Abstract
Small cell carcinoma of the bladder is an uncommon and rather aggressive bladder tumor, representing less than 1% of all vesical tumors. Small cell carcinoma of different organs has been shown to express markers of neuroendocrine differentiation, and also thyroid transcription factor 1 (TTF-1). TTF-1 is a transcription factor and its expression has been shown mainly in pulmonary small cell carcinomas and adenocarcinomas and in thyroid tumors. Although it was initially proposed as a useful marker to delineate the origin of metastatic adenocarcinomas from the lung, its expression is being increasingly reported in tumors from different origins. The goal of this review is to analyse the immunohistochemical profile of small cell carcinoma of the bladder and to compare it to classical urothelial cell carcinomas. With this aim we have reviewed the small cell bladder carcinomas diagnosed in a single tertiary hospital in Madrid (Fundación Jiménez Díaz) in the last 12 years. We have found 6 pure small cell carcinomas and performed a wide panel of immunohistochemistry, including cytokeratins 7 and 20, enolase, chromogranin, synaptophysin, CD56 and TTF-1 to these tumors and also to 30 high grade urothelial cell carcinomas of usual type. Only one of our small cell carcinoma cases showed positivity for TTF-1, while five expressed CD56 and four neuron-specific enolase. None of our cases expressed cytokeratin 20 or 7. To our surprise we found a case of conventional urothelial cell carcinoma expressing focally TTF-1. These results are in accordance with the current literature, although our rate of TTF-1 expression (16.6%) is on the low end of the spectrum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small cell carcinoma of the bladder is a rare aggressive neoplasm, comprising less than 1% of all bladder carcinomas in most series [1, 2]. These tumors are morphologically identical to their more common pulmonary counterparts and a neuroendocrine origin has been postulated [3]. The frequent association with otherwise usual urothelial cell carcinomas has led some authors to postulate a common origin for both tumors, suggesting small cell presence only represents a dedifferentiation within urothelial cell lesions [4]. Most reports agree that these tumors behave aggresively and can kill the patient despite therapy, most like its pulmonary counterpart. Several studies have analysed the immunohistochemical profile of small cell carcinomas of the bladder [5, 6] and showed a variable rate of positivity for neuroendocrine markers and thyroid transcription factor 1 (TTF-1). The aim of the present study is to review our experience with small cell carcinomas of the bladder and to compare them with usual urothelial cell carcinomas.
Material and Methods
We have reviewed the electronic files of the Department of Surgical Pathology of the Fundación Jiménez Díaz Hospital in Madrid (Spain). It is a tertiary hospital covering an approximate population of 350,000 people living in Madrid metropolitan area. During the period between 1997 and 2009 we have found 987 samples of urothelial carcinomas and 6 of them (0.6%) were pure small cell carcinoma with no foci of typical urothelial cell carcinoma. We have reviewed the clinical files from our patients to determine outcome and we have also performed immunohistochemistry on paraffin-embedded archival tissue from the tumors and also from 30 classical urothelial cell high grade pT2 carcinomas (Fig. 1a and b). The immunohistochemical panel included cytokeratins 7 and 20 (markers for urothelial cell tumors); enolase, chromogranin, synaptophysin and CD56 (markers for neuroendocrine differentiation) and TTF-1 (all reagents from Dako, Denmark). All the immunohistochemical reactions were performed with an automated system (Autostainer, Dako, Denmark) with antigen retrieval.
Results
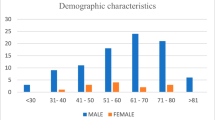
Table 1 summarizes the demographic data of the small cell carcinoma patients and Table 2 summarizes the immunohistochemical results for both small cell and classical urothelial cell carcinomas (Figs. 2a and b and 3a and b). It can be noted that most cases in the small cell group behaved very aggressively and killed the patient in a median time of 7.5 months (range: 3–13). Most patients were men and the mean age was 61 years for the whole series (range 44–73). These demographic data are in complete accordance with those described in the world literature regarding this tumor. We only had information regarding smoking in four of the cases and three of them were active cigarette smokers and one was a former smoker for 1 year.
Immunohistochemistry allows a clearcut distinction between these two lesions for urothelial cell carcinomas express cytokeratins 7 and/or 20 and show no expression of the markers of neuroendocrine differentiation, while the opposite holds true for small cell carcinomas, which have never expressed cytokeratin 20. All our cases, but one, have expressed at least one of the neuroendocrine differentiation markers, mainly CD56. One case was negative for all the imunohistochemical markers, but it was showed to express p53 in a wide and diffuse manner.
