Abstract
Purpose
The objective of present study was to increase solubility and dissolution performance of a poorly water soluble antidiabetic drug, Nateglinide (NAT), through formation of inclusion complexes with hydroxypropyl-beta-cyclodextrin (HP–β–CD). The effect of L-arginine (ARG), an amino acid, on the complexation efficiency and solubility enhancing power of HP–β–CD was investigated by preparing ternary inclusion complexes.
Methods
The binary and ternary inclusion complexes were prepared by physical mixing, kneading, co-evaporation, and spray drying methods containing NAT, HP–β–CD, and ARG. The complexes were characterized by FTIR, DSC, PXRD, and 1H–NMR. Molecular modeling study revealed that introduction of ternary agent ARG have improved the interactions of NAT and HP–β–CD.
Results
The complex prepared by spray drying method showed the highest increase in solubility and dissolution rate compared to other methods. Molecular docking study revealed that ARG interactions plays an essential role in increasing the stability and solubility of the complex.
Conclusions
The present study demonstrated increase in solubility and dissolution of NAT. Hence, ternary complexes of NAT can be used as an efficient tool for the delivery of insoluble drug, NAT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor solubility has limited many therapeutic agents in exhibiting the desired pharmacological response. These drawbacks can be overcome by molecular encapsulation of drug with cyclodextrin to form complex which facilitates safe and efficient delivery of the drugs [1–6]. According to the literature, cyclodextrin inclusion complexation technique has wide commercial applicability, but some are associated with toxicity. Various derivatives of β-CD, e.g., hydroxypropyl–beta–cyclodextrin (HP–β–CD), have demonstrated low toxicity but are expensive. To overcome disadvantages of these cyclodextrin derivatives, it is proposed to incorporate the selected API in a ternary complex along with cyclodextrin and the ternary agent. This would help in enhancing the solubility, dissolution rate, and stability of poorly soluble drugs by synergistic effects as compared to the binary inclusion complexation techniques while reducing the amount of cyclodextrin by 80% [7, 8]. Cyclodextrins (CDs) are cyclic torus-shaped molecules which have a hydrophilic outer surface and a lipophilic central cavity that can accommodate a variety of lipophilic drugs [9]. Many favorable changes in the physicochemical properties of the drug, such as solubility, dissolution rate, stability and bioavailability, take place due to the formation of complexes. It is emphasized, however, that pharmaceutical dosage forms should contain as little CD as possible, because excess amount of CD can cause some problems of potential toxicity [10]. Also, there are cases where low complexation efficiency requires a larger amount of CD than that acceptable for various dosage forms. Hence, enhancement of the complexation capacity of the chosen CD is very crucial. Positive effect of the addition of small amounts of a ternary agent such as polymers to a drug-CD system to improve the complexation and solubilization efficiencies of the drugs has been reported [11, 12]. The ternary agents enhance the cyclodextrin complexation through the formation of the ternary complexes. Thus, reducing the amount of CD required in the formulation with improved absorption of the drug-CD complex [13]. Similarly, positive effects on CD solubilization after the addition of certain low molecular weight acids or hydroxyacids have also been reported for base-type drugs, e.g., the combined use of hydroxyacids and CDs shows synergistic effect on the hydrosolubility of econazole [14–16]. Thus, the cyclodextrins have been widely used to enhance the solubility of several drugs, and the improvement is attributed to the formation of inclusion complexes [17–19].
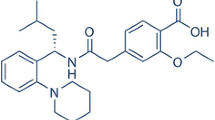
Nateglinide (NAT), a poorly water soluble anti-diabetic agent belonging to BCS class II was selected for the study. It is used for the treatment of type 2 diabetes (non-insulin dependent diabetes mellitus, NIDDM) and belongs to the meglitinide class of blood glucose lowering drugs. The molecular structure of NAT is depicted in Fig. 1a. [20, 21] The effect of CD on the solubility of NAT was studied by the methods described by Loftsson et al. who investigated ways of improving the solubility of the drug [22]. In current investigation, L-arginine (ARG, Fig. 1b), a ternary agent [23–34] was selected to enhance the solubility of drug. The study investigated the effect of a CD and an amino acid on solubility of this drug in the solid state. The influence of HP–β–CD (Fig. 1c) and the amino acid on the physicochemical properties of NAT was evaluated by preparing inclusion complexes with equimolar quantities of HP–β–CD, NAT, and ARG by physical mixing, kneading, co-evaporation, and spray-drying methods. Nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), and powder X-ray diffractometry (XRD) analyses were used to characterize the binary and ternary systems and to confirm the solid state interactions. To rationalize the experimental results and to get structural insights to inclusion complex formation, the molecular modeling was performed using the Genetic Algorithm (GA).
