Abstract
Neuroendocrine and small cell carcinomas of the urinary tract are rare diseases which result in early metastasis with a poor outcome. The most frequent presentation in the urinary tract is small cell bladder cancer which is best treated by neoadjuvant chemotherapy and radiotherapy or cystectomy if the disease is limited to the pelvic area. In patients with distant metastasis, the prognosis is dismal despite palliative chemotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neuroendocrine
- Small cell
- Bladder cancer
- Cisplatin
- Chemotherapy
- Cystectomy
- Chemoradiotherapy
- Bladder sparing
Introduction
Neuroendocrine and small cell carcinoma of the bladder (SCCB) are rare conditions, accounting for approximately 0.5–0.7 % of urothelial malignancies [1, 2, 11, 26] Due to this low incidence, the published single-institution reports on this topic contain limited numbers of patients (ranging from 5 to 125) and are mainly retrospective. A consensus on the optimal treatment strategy has not been reached [5, 36, 57], though attempts at national guidelines have been made. Initially radical cystectomy was considered the standard of care for patients with clinically localized disease. However, the high rate of metastases has led to the introduction of multimodality approaches with systemic chemotherapy combined with either surgery or radiotherapy [2, 5, 8, 36, 57].
In the 1980s it was recognized that the biological and clinicopathological features of SCCB are similar to those of small cell lung carcinoma (SCLC) [24, 43]. Thus the treatment approach to this rare tumor has been greatly influenced by the treatment of the far more common SCLC. In the approach of SCLC, a distinction is made between patients with limited disease (tumor confined to the hemithorax, mediastinum, or supraclavicular lymph nodes) and patients with extensive disease (tumor outside these areas) [55]. Patients with limited disease (LD) SCLC are generally treated with a combination of systemic chemotherapy and local radiotherapy [22, 32, 59, 60]. This multimodality treatment is applied to address the risk of occult micrometastases at the time of diagnosis. Patients with extensive disease (ED) SCLC are treated with palliative chemotherapy only [55]. Considering the similar tumor biology of SCCB and SCLC, some authors define limited (LD) and extensive disease (ED) SCCB in analogy to SCLC to guide treatment decisions [8, 35, 44]. It has been suggested by some institutions that the treatment approach for LD-SCCB should follow the multimodality treatment applied for LD-SCLC (i.e., systemic chemotherapy combined with external beam radiotherapy) rather than cystectomy subsequent to neoadjuvant therapy [8, 36].
Definition of Neuroendocrine and Small Cell Carcinoma of the Bladder
The first case of primary small cell bladder cancer (SCCB) was reported in 1981 [16]. Since then approximately 800–1,000 cases diagnosed according to World Health Organization (WHO) criteria [23, 24] have been published in small single-arm prospective studies, retrospective series, and case reports. Based on the WHO classification, SCCB is defined as appearance of typical oat cell-shaped tumor cells at light microscopy. SCCB may consist of additional other bladder cancer subtypes and neuroendocrine cells but the diagnostic leading feature is the presence of small cells. In the literature, neuroendocrine tumors and SCCB are occasionally grouped together to describe outcome of treatment approaches [10]. However, they are not a single disease entity. SCCB may often contain neuroendocrine cells, but not exclusively and not consistently [21] (see also chapter “Diagnosis” and Table 8.1). The concomitant occurrence of other bladder cancer subtypes and neuroendocrine cells has prompted several theories about the origin of SCCB of which the theory of a common pluripotent stem cell in the urothelium leading to heterogeneity of tumor subtypes and a variety of epithelial and endocrine markers is favored [11]. Large cell neuroendocrine carcinoma (LCNC) is defined in the urinary bladder, as in other sites, as a high-grade neoplasm exhibiting neuroendocrine features at light microscopy with hematoxylin-eosin staining (H&E), high mitotic activity, and evidence of neuroendocrine differentiation by immunohistochemistry [15, 53]. Paraganglioma (PG) of the urinary bladder is a rare neuroendocrine neoplasm, accounting for <0.1 % of all bladder tumors [42].
Epidemiology
Neuroendocrine bladder carcinoma and SCCB are rare diseases. Of all bladder cancers, their frequency is less than 1 %. Based on the WHO definition of small cell carcinoma, which includes neuroendocrine variants, SCCB is a form of extrapulmonary small cell carcinoma (ESPCC). Small cell carcinoma accounts for one fifth of lung cancer cases but is rarely observed in extrapulmonary tumors [27]. In a recent Surveillance, Epidemiology and End Results (SEER) Program analysis, 55,722 cases of small cell carcinoma were diagnosed among the analyzed population between 1992 and 2010 (incidence rate = 81.8/million patient years). The incidence of SCLC (n = 51,959; incidence rate = 76.3) was 22 times more than that of extrapulmonary SCC (n = 2,438; incidence rate = 3.5). While SCLC accounted for 93 % of cases of small cell carcinoma, the urinary bladder seems to be among the most common extrapulmonary site. Of the extrapulmonary sites, incidence rates were low for the renal pelvis and ureter (incidence rate of urinary bladder 1.48 for men and 0.30 for women versus 0.07 for men and not assessable for women in the upper urinary tract). Small cell carcinoma IR was 35 % higher among men than women, with the greatest gender disparities for urinary bladder (male-to-female incidence rate ratio = 4.91) [18].
Extrapulmonary neuroendocrine tumors are rare. Neuroendocrine bladder cancer has been reported in only eight cases over a period of 3 years (2010–2012) in collective data from ten oncological centers in Germany [39]. This report did not distinguish between LCNC and neuroendocrine SCCB. Pure LCNC seems to be a rare disease and possibly underreported in the literature. A recent case report reviewed the literature and found only 12 cases of pure LCNC. The authors hypothesized that prior to the introduction of immunohistochemistry, most of these tumors which have a very aggressive course of disease were probably being diagnosed as high-grade undifferentiated urothelial cell carcinoma [51]. Due to the rarity of LCNC and absence of treatment recommendations, this chapter will predominantly focus on SCCB.