TTF-1 expression rate has been low in our small cell carcinomas. The rates of positivity have widely varied in the literature from 7% to 50%, and our results seem to be on the low range of this spectrum. To our surprise, we have found a definite, despite focal, positivity for TTF-1 in an otherwise conventional cytokeratin 20 expressing urothelial cell carcinoma, what represents a rather novel finding.
Discussion
Small cell carcinoma is a rather aggressive and infrequent type of bladder tumor [1, 2, 7]. The largest series of patients reported has followed 64 patients from different hospitals all over the world and confirms a survival of few months after diagnosis and an advanced stage at diagnosis [8]. Prognosis does not seem to be changed by therapy and patients undergoing cystectomy have died of disease after a similar median time to non operated patients.
Many studies have analyzed the possible origin of this neoplasia and some clonal-based ones seem to indicate that small cell carcinoma shares a common origin with urothelial cell carcinoma as they share many molecular abnormalities [4, 9]. Recent reports have analysed the role of some growth factors in these tumors, mainly EGFR, but have failed to show either prognostic or pathogenic significance of its expression [10].
Small cell carcinoma is most frequently found in the lungs, mainly of chronic smokers and is usually a lethal and rapidly evolving disease. However, there are many reports on small cell carcinoma in extrapulmonary locations. These have included the breast, [11] the uterine cervix, the gastrointestinal tract, the liver, [12] and many other organs [3, 13]. In a large review of 9 cases of small cell carcinoma of the breast by Shin et al. [9] prognosis was not as dismal in other locations; behaviour of small cell carcinoma might therefore vary according to the location of the lesions.
Thryoid transcription factor 1 (TTF-1) is a 38 kD homeodomain containing transcription factor, expressed in the thyroid, in some lung cells (mainly Clara cells and type II pneumocytes) and in certain brain areas [5]. TTF-1 has been identified by immunohistochemistry in most small cell and adenocarcinomas of the lung and has even been proposed as an specific marker to determine the origin of metastasis from these tumor types to distant organs. However, this alleged specificity of TTF-1 for lesions of pulmonary and thyroid origin is being increasingly questioned. In 2000 Ordoñez [13] reported the first series of patients [14] with extrapulmonary small cell carcinomas comparing expression of TTF-1 with pulmonary ones. He found that 96% of lung small cell carcinomas expressed TTF-1 as compared with only 5 of 54 extrapulmonary ones, including vesical, prostatic and gastrointestinal cases. He postulated TTF-1 could serve as a useful marker to determine pulmonary origin of metastasis from small cell carcinomas. However, subsequent reports by Cheuk [15] and Agoff [3] did not support this hypothesis. Cheuk found TTF-1 positivity in 42% (21/50) of his extrapulmonary small cell carcinomas, including two bladder ones. He concluded that TTF-1 could not be relied on to determine the origin of small cell carcinomas.
TTF-1 seemed to remain a useful tool for the diagnosis of lung adenocarcinomas, but a recent report by Siami et al. [16] has also rendered doubts on this, for they have shown TTF-1 immunoreactivity in 6/32 (19%) endometroid endometrial carcinoma independently of the differentiation grade, in 1/28 (4%) of undifferentiated adenocarcinoma of the uterine cervix and in 3/13 (23%) serous carcinomas of the uterine corpus. Although these results should be confirmed in larger series of patients, it seems that TTF-1 expression can also be found with some frequency in adenocarcinomas of other origins. In this sense, the surprising identification of TTF-1 focal positivity in one urothelial carcinoma in our series seems to confirm the possible aberrant expression of TTF-1 in tumors of different locations. We have not found any reference to this positivity in the world literature. In the series of 44 bladder small cell carcinomas by Jones et al. [5] they report that they found no TTF-1 positivity in the areas of classical urothelial cell carcinomas found adjacent to the small cell lesions in 50% of their cases. We have only found a recent report of the coexpression of cytokeratin 20 and TTF-1 in a cutaneous metastasis from a large cell neuroendocrine carcinoma of vesical origin [16]. Our case expressed cytokeratin 20 and none of the neuroendocrine markers analysed in our series, so we cannot consider it a neuroendocrine tumor. It remains to be shown in other larger series whether TTF-1 is aberrantly expressed in high grade urothelial cell carcinomas. TTF-1 is still useful for the differential diagnosis of cutaneous metastasis from small cell tumors of any origin and Merkel cell carcinoma, for all the studies performed seem to confirm the latter is consistently negative for TTF-1 [17].
As for the other markers performed in our cases, the immunohistochemical profile confirms the neuroendocrine nature of small cell carcinomas of the bladder. In a recent report by Alijo et al. [6] on 44 small cell and two large cell undifferentiated carcinomas of the bladder, they found TTF-1 expression in 25% of the small cell tumors, while synaptophysin was positive in 50% and enolase in 80%. They did not include CD56. In our series CD56 was positive in all the cases, but one. The small cell carcinoma with no positive reactivity for any of the analysed markers showed positivity for p53 in 100% of the tumor cells and run an accelerated course, with the patient dying of disease in 3 months.
