215 large-scale and long-term follow-up studies are needed.
References
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Calin GA, Croce CM (2006) MicroRNA-cancer connection: the beginning of a new tale. Cancer Res 66:7390–7394
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Hwang HW, Mendell JT (2006) MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 94:776–780
Cho WC (2007) OncomiRs: the discovery and progress of microRNAs in cancers. Mol Canc 6:60
Calin GA, Sevignani C, Dumitru CD et al (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 101:2999–3004
Bandi N, Vassella E (2011) miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol Canc 10:55
Feber A, Xi L, Pennathur A et al (2011) MicroRNA prognostic signature for nodal metastases and survival in esophageal adenocarcinoma. Ann Thorac Surg 91:1523–1530
Guo J, Miao Y, Xiao B et al (2009) Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol 24:652–657
Park JK, Henry JC, Jiang J et al (2011) miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun 406:518–523
Chiang Y, Song Y, Wang Z et al (2011) Aberrant expression of miR-203 and its clinical significance in gastric and colorectal cancers. J Gastrointest Surg 15:63–67
Marchini S, Cavalieri D, Fruscio R et al (2011) Association between miR-200c and the survival of patients with stage I epithelial ovarian cancer: a retrospective study of two independent tumour tissue collections. Lancet Oncol 12:273–285
Tsukamoto Y, Nakada C, Noguchi T et al (2010) MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res 70:2339–2349
Chen Y, Song Y, Wang Z, Yue Z, Xu H, Xing C (2010) Altered expression of miR-148a and miR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg 14:1170–1179
Song YX, Yue ZY, Wang ZN et al (2011) MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol Canc 10:1
Xiong X, Ren HZ, Li MH, Mei JH, Wen JF, Zheng CL (2011) Down-regulated miRNA-214 induces a cell cycle G1 arrest in gastric cancer cells by up-regulating the PTEN protein. Pathol Oncol Res 17:931–937
Shi R, Chiang VL (2005) Facile means for quantifying microRNA expression by real-time PCR. Biotechniques 39:519–525
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative pcr and the 2 (delta delta c(T)) method. Methods 25:402–408
Du Y, Xu Y, Ding L et al (2009) Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J Gastroenterol 44:556–561
Shah AA, Leidinger P, Blin N, Meese E (2010) miRNA: small molecules as potential novel biomarkers in cancer. Curr Med Chem 17:4427–4432
Yanaihara N, Caplen N, Bowman E et al (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Canc Cell 9:189–198
Mathé EA, Nguyen GH, Bowman ED et al (2009) MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res 15:6192–6200
Gui J, Tian Y, Wen X et al (2011) Serum microRNA characterization identifies miR-885-5p as a potential marker for detecting liver pathologies. Clin Sci (Lond) 120:183–193
Braun CJ, Zhang X, Savelyeva I et al (2008) p53-responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res 68:10094–10104
Georges SA, Biery MC, Kim SY et al (2008) Coordinated regulation of cell cycle transcripts by p53-Inducible microRNAs, miR-192 and miR-215. Cancer Res 68:10105–10112
Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS (2009) n-3 polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 30:2077–2084
Baffa R, Fassan M, Volinia S et al (2009) MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol 219:214–221
Jin Z, Selaru FM, Cheng Y et al (2011) MicroRNA-192 and -215 are upregulated in human gastric cancer in vivo and suppress ALCAM expression in vitro. Oncogene 30:1577–1585
Volinia S, Calin GA, Liu CG et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103:2257–2261
Lee EJ, Gusev Y, Jiang J et al (2007) Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 120:1046–1054
Quach H, Barreiro LB, Laval G et al (2009) Signatures of purifying and local positive selection in human miRNAs. Am J Hum Genet 84:316–327
Kim DY, Joo JK, Park YK, Ryu SY, Kim YJ, Kim SK (2007) Predictors of long-term survival in node-positive gastric carcinoma patients with curative resection. Langenbeck Arch Surg 392:131–134
Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S (2000) Early and late recurrence after gastrectomy for gastric carcinoma. Cancer 89:255–261
Kim JP, Kim YW, Yang HK, Noh DY (1994) Significant prognostic factors by multivariate analysis of 3926 gastric cancer patients. World J Surg 18:872–878
Pichiorri F, Suh SS, Rocci A et al (2010) Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Canc Cell 18:367–381
Acknowledgement
This work was supported by National Science Foundation of China (No. 30972879 and No. 81172370), Specialized Research Fund for the Doctoral Program of Higher Education (No. 200801590006) and Natural Science Foundation of Liaoning Province (No. 20092129).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yeunpo Chiang and Xin Zhou contributed equally to this work
Rights and permissions
About this article
Cite this article
Chiang, Y., Zhou, X., Wang, Z. et al. Expression Levels of MicroRNA-192 and -215 in Gastric Carcinoma. Pathol. Oncol. Res. 18, 585–591 (2012). https://doi.org/10.1007/s12253-011-9480-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-011-9480-x