Clinical Presentation
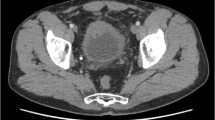
SCCB often presents with large bladder tumors (Fig. 8.1a) and, in elderly patients, gross hematuria in up to 94 % and early metastasis [21]. This is similar to the presentation of neuroendocrine bladder tumors, both SCCB or LCNC. A case series demonstrated that neuroendocrine bladder cancer is predominantly a disease of the elderly, who present with distant metastatic disease at the time of diagnosis in up to 50 % cases [10]. Patients with SCCB are typically elderly men and in some series more than half of the patients were over 70 years of age [13, 14]. A SEER study of SCCB (n = 642) confirmed the predominance of elderly Caucasian men with a median age of 73 years. Thirty-six percent of the patients presented with distant metastatic disease at the time of diagnosis [35]. Advanced disease stage in an elderly population poses particular problems regarding treatment options. In a series of 32 patients with LD-SCCB, 4 patients (12.5 %) with a median age of 80 years (range 79–87 years) did not receive chemotherapy due to age-related comorbidity and were treated with radiotherapy only. One patient refused any treatment [7]. Preferred metastatic sites are the pelvic and retroperitoneal lymph nodes (Figs. 8.1d and 8.2b), liver, lung, bone, and brain [44]. With up to 12 % brain metastases, intracranial secondaries are more common than in conventional transitional cell carcinoma but less common than in SCLC [9]. In comparison to the already high percentage of clinically evident metastatic disease, occult micrometastases are a very common feature of SCCB and responsible for the poor outcome reported.
Computed tomography (CT) of the abdomen demonstrating a large small cell carcinoma of the bladder (SCCB) with extravesical extension (a); CT of the abdomen with extensive liver metastasis at diagnosis in a patient with extensive disease stage (b); fluorodeoxyglucose (FDG)-positron emission tomography (PET) of a primary SCCB after image attenuation for physiological excretion in the urine (c); FDG-PET in a patient with limited disease stage demonstrating pelvic lymph node metastasis
Typical oat cell-shaped appearance of small cell bladder cancer in a transurethral resection (TUR) specimen (20×) (a); lymph node metastasis of SCCB with peripheral remnants of lymph node tissue (10×) (b); TUR specimen of SCCB staining positive for chromogranin A (20×) (c); TUR specimen of SCCB positive for synaptophysin (20×) (d)
Another difference between SCLC and SCCB is observed in the percentage of patients with extensive disease. Sixty to 70 % have extensive SCLC at presentation, whereas some authors reported only 30 % in SCCB. This may be due to a difference in definition or clinical signs such as hematuria leading to an early diagnosis, but it is known that extent of disease and prognosis is partially depending on the primary disease site [38]. Whether this is due to distinct anatomical features of a particular site or underlying differences in genetic patterns remains to be determined.
Paraneoplastic symptoms have been described such as hypercalcemia, Lambert-Eaton myasthenic syndromes, or symptoms originating from ectopic ACTH secretion [14, 44, 54].
Diagnosis
Usually the diagnosis is made by histopathological examination of the transurethral resection (TUR) specimen revealing appearance of typical oat cell-shaped tumor cells at light microscopy (Fig. 8.2a). This can be challenging in case of smaller tumors and non-muscle invasive bladder cancer (NMIBC) because of limited tissue sample sizes and because the clinical appearance does not suggest the presence of a more aggressive variant [56]. In these cases immunohistochemistry may be of additional value as SCCB expresses a variety of neuroendocrine markers (Table 8.1, Fig. 8.2c–d). The presence of neuroendocrine and small cell components is of poor prognostic relevance with increased risk for recurrence and progression. However, the prognostic significance of neuroendocrine marker expression in addition to an existing small cell cancer type remains controversial. Nevertheless, patients with small cell NMIBC need to be clearly identified as they are not candidates for bladder instillation therapies but should receive treatment as outlined in the following sections. Unfortunately, this is often not the case in both non-muscle invasive and muscle-invasive bladder cancer. Paraganglioma or LCNC of the bladder may be misdiagnosed as undifferentiated high-grade urothelial cell carcinoma [42]. Often small cell components are not recognized in the specimen collected at TUR or are not part of the resected material. In a series of 32 patients with LD-SCCB, 7 patients (21.9 %) were treated with cystectomy without neoadjuvant chemotherapy because the small cell component was only revealed in the final specimen and not at TUR [7]. Combined SCCB is observed between 23 % and 75 % and the transitional cell carcinoma, adenocarcinoma, or squamous cell components have no apparent prognostic influence [11, 13, 21, 26]. The proportion of the non-SCCB component may exceed the resected volume. There are several reports in the literature suggesting that the presence of SCCB in combined bladder tumors is the leading prognosticator and that these combined tumors should be managed like pure SCCB [6, 38].
Staging and Prognosis
Bladder cancer, including neuroendocrine and SCCB, is staged according to the UICC TNM classification. Clinical staging depends on imaging with computed tomography (CT) of the chest and abdomen. Fluorodeoxyglucose (FDG)-positron emission tomography (PET) may further help to identify systemic disease (Fig. 8.1c–d). Several TNM versions have been used in the past and differences need to be taken into account when evaluating the reported outcome in the literature. However, in a large retrospective analysis of SCCB [38], tumor stage was not independently associated with survival suggesting that micrometastases are often present in clinically localized disease. Due to very early micrometastasis at diagnosis, some authors favor the division of patients with SCCB into groups with limited (LD-SCCB) and extensive disease (ED-SCCB) in analogy to the far more common SCLC [44].