In summary in this report we comment on the immunohistochemical profile of pure small cell carcinoma. Cytokeratin negativity and CD56 expression can, apart from the histopathological features, confirm diagnosis in doubtful cases. Some bladder small cell carcinomas express TTF-1, and the bladder should be taken into account as another possible source of metastasis from small cell carcinoma expressing this marker. In our series we have also found the rather infrequent expression of TTF-1 in a typical urothelial cell carcinoma, which should be confirmed in larger series.
References
Blomjous CE, Vos W, De Voogt HJ, Van der Valk P (1989) Small cell carcinoma of the urinary bladder. A clinicopathological, morphometric, immunohistochemical and ultrastructural series of 18 cases. Cancer 64:1347–1357
Lopez JL, Angulo JC, Flores N (1994) Small cell carcinoma of the urinary bladder. A clinicopathological study of six cases. Br J Urol 73:43–49
Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, Folpe AL (2000) Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol 13:238–242
Cheng L, Jones TD, McCarthy RP, Eble JN, Wang M, MacLennan GT, Lopez-Beltran A, Yang XJ, Koch MO, Zhang S, Pan CX, Baldridge LA (2005) Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol 166:1533–1539
Jones TD, Kernek KM, Yang XJ, López-Beltrán A, MacLennan GT, Eble JN, Lin H, Pan CX, Tretiakova M, Baldridge LA, Cheng L (2005) Thyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: an immunohistochemical profile of 44 cases. Hum Pathol 36:718–723
Alijo Serrano F, Sánchez-Mora N, Angel Arranz J, Hernández C, Alvarez-Fernández E (2007) Large cell and small cell neuroendocrine bladder carcinoma: immunohistochemical and outcome study in a single institution. Am J Clin Pathol 128:733–739
Mackey JR, Au HJ, Hugh J, Venner P (1998) Genitourinary small cell carcinoma: determination of clinical and therapeutic factor associated with survival. J Urol 159:1624–1629
Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H, Kuzel TM, Papavero V, Tretiakova M, Nigro K, Koch MO, Eble JN (2004) Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer 101:957–962
Abbosh PH, Wang M, Eble JN, Lopez-Beltran A, Maclennan GT, Montironi R, Zheng S, Pan CX, Zhou H, Cheng L (2008) Hypermethylation of tumor-suppressor gene CpG islands in small-cell carcinoma of the urinary bladder. Mod Pathol 21:355–362
Wang X, Zhang S, MacLennan GT, Eble JN, Lopez-Beltran A, Yang XJ, Pan CX, Zhou H, Montironi R, Cheng L (2007) Epidermal growth factor receptor protein expression and gene amplification in small cell carcinoma of the urinary bladder. Clin Cancer Res 13:953–957
Shin SJ, DeLellis RA, Ying L, Rosen PP (2000) Small cell carcinoma of the breast. A clinicopathological and immunohistochemical study of nine patients. Am J Surg Pathol 24:1231–1238
Choi SJ, Kim JM, Han JY, Ahn SI, Kim JS, Kim L, Park IS, Chu YC (2007) Extrapulmonary small cell carcinoma of the liver: clinicopathological and immunohistochemical findings. Yonsei Med J 48:1066–1071
Ordóñez NG (2000) Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 24:1217–1223
Cheuk W, Kwan MY, Suster S, Chan JK (2001) Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the detection of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med 125:228–231
Siami K, McCluggage WG, Ordonez NG, Euscher ED, Malpica A, Sneige N, Silva EG, Deavers MT (2007) Thyroid transcription factor-1 expression in endometrial and endocervical adenocarcinomas. Am J Surg Pathol 31:1759–1763
Lee WJ, Kim CH, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK (2009) Cutaneous metastasis from large-cell neuroendocrine carcinoma of the urinary bladder expressing CK20 and TTF-1. Am J Dermatopathol 31:166–169
Byrd-Gloster AL, Khoor A, Glass LF, Messina JL, Whitsett JA, Livingston SK, Cagle PT (2000) Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol 31:58–62
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors acknowledge no conflict of interest in the elaboration of the present manuscript.
Rights and permissions
About this article
Cite this article
Fernández-Aceñero, M.J., Córdova, S., Manzarbeitia, F. et al. Immunohistochemical Profile of Urothelial and Small Cell Carcinomas of the Bladder. Pathol. Oncol. Res. 17, 519–523 (2011). https://doi.org/10.1007/s12253-010-9341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-010-9341-z