Materials and Methods
Materials
Nateglinide was supplied by Biocon Ltd., Bangalore, India as a gift sample. HP–β–CD was kindly provided by Gangwal Chemicals Pvt. Ltd., Mumbai, India. L-arginine, Soluplus, Hydropropyl methyl cellulose (HPMC), and citric acid were purchased from S.D. Fine Chemicals, Mumbai, India. All the reagents were of analytical grade. Distilled water was used throughout the experiment.
Saturation Solubility Studies of NAT
The saturation solubility of NAT in water and HCl buffer (pH 1.2) was determined by classical shake-flask method [35]. An excess amount of drug was added to conical flask containing 10 ml of distilled water. The flask was kept in thermostatically controlled orbital flask shaker (Electrolab, India) at 25 °C for 48 h. The resultant dispersion was filtered through 0.45 μm filter. The quantification of NAT was done by High Performance Liquid Chromatography (HPLC) (Agilent 1200 series system) with UV detection at 210 nm using a C18 Princetonspher (4.6 × 250 mm, 5 μm) column and a mobile phase containing potassium dihydrogen phosphate buffer (pH 2.3): acetonitrile in 60:40 v/v ratio and flow rate of 1.0 ml/min. Similarly, saturation solubility of NAT was determined in pH 1.2 HCl buffer.
Selection of Ternary Agents
To demonstrate the effect of ternary agent on complexation efficiency of HP–β–CD, this study was performed. L-arginine, Soluplus, hydropropyl methyl cellulose, and citric acid were analyzed for solubility of NAT. NAT in excess was added to the vials containing varying concentrations of ternary agents prepared in water. These dispersions were shaken on orbital shaker at a temperature of 25 °C for 24 h. The amount of NAT solubilized in ternary agents was estimated by using HPLC.
Phase Solubility Studies and Complexation Efficiency
Phase solubility studies were carried out according to the method reported by Higuchi and Connors [36]. An excess amount of NAT was added to a series (4–24 mM) of HP–β–CD solutions. Flasks were sealed to avoid changes caused by evaporation, and the suspensions were vigorously shaken at 25 °C in a thermostatically controlled orbital flask shaker (Electrolab, India) for 48 h. The resultant dispersion was filtered using 0.45 μ syringe filter. The filtrates were analyzed using HPLC as mentioned above. The phase solubility study for ternary system was studied in the similar way of binary system with addition of 3 mM L-arginine solution (concentration obtained from ternary agent screening study). The apparent stability constants (K c) for binary and ternary systems were calculated from the slope of the phase solubility diagrams and solubility of the drug in water (S 0).
Where, K c is stability constant; S 0 is the solubility of NAT in absence of CD and ARG.
Complexation efficiency (CE) is defined as the solubilizing efficiency of CD for guest molecule. For selection of complexation conditions, it is more convenient to obtain complexation efficiency (CE) values for CDs [37]. Hence, the CE was also calculated using the following formula-
Preparation of Inclusion Complexes of NAT
Equimolar binary and ternary systems were prepared from previously sieved components. The stoichiometric ratio of drug, cyclodextrin, and auxiliary substance used for binary system was 1:1 (NAT: HP–β–CD) and for ternary system was 1:1:1 (NAT: HP–β–CD: ARG). The binary and ternary inclusion complexes were prepared by physical mixing, kneading, co-evaporation, and spray drying method as described below.
Physical Mixing Method (PM)
The binary and ternary physical mixtures (PM) were prepared by geometric mixing of NAT: HP–β–CD and NAT: HP–β–CD: ARG, respectively. This mixture was passed through (80#) sieve with minimum abrasion.
Kneading Method (KM)
For binary system, NAT and HP–β–CD were accurately weighed, mixed geometrically, and transferred to mortar. Similar step was followed for ternary system with ARG. Small portion of water: ethanol (1:1 v/v) solution was added to this mixture and triturated for 45 min to form a homogenous paste. Paste was dried at 45 °C in an oven. The dried mass was pulverized and passed through mesh 80 # sieve.