In this staging approach patients with tumor confined to the pelvis are defined as limited disease. Furthermore, it provides a useful classification for both treatment and prognosis. Patients with LD-SCCB have a significantly better outcome compared to patients with ED-SCCB.
In general the prognosis of SCCB is poor, with 5-year OS ranging from 8 % to 44 % for limited disease [2, 7, 36]. In earlier reports only platinum-based combination chemotherapy has been associated with significant improvement of survival regardless of the regimen used [37, 38, 57].
Neuroendocrine variants and SCCB with components of other bladder tumor subtypes have been studied with regard to a different prognosis. Evidence from the literature supports that the presence of SCCB in combined bladder tumors is the leading prognosticator [21, 38] and that it should be managed like pure SCCB with cisplatin-based chemotherapy. If the component obtained at TUR was predominantly transitional cell carcinoma (>50 %), some authors applied MVAC as suggested in the literature [7, 8, 41]. The transitional cell carcinoma, adenocarcinoma, or squamous cell components have no apparent prognostic influence, although a retrospective series in which patients were treated with cystectomy only suggested that mixed subtypes tended to have a better outcome than pure SCCB (p = 0.064) [52]. However, this series of 25 patients also included 5 LCNC. In a more recent series of 18 neuroendocrine tumors, 14 of which were SCCB including other various subtypes, an OS analysis revealed no difference between pure neuroendocrine tumors and those with mixed subtypes [10].
Due to the paucity of LCNC bladder cancer, there is considerable uncertainty regarding the prognosis of this disease in comparison to SCCB or neuroendocrine SCCB. A retrospective analysis of 572 bladder tumors revealed 14 cases of neuroendocrine SCCB and 4 LCNC bladder cancers [10]. The authors compared the course of disease and outcome of these patients. Interestingly, one patient had SCCB on the primary site and large LCNC on the metastatic site. Overall survival did not differ between SCCB and LCNC; however, the study was limited by different treatment modalities and very low numbers of patients with LCNC [10].
Treatment
The paucity of SCCB has not encouraged to design and conduct prospective randomized trials and the optimal therapeutic strategy is still unknown. Sufficient data demonstrate a similarity of the clinical course of SCCB and SCLC which in the past has been used as a rationale to introduce chemotherapy into the treatment algorithm of SCCB [6]. In SCLC survival increased only after the introduction of multi-agent chemotherapy regimens. Most of the benefit occurred in patients less than 65 years of age [22]. The definition of limited SCLC takes early metastasis into account with tumor confined to the hemithorax of origin, the mediastinum, or the supraclavicular lymph nodes [32]. All patients with tumor beyond these limits are considered to have extensive disease. The current treatment of limited SCLC consists of a combination of cisplatin and etoposide plus irradiation of the chest preferably during the first or second cycle of chemotherapy [25, 49]. Prophylactic brain radiation follows in patients with a complete response [32]. This strategy leads to median survival of 18–24 months and 50 % 2-year survival. Due to early micrometastasis, the overall survival of SCLC remains poor with 5–10 % after 5 years [55].
Currently, there is no consensus regarding the optimal treatment for limited disease SCCB. A 12-year National Cancer Database analysis on clinical characteristics and treatment patterns of 625 patients with SCCB revealed that most patients were treated with a multimodal bladder-preserving approach [48]. Upfront cystectomy with adjuvant chemotherapy has been propagated [21] as well as combinations of neoadjuvant chemotherapy with transurethral resection (TUR), cystectomy, and partial cystectomy and radiotherapy [26, 38, 43]. A contemporary report on 107 cases from an International Rare Cancer Network revealed a broad range of surgical or bladder-sparing approaches with or without adjuvant or neoadjuvant chemotherapy in the current era [47]. A Canadian consensus guideline from 2013 established the evidence base in a robust narrative review from the English language literature from 1946 until 2013 for the role of neoadjuvant chemotherapy in combination with either cystectomy or radiotherapy of the bladder [44]. Retrospective series suggest that platinum-based chemotherapy is essential. The benefit of cisplatin-based chemotherapy for SCCB has been observed in early studies [36, 38, 57]. Conversely, in SCLC, two or more drugs are needed for maximal effect but most regimens produced similar survival outcomes regardless of cisplatin [25]. In SCCB, combinations without cisplatin were not associated with prolonged survival [38], though this should be interpreted with caution. Good performance status required for cisplatin-based chemotherapy may explain the observed association of this drug with improved survival in retrospect. Chemotherapy is the only treatment option for patients with distant metastatic disease (extensive SCCB).
Limited Disease SCCB
Cystectomy as Single Treatment Modality
Historically, cystectomy was the preferred treatment for SCCB although the poor prognosis of the disease became rapidly apparent after its first description in 1981. Due to early micrometastases, cystectomy only is no longer recommended in reviews of the literature or national consensus documents [44]. This applies mainly to SCCB but is probably also true for LCNC of the bladder. There are patients, particularly in earlier stages (pT1–2 N0M0) in whom cure has been reported after cystectomy only [34]. In a retrospective analysis of the Mayo Clinic on 44 patients with SCCB, 12 patients had pT1–2 N0M0. Five-year survival for this group was reported at 63.3 % and six of eight who underwent cystectomy only were considered cured [14]. Finally, retrospective studies that compared patients with neoadjuvant chemotherapy and cystectomy to those with cystectomy only or adjuvant chemotherapy clearly suggest that cystectomy as single treatment modality or followed by adjuvant chemotherapy is far inferior in terms of survival and downstaging, including lower stages who are often clinically understaged [57] (Table 8.2).