Co-Evaporation Method (CE)
For preparation of binary complexes, minimum quantity of NAT and HP–β–CD was dissolved in ethanol and water to form an aqueous phase and organic phase, respectively. The aqueous phase was added to alcoholic phase and mixture was stirred using a magnetic stirrer. The solvent was evaporated till a dried mass was formed and then passed through mesh 80# sieve. For ternary complex, NAT was dissolved in minimum quantity of ethanol and aqueous phase containing equimolar mixture of HP–β–CD and ARG was added with stirring on magnetic stirrer and similar procedure was followed.
Spray Drying
For binary complex, NAT was dissolved in ethanol and HP–β–CD was dissolved in distilled water, respectively, and both solutions were mixed slowly by adding the aqueous solution to the alcoholic solution on the magnetic stirrer for 30 min. The resultant solution was fed to Lab spray dryer (LV222 advanced SD-1000) and sprayed in the chamber from the nozzle. The product obtained was collected. For ternary complex, ARG was also added to the aqueous phase and similar procedure was followed. The optimized parameters of spray drying method are depicted in Table 1 .
Characterization of Complexes
Saturation Solubility Studies of Complexes and Drug Content
Saturation solubility of the complexes in distilled water and pH 1.2 buffer was determined by taking 10 ml of each solvent in conical flasks followed by the addition of NAT complexes. The flasks were kept in an orbital shaker at 25 °C for 48 h. The resultant dispersions were then filtered through 0.45 μ syringe filter, and the filtrates were subjected to quantification by HPLC.
To ensure that there is no loss of NAT during the preparation of ICs, the drug content in complexes was examined. Complex equivalent to 4 mg of NAT was dissolved in 10 ml methanol. After suitable dilution of the samples, the concentration of NAT was determined by HPLC, and percent drug content was calculated from the following equation. The determinations were performed in triplicate.
% drug content = (Practical drug content/Theoretical drug content) × 100.
In Vitro Dissolution Studies
The improvement in release profile of NAT was assessed by in vitro dissolution studies. A complex equivalent to 60 mg of pure NAT was taken for dissolution studies. Dissolution studies on the formulations were performed in triplicate in 900 ml of pH 1.2 HCl buffer using USP type II paddle type dissolution apparatus at 37 ± 0.5 °C and stirred at 75 rpm. Aliquots (5 ml) were withdrawn after fixed interval of time and filtered through Whatman filter paper no. 41. An equal volume of fresh dissolution medium was replaced to maintain the volume of dissolution medium. The quantification of NAT was done by HPLC.
Based on the results of saturation solubility and in vitro dissolution studies, the complexes prepared by spray drying methods were subjected to other characterization studies such as FTIR, DSC, PXRD, and NMR.
Fourier Transform Infrared Spectroscopy (FT-IR)
The FT-IR spectra of the pure components, PM, and IC were recorded on an IR spectrophotometer (Perkin Elmer) using the KBr disk technique. Sample preparation involved mixing 1 mg of sample with 100 mg of KBr, triturating in Agate mortar to form a compact disk. The sample was scanned in range of 400–4000 cm−1 at ambient temperature.
Differential Scanning Calorimetry (DSC) Studies
The DSC curves of the samples were measured by a differential scanning calorimeter using STARe software (version 5.21). The thermal behavior was studied by heating 2–5 mg samples in a sealed aluminum pan under a stream of nitrogen, using an empty sealed pan as reference, over the temperature range 30–300 °C, at a scan rate of 10 °C/min. The thermograms of NAT, HP–β–CD, ARG, and ICs were compared to confirm the formation of new solid complexes.
Powder X-Ray Diffraction Studies (PXRD)
The PXRD spectra of the samples were recorded using high power powder X-ray diffractometer (PANalytical) with Cu as target filter having a voltage/current of 40 kV/40 mA at a scan speed of 4°/min. The samples were analyzed at 2θ angle range of 5 to 40°. Step time was 0.5 s and time of acquisition was 1 h. The XRD graphs of all pure components, binary and ternary systems were compared with regard to the peak position and relative intensity, the presence and/or absence of peaks in certain regions of 2θ values. Crystallinity was determined by comparing representative peak heights in the diffraction patterns of the ternary systems with the reference drug.
Nuclear Magnetic Resonance Studies (NMR)
To determine the nature of proton or protonated group in NAT and complexes of NAT, the proton NMR spectrum (1H–NMR) in dimethyl sulphoxide (DMSO-d6) were recorded on Bruker Avance 400, FT-NMR spectrometer, 300 MHz, using TMS (Tetra Methyl Silane) as internal standard. Chemical shifts of NAT and IC were compared to illustrate the mechanism of IC formation.