Neoadjuvant Chemotherapy
Due to the inferior results with cystectomy, only some institutions have propagated neoadjuvant chemotherapy as an essential modality in the treatment of SCCB. Neoadjuvant chemotherapy has been chosen in analogy to regimens accepted in the treatment of SCLC (Table 8.3). Some institutions chose methotrexate 30 mg/m2, vinblastine 3 mg/m2, doxorubicin 30 mg/m2, and cisplatin 70 mg/m2 (M-VAC) for patients with <50 % SCCB in combination with urothelial carcinoma in the primary TUR-BT specimen. The number of courses differs, but often four courses were given with response evaluation after the first two courses. The optimal number of cycles of neoadjuvant chemotherapy is currently unknown. In a clinical trial of neoadjuvant chemotherapy for SCCB [58], 18 patients received four cycles of alternating chemotherapy. While patients with cT2N0 disease had a high likelihood of cure with this approach, those with stage cT3a-4 N0 did not fare as well, with SCCB remaining at cystectomy. This may reflect either poor biology or the need for additional chemotherapy in the setting of more bulky disease. Over the past years the cisplatin-based SCLC regimens changed. Four courses of ifosfamide 1.2 g/m2 (maximum 1.75 g), VP-16 (etoposide) 75 mg/m2, and cisplatin 20 mg/m2 (VIP) on days 1–4 repeated after 21 days were later replaced by 4 courses of cisplatin 75 mg/m2 day 1 with etoposide 100 mg/m2 intravenous (CE) on days 1–3, repeated after 21 days [32]. In one study patients with SCCB and contraindications for cisplatin but a performance score of WHO ≤2 received five courses of cyclophosphamide 1 g/m2 (day 1), doxorubicin 45 mg/m2 (day 1), and etoposide 100 mg/m2 (days 1–3) (CDE) repeated after 21 days. Later that regimen was changed to carboplatin AUC 5 (day 1) with etoposide 100 mg/m2 intravenous (CaE) on days 1–3, repeated after 21 days [7]. One prospective phase II trial investigated alternating chemotherapy with cisplatin/etoposide and doxorubicin/ifosfamide until cystectomy [58].
Response to neoadjuvant chemotherapy should be evaluated according to RECIST 1.1 and based on CT scan and cystoscopy. In doubtful cases TUR-BT or biopsy should be performed [19].
Two strategies are currently followed and recommended with level 3, grade C according to Oxford Centre of Evidence-Based Medicine (OCEBM) [44].
Neoadjuvant Chemotherapy and Cystectomy
Several studies including a prospective single-arm phase II trial revealed that for LD-SCCB, neoadjuvant chemotherapy followed by surgery can result in a 5-year survival of up to 80 % as reported in a subset of patients with resectable LD-SCCB [57, 58] (Table 8.2). In a series of 88 patients with neuroendocrine SCCB, 46 underwent cystectomy including 21 after neoadjuvant chemotherapy. Of the 25 patients with cystectomy, only 7 were treated with adjuvant chemotherapy. Independent of the fact that adjuvant therapy did not improve outcome, median cancer-specific survival (CSS) for initial cystectomy was 23 months, with only 36 % disease-free at 5 years. Contrary, for patients receiving neoadjuvant chemotherapy, median CSS had not been reached (p = 0.026) at the time the study reported, with a CSS at 5 years of 78 % and no cancer-related deaths observed beyond 2 years [57]. The most impressive outcome was reported in a large retrospective comparison performed by the authors of the prospective phase II trial. In a series of 95 patients with LD-SCCB who underwent cystectomy, 48 received neoadjuvant chemotherapy, and 47 underwent initial cystectomy. Neoadjuvant treatment was associated with improved OS and disease-specific survival compared with patients who underwent initial cystectomy. Median OS was 159.5 months versus 18.3 months, (p < 0.001) and the 5-year disease-specific survival (DSS) 79 % versus 20 % (p < 0.001). Moreover, neoadjuvant chemotherapy resulted in pathologic downstaging to ≤ pT1N0 in 62 % of tumors compared with only 9 % in patients treated with initial cystectomy and lymphadenectomy. Even in patients with clinically node-positive disease, neoadjuvant therapy and cystectomy led to clinical complete responses by chemotherapy and surgery in eight patients with a median OS of 23.3 months and 5-year OS of 38 % [37]. The majority of patients in these studies received cisplatin/etoposide or ifosfamide/doxorubicin alternating with cisplatin/etoposide [37, 57, 58]. Of note, these impressive survival outcomes are better than those reported after neoadjuvant chemotherapy for conventional urothelial bladder cancer and may be due to selection. In the randomized phase three trial of neoadjuvant chemotherapy for bladder cancer, the median OS for those receiving chemotherapy was 56 months at inclusion of cT1–4a cN0/x cM0 patients [20].
However, the results clearly suggest the beneficial roles of neoadjuvant chemotherapy in combination with cystectomy. Neoadjuvant chemotherapy results in significant pathological downstaging which may not only improve outcome but facilitate surgery [37]. Conversely, adjuvant chemotherapy following cystectomy was not shown to be superior to cystectomy alone although the numbers of patients receiving adjuvant therapy were small (7 of 25 and 21 of 47) [37, 57] (Table 8.2). In a retrospective SEER database analysis, chemotherapy improved outcome across all stages, but not in addition to cystectomy [35].
Bladder Preservation with Chemoradiotherapy
Despite cystectomy after neoadjuvant chemotherapy, SCCB remains to portend a dismal prognosis. Most institutions have reported a median OS for nonmetastatic SCCB of 13–23 months [44], although exceptional median OS of 58 months has been reported with this approach [58]. This has prompted investigation of bladder preservation with chemoradiotherapy (Table 8.4). Sequential chemoradiation for LD-SCCB results in a reasonable outcome with a high bladder preservation rate [4]. In general in SCLC patients, radiotherapy is applied concomitantly with the chemotherapy [29]. However, experience with an increased risk for local toxicity in the bladder after concurrent chemoradiation has led some institutions to schedule external beam radiotherapy (EBRT) after the neoadjuvant chemotherapy. EBRT has been applied using 8–18 MV photons with a three- or four-field technique. The median dose was 60 Gy. The target area consisted of the bladder and the tumor. When the total dose was 70 Gy, 50 Gy was given to the bladder and tumor with a 20 Gy boost to the tumor area only [7]. Early reports of small patient series with LD-SCCB reported long-term survival with three of five patients alive and free of disease 60, 48, and 27 months after diagnosis [5]. In another series of ten patients treated with sequential chemoradiation from British Columbia, five patients were alive and disease-free an average of 82 months following diagnosis [36].
Neoadjuvant Chemotherapy Followed by Cystectomy Versus Bladder Sparing with Sequential Chemoradiation
There are no prospective randomized studies comparing treatment modalities for SCCB. Recently Koay et al. reported on a large subset of patients from the Surveillance, Epidemiology, and End Results (SEER) Medicare database with SCCB treated with chemotherapy and radiotherapy versus cystectomy with chemotherapy, showing no significant differences in OS between the two treatment modalities [35]. Chemotherapy was shown to improve outcome in all stages of disease including those patients who were treated with TUR as their only surgical procedure. A bladder-sparing approach involving TUR combined with chemotherapy and radiation showed no significant difference in OS compared with patients undergoing at least a cystectomy (of whom over 90 % received radical cystectomy) with chemotherapy (p > 0.05). However, this report has several limitations and did not distinguish between neoadjuvant or adjuvant chemotherapy in combination with cystectomy. Nevertheless, outcome data of several studies suggest that upfront chemotherapy may be the most important therapeutic modality with local therapeutic treatment options such as cystectomy, radiotherapy, or even complete TUR being secondary.
As with conventional transitional cell carcinoma of the bladder, the risk of bladder sparing has to be balanced against the local recurrence rate. The risk of local recurrence of transitional cell carcinoma after primary mixed tumors has been reported in several studies, especially in long-term survivors after chemoradiation [5, 36]. Though 5-year OS following bladder sparing with chemoradiation has been reported in a small case series [36], this approach has been criticized for the relatively high rate of local recurrences. Local recurrence rates of 20–69 % have been reported [5, 36] in small series of five and eight patients, respectively. In a larger retrospective analysis of 27 LD-SCCB treated with sequential chemoradiation, local recurrence in the bladder was seen in 29.6 % of patients [41]. Histopathology of the recurrences in the bladder revealed small cell carcinoma in two patients (7.4 %) and transitional cell carcinoma in six patients (22.2 %). The median time to local recurrence was 29 months. In some cases local recurrence in the bladder can be treated with conservative therapy (e.g., TUR-BT and adjuvant intravesical BCG instillations). In the group with LD-SCCB and sequential chemoradiation, the bladder preservation rate was 85.2 %.
Considering the nature of retrospective analyses with their inherent bias which influences the comparability of data, it appears that overall and progression-free survival is similar for local therapies such as cystectomy and radiotherapy as long as systemic chemotherapy had been applied. Cheng et al. retrospectively analyzed 64 cases and found no survival difference between those who had cystectomy and those who had not. Interestingly, none of the parameters age, gender, presenting symptoms, smoking history, the presence of a non-small cell carcinoma component, chemotherapy, or radiation therapy were associated with survival. Consequently, the authors raised doubt about the effectiveness of cystectomy as treatment modality. The 1- and 5-year survival times of those who had a cystectomy were 57 % and 16 % versus 55 % and 18 % for those who had no cystectomy [13]. However, the chemotherapy in those who underwent cystectomy was applied as adjuvant therapy which does not appear to be as effective as the neoadjuvant approach. As has been discussed in the previous paragraph, the majority of retrospective studies and one prospective study support neoadjuvant therapy when cystectomy is planned. Consequently, these data should be compared to the bladder-sparing chemoradiotherapy data. In a retrospective series of 17 patients with LD-SCCB treated with sequential chemoradiation, the 1- and 5-year survival estimate was 82 % (C.I. 0.56–0.92) and 36 % (C.I. 0.14–0.61), respectively [7].
Treatment Options for Elderly Comorbid Patients
Patients with SCCB are predominantly elderly men and in some series more than half of the patients were over 70 years of age [48]. Though there have been reports that chemotherapy for SCLC is feasible in elderly patients, a high rate of age-related comorbidity among patients older than 70 years has been observed [8]. In a series of 25 patients with SCCB, 48 % of patients were older than 70 years (12/25). In patients with limited disease unfit for chemotherapy, radiotherapy subsequent to a macroscopically complete TUR can be considered as a treatment option, especially if the disease is localized. Long-term survivors have been reported in a retrospective series with this strategy [26]. A more recent SEER database analysis of 533 patients with SCCB revealed that the majority of patients (54 %) received a TUR as their only surgical treatment [35]. A subset analysis of these patients indicated that chemotherapy played a role in all stages of disease (p < 0.05) whereas radiation improved overall survival in regional-stage disease (p < 0.05) [35]. These data however are retrospective and prone to selection bias. Exceptionally, cystectomy as single treatment modality can be considered if severe locoregional symptoms and/or contraindications for radiotherapy were present. In a series of 17 patients with LD-SCCB, ultimately 9 patients (52.9 %) could not be treated with chemotherapy and sequential radiotherapy, mostly because of PS WHO 3 (n = 7) [8].
Distant Metastatic SCCB (Extensive Disease)
Patients with clinically evident extensive disease or distant metastasis have a poor outcome. The mainstay of therapy is systemic chemotherapy in analogy with the regimen given for SCLC described in the section on neoadjuvant chemotherapy. Reported median OS in the literature does not exceed 5–8 months and palliation is the main objective of therapy [30, 35].
Follow-Up and Prophylactic Cranial Irradiation
Due to frequent and late local recurrences in case of a bladder-sparing approach, regular follow-up with cystoscopy is mandatory for a prolonged period. In some instances, recurrences were diagnosed after almost 5 years of follow-up. No general imaging recommendations exist but cross-sectional imaging with computed tomography of chest and abdomen as for conventional bladder carcinoma is suggested. Patients with SCLC have a significant risk for the development of brain metastases (up to 60 % within 2–3 years after starting treatment). Therefore patients with complete response to chemotherapy are offered prophylactic cranial irradiation [32]. Similarly patients with SCCB show a risk for the development of brain metastases. Siefker-Radtke et al. reported up to 26.7 % brain metastases in patients with SCCB [58]. In a retrospective long-term analysis of patients with SCCB, 12.1 % developed symptomatic brain metastases [41]. An earlier analysis and review of the literature reported a pooled estimate of cumulative incidence of symptomatic brain metastases of 10.5 % [9]. This incidence is higher than brain metastases from transitional cell carcinoma of the bladder (approximately 3 %) but far lower than for SCLC. Differences in frequency of brain metastasis reported in the literature can be explained by routine brain scanning during follow-up versus cross-sectional imaging performed in symptomatic patients only. There are no studies indicating superiority of prophylactic cranial irradiation to cranial irradiation in SCCB patients with symptomatic brain metastases.
Conclusion: Future Therapeutic Strategies
There have been reports on the beneficial effects of concurrent administration of radiosensitizing agents (e.g., chemotherapy) potentiating the cytotoxic effect of radiotherapy for bladder cancer [31]. As the techniques of EBRT have evolved in recent years and the risks of local toxicity have been further reduced, the use of concurrent chemoradiation may be expected to gain terrain. Regarding chemotherapy for SCCB, new regimens are primarily investigated in the more common SCLC. Some authors suggest that PEI (platinum, etoposide, ifosfamide) is more effective than PE based on a randomized trial [12] but this is not supported by a systematic review [61]. Somatostatin may increase the efficacy of chemotherapy in SCLC [17].
There are very limited data on the second-line therapeutic options for patients who fail platinum-based chemotherapy. In a series including three patients with SCCB, single-agent weekly vinorelbine had shown promising safety and efficacy profile [33]. Targeted agents are being investigated but the paucity of the disease may require comparison with SCLC [50]. Expression of c-kit was investigated in 52 cases of SCCB [46]. Overall, 14 of 52 (27 %) SCCB were positive for c-kit expression when defining less than 10 % staining as negative. Outcome in the entire series was as reported previously. During a median follow-up of 11 months, 60 % of the patients died of disease. While no association was found between c-kit expression and survival or other clinicopathological parameters, 27 % of SCCB expressed c-kit, which may be a therapeutic target for imatinib. In addition, mTOR inhibitors have been investigated in preclinical models as has been the mechanism of resistance to everolimus [40].
References
The World Health Organization histological typing of lung tumours. 2nd ed. Am J Clin Pathol. 1982;77(2):123–36.
Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995;46:617–30.
Abrahams NA, Moran C, Reyes AO, Siefker-Radtke A, Ayala AG. Small cell carcinoma of the bladder: a contemporary clinicopathological study of 51 cases. Histopathology. 2005;46:57–63.
Asmis TR, Reaume MN, Dahrouge S, Malone S. Genitourinary small cell carcinoma: a retrospective review of treatment and survival patterns at the Ottawa Hospital Regional Cancer Center. BJU Int. 2006;97:711–5.
Bastus R, Caballero JM, Gonzalez G, Borrat P, Casalots J, Gomez De Segura G, Marti LI, Ristol J, Cirera L. Small cell carcinoma of the urinary bladder treated with chemotherapy and radiotherapy: results in five cases. Eur Urol. 1999;35:323–6.
Bex A. Review: treatment of small cell carcinoma of the urinary bladder: can we learn from small cell lung cancer? Clin Adv Hematol Oncol. 2008;6:385–6.
Bex A, DE Vries R, Pos F, Kerst M, Horenblas S. Long-term survival after sequential chemoradiation for limited disease small cell carcinoma of the bladder. World J Urol. 2009;27:101–6.
Bex A, Nieuwenhuijzen JA, Kerst M, Pos F, Van Boven H, Meinhardt W, Horenblas S. Small cell carcinoma of bladder: a single-center prospective study of 25 cases treated in analogy to small cell lung cancer. Urology. 2005;65:295–9.
Bex A, Sonke GS, Pos FJ, Brandsma D, Kerst JM, Horenblas S. Symptomatic brain metastases from small-cell carcinoma of the urinary bladder: the Netherlands Cancer Institute experience and literature review. Ann Oncol. 2010;21:2240–5.
Bhatt VR, Loberiza Jr FR, Tandra P, Krishnamurthy J, Shrestha R, Wang J. Risk factors, therapy and survival outcomes of small cell and large cell neuroendocrine carcinoma of urinary bladder. Rare Tumor. 2014;6:5043.
Blomjous CE, Vos W, de Voogt HJ, van der Valk P, Meijer CJ. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer. 1989;64:1347–57.
Boni C, Pagano M, Baldi L, Gnoni R, Braglia L, Savoldi L, Zanelli F. Pei regimen: a therapeutic option in small cell lung cancer? A retrospective monoinstitutional analysis of 46 consecutive cases. J Transl Med. 2015;13:130.
Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, Maclennan GT, Lin H, Kuzel TM, Papavero V, Tretiakova M, Nigro K, Koch MO, Eble JN. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer. 2004;101:957–62.
Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer. 2005;103:1172–8.
Coelho HM, Pereira BA, Caetano PA. Large cell neuroendocrine carcinoma of the urinary bladder: case report and review. Curr Urol. 2013;7:155–9.
Cramer SF, Aikawa M, Cebelin M. Neurosecretory granules in small cell invasive carcinoma of the urinary bladder. Cancer. 1981;47:724–30.
Domvri K, Bougiouklis D, Zarogoulidis P, Porpodis K, Xristoforidis M, Liaka A, Eleutheriadou E, Lampaki S, Lazaridis G, Organtzis J, Kyriazis G, Hohenforst-Schmidt W, Tsirgogianni K, Karavasilis V, Baka S, Darwiche K, Freitag L, Trakada G, Zarogoulidis K. Could somatostatin enhance the outcomes of chemotherapeutic treatment in SCLC? J Cancer. 2015;6:360–6.
Dores GM, Qubaiah O, Mody A, Ghabach B, Devesa SS. A population-based study of incidence and patient survival of small cell carcinoma in the United States, 1992–2010. BMC Cancer. 2015;15:185.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7.
Grignon DJ, Ro JY, Ayala AG, Shum DT, Ordonez NG, Logothetis CJ, Johnson DE, MacKay B. Small cell carcinoma of the urinary bladder. A clinicopathologic analysis of 22 cases. Cancer. 1992;69:527–36.
Hand S, Baker J, Smith AP, Macbeth FR. Outpatient intensive chemotherapy for small cell lung cancer: five years experience of modified ‘ICE’ ifosfamide carboplatin and etoposide. Clin Oncol (R Coll Radiol). 2002;14:367–71.
Hirsch FR, Matthews MJ, Aisner S, Campobasso O, Elema JD, Gazdar AF, MacKay B, Nasiell M, Shimosato Y, Steele RH, et al. Histopathologic classification of small cell lung cancer. Changing concepts and terminology. Cancer. 1988;62:973–7.
Hirsch FR, Matthews MJ, Yesner R. Histopathologic classification of small cell carcinoma of the lung: comments based on an interobserver examination. Cancer. 1982;50:1360–6.
Holbrechts S, Roelandts M. Treatment of small cell lung cancer: limited disease. Guidelines of clinical practice made by the European Lung Cancer Working Party. Rev Med Brux. 2014;35:160–3.
Holmang S, Borghede G, Johansson SL. Primary small cell carcinoma of the bladder: a report of 25 cases. J Urol. 1995;153:1820–2.
Ibrahim NB, Briggs JC, Corbishley CM. Extrapulmonary oat cell carcinoma. Cancer. 1984;54:1645–61.
Iczkowski KA, Shanks JH, Allsbrook WC, Lopez-Beltran A, Pantazis CG, Collins TR, Wetherington RW, Bostwick DG. Small cell carcinoma of urinary bladder is differentiated from urothelial carcinoma by chromogranin expression, absence of CD44 variant 6 expression, a unique pattern of cytokeratin expression, and more intense gamma-enolase expression. Histopathology. 1999;35:150–6.
Ismaili N, Elkarak F, Heudel PE, Flechon A, Droz JP. Small cell cancer of the bladder: the Leon-Berard cancer centre experience. Indian J Urol. 2008;24:494–7.
Ismaili N, Heudel PE, Elkarak F, Kaikani W, Bajard A, Ismaili M, Errihani H, Droz JP, Flechon A. Outcome of recurrent and metastatic small cell carcinoma of the bladder. BMC Urol. 2009;9:4.
James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–88.
Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e400S–19.
June RR, Dougherty DW, Reese CT, Harpster LE, Hoffman SL, Drabick JJ. Significant activity of single agent vinorelbine against small-cell cancer of the bladder as second line chemotherapy: a case series and review of the literature. Urol Oncol. 2012;30:192–5.
Kaushik D, Frank I, Boorjian SA, Cheville JC, Eisenberg MS, Thapa P, Tarrell RF, Thompson RH. Long-term results of radical cystectomy and role of adjuvant chemotherapy for small cell carcinoma of the bladder. Int J Urol. 2015;22:549–54.
Koay EJ, Teh BS, Paulino AC, Butler EB. A surveillance, epidemiology, and end results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer. 2011;117:5325–33.
Lohrisch C, Murray N, Pickles T, Sullivan L. Small cell carcinoma of the bladder: long term outcome with integrated chemoradiation. Cancer. 1999;86:2346–52.
Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE, Dinney CP, Siefker-Radtke A. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013;64:307–13.
Mackey JR, Au HJ, Hugh J, Venner P. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998;159:1624–9.
Maschmeyer G, Mugge LO, Kampfe D, Kreibich U, Wilhelm S, Assmann M, Schwarz M, Kahl C, Kohler S, Grobe N, Niederwieser D. A retrospective review of diagnosis and treatment modalities of neuroendocrine tumors (excluding primary lung cancer) in 10 oncological institutions of the East German Study Group of Hematology and Oncology (OSHO), 2010–2012. J Cancer Res Clin Oncol. 2015.
Matsumoto M, Seike M, Noro R, Soeno C, Sugano T, Takeuchi S, Miyanaga A, Kitamura K, Kubota K, Gemma A. Control of the MYC-eIF4E axis plus mTOR inhibitor treatment in small cell lung cancer. BMC Cancer. 2015;15:241.
Meijer RP, Meinhardt W, van der Poel HG, van Rhijn BW, Kerst JM, Pos FJ, Horenblas S, Bex A. Local control rate and prognosis after sequential chemoradiation for small cell carcinoma of the bladder. Int J Urol. 2013;20:778–84.
Menon S, Goyal P, Suryawanshi P, Tongaonkar H, Joshi A, Bakshi G, Desai S. Paraganglioma of the urinary bladder: a clinicopathologic spectrum of a series of 14 cases emphasizing diagnostic dilemmas. Indian J Pathol Microbiol. 2014;57:19–23.
Mills SE, Wolfe 3rd JT, Weiss MA, Swanson PE, Wick MR, Fowler Jr JE, Young RH. Small cell undifferentiated carcinoma of the urinary bladder. A light-microscopic, immunocytochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1987;11:606–17.
Moretto P, Wood L, Emmenegger U, Blais N, Mukherjee SD, Winquist E, Belanger EC, Macrae R, Balogh A, Cagiannos I, Kassouf W, Black P, Czaykowski P, Gingerich J, North S, Ernst S, Richter S, Sridhar S, Reaume MN, Soulieres D, Eisen A, Canil CM. Management of small cell carcinoma of the bladder: consensus guidelines from the Canadian Association of Genitourinary Medical Oncologists (CAGMO). Can Urol Assoc J. 2013;7:E44–56.
Oesterling JE, Brendler CB, Burgers JK, Marshall FF, Epstein JI. Advanced small cell carcinoma of the bladder. Successful treatment with combined radical cystoprostatectomy and adjuvant methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer. 1990;65:1928–36.
Pan CX, Yang XJ, Lopez-Beltran A, Maclennan GT, Eble JN, Koch MO, Jones TD, Lin H, Nigro K, Papavero V, Tretiakova M, Cheng L. c-kit expression in small cell carcinoma of the urinary bladder: prognostic and therapeutic implications. Mod Pathol. 2005;18:320–3.
Pasquier D, Barney B, Sundar S, Poortmans P, Villa S, Nasrallah H, Boujelbene N, Ghadjar P, Lassen-Ramshad Y, Senkus E, Oar A, Roelandts M, Amichetti M, Vees H, Zilli T, Ozsahin M. Small cell carcinoma of the urinary bladder: a retrospective, multicenter rare cancer network study of 107 patients. Int J Radiat Oncol Biol Phys. 2015;92:904–10.
Patel SG, Stimson CJ, Zaid HB, Resnick MJ, Cookson MS, Barocas DA, Chang SS. Locoregional small cell carcinoma of the bladder: clinical characteristics and treatment patterns. J Urol. 2014;191:329–34.
Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21:2244–55.
Puglisi M, Dolly S, Faria A, Myerson JS, Popat S, O’Brien ME. Treatment options for small cell lung cancer – do we have more choice? Br J Cancer. 2010;102:629–38.
Pusiol T, Morichetti D, Zorzi MG. “Pure” primary large cell neuroendocrine carcinoma of the urinary bladder: case report, literature review and diagnostic criteria. Pathologica. 2014;106:82–5.
Quek ML, Nichols PW, Yamzon J, Daneshmand S, Miranda G, Cai J, Groshen S, Stein JP, Skinner DG. Radical cystectomy for primary neuroendocrine tumors of the bladder: the university of southern California experience. J Urol. 2005;174:93–6.
Radović N, Turner R, Bacalja J. Primary “Pure” large cell neuroendocrine carcinoma of the urinary bladder: a case report and review of the literature. Clin Genitourin Cancer. 2015;13(5):e375–7. doi: 10.1016/j.clgc.2015.03.005. Epub 2015 Mar 30.
Reyes CV, Soneru I. Small cell carcinoma of the urinary bladder with hypercalcemia. Cancer. 1985;56:2530–3.
Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, Pietanza MC, Ramalingam SS, Turrisi 3rd AT, Giaccone G. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol. 2015;33:4106–11.
Seisen T, Comperat E, Leon P, Roupret M. Impact of histological variants on the outcomes of nonmuscle invasive bladder cancer after transurethral resection. Curr Opin Urol. 2014;24:524–31.
Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL, Grossman HB, Swanson DA, Millikan RE. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481–4.
Siefker-Radtke AO, Kamat AM, Grossman HB, Williams DL, Qiao W, Thall PF, Dinney CP, Millikan RE. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27:2592–7.
Turrisi 3rd AT. Limited stage small cell lung cancer: treatment and therapy. Curr Treat Options Oncol. 2003;4:61–4.
Wolff AC, Ettinger DS, Neuberg D, Comis RL, Ruckdeschel JC, Bonomi PD, Johnson DH. Phase II study of ifosfamide, carboplatin, and oral etoposide chemotherapy for extensive-disease small-cell lung cancer: an Eastern Cooperative Oncology Group pilot study. J Clin Oncol. 1995;13:1615–22.
Yang H, Ma Y, Liu Z, Wang Z, Han B, Ma L. Benefit from ifosfamide treatment in small-cell lung cancer: a meta-analysis. Mol Clin Oncol. 2015;3:420–4.
Acknowledgments
We thank Dr. J. de Jong, Department of Pathology of the Netherlands Cancer Institute, Amsterdam, for providing high-power field photomicrographs of SCCB.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Meijer, R., Bex, A. (2016). Neuroendocrine and Small Cell Carcinoma of the Urinary Tract. In: Pagliaro, L. (eds) Rare Genitourinary Tumors. Springer, Cham. https://doi.org/10.1007/978-3-319-30046-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-30046-7_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30044-3
Online ISBN: 978-3-319-30046-7
eBook Packages: MedicineMedicine (R0)