Molecular Docking
The X-ray crystallographic structure of HP–β–CD was retrieved from the Protein Data Bank (PDB) with PDB ID: 1BFN. For the experimental work related to HP–β–CD, four 2-hydroxypropyl groups were added to the primary hydroxyl group of β-cyclodextrin [38, 39]. Resulting structure of HP–β–CD was minimized and optimized as per standard procedure using Sybyl X.2.1 molecular modeling software [40]. Ligand structures (L-arginine and NAT) were drawn in ACD Chem sketch [41] and optimized by Sybyl X.2.1 standard protocol. The optimized HP–β–CD and ligands were taken for the molecular docking by GOLD v5.2 program by CCDC.
Docking simulations were performed in GOLD. The missing hydrogens were added, and 12 Å grid was generated around the HP–β–CD from the center. H-bond donors/acceptors were treated as solvent accessible. The gold ChemPLP scoring function, best and most reliable, was used to rank the generated binding modes. The ChemPLP score uses the ChemScore hydrogen bonding term and multiple linear potentials to model van der Waals and repulsive terms [42].
Statistics
Data was evaluated using one way analysis of variance (ANOVA), and means were compared with Dunnet test (p < 0.05) or paired t test using the software GraphPad Instat software (Version 3.03).
Results and Discussion
Screening of Ternary Agents
In screening of ternary agents, the solubility of NAT in ARG solution increases with increase in concentration of ARG, and the saturation solubility of NAT in arginine solution was maximum compared to other agents (data not shown). Hence, ARG was selected as a ternary agent for phase solubility study and preparation of ternary inclusion complexes.
Phase Solubility Studies
The ternary agents are known to interact with the outer surface of CDs and also with the drug-CD complexes, to form co-complexes or aggregates which show higher stability constants (K c) values than those for the binary drug-CD system. The ternary agents increase the complexation efficiency, thus allowing the use of smaller amount of CD in the preparation of the complex. Only complexes with a K c between 100 and 1000 mol L−1 have industrial applications. Complexes with a lower K c than 100 mol L−1 represent drug-CD systems which are highly unstable, whereas complexes with a K c higher than 1000 mol L−1 could adversely affect the absorption of the drug [43]. The K c values found for all the systems studied indicate that inclusion complexes with suitable stability were formed. Loftsson et al. proposed another method to evaluate the solubilizing effects of CDs, in which the complexation efficiency (CE) is determined.
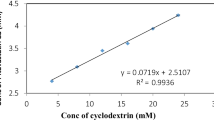
The effect of solubility of NAT in aqueous solution by varying the concentrations of HP–β–CD and ARG was studied to reduce the concentration of HP–β–CD without effecting the solubility or to increase and develop a stable ternary complex. The phase solubility diagrams were all found to be Higuchi AL type, that is, they indicated a linear increase in drug solubility as a function of HP–β–CD concentration, indicating the formation complex of first order with respect to HP–β–CD [Fig. 2 ]. In addition, the slopes of all the phase solubility diagrams less than 1, indicates formation of 1:1 NAT: HP–β–CD complex. There was no changes in the phase solubility diagram for ternary system, hence, existence of 1:1:1 stoichiometry for NAT, HP–β–CD and ARG was assumed. [44–46] The estimated values of the slopes of the phase solubility diagrams and K c values are shown in Table 2 . The phase solubility curve of ARG in 1% (w/v) shows significant increase in the slope of the curve with little increase in the intrinsic solubility of drug. This indicated that ARG has improved the stability constant remarkably when used in the concentration of 1% (w/v) (p < 0.001) as the equilibrium was achieved within 2 days. The significant enhancement obtained in stability constant of NAT might be attributed to electrostatic effect of arginine with drug and HP–β–CD. ARG interacts simultaneously with the drug (via electrostatic interaction and salt formation) and HP–β–CD (via hydrogen bonding) [47]. From these studies, it could be concluded that ARG has not only improved the complexation efficiency of HP–β–CD towards NAT but also promoted the rate of complex formation.
Saturation Solubility Studies and Drug Content of Complexes
The results of the saturation solubility of NAT and inclusion complexes in water and pH 1.2 buffer are depicted in Fig. 3 . The formation of binary and ternary inclusion complexes of NAT with HP–β–CD resulted in improved water solubility of NAT. The extent of solubility of NAT in ternary complexes was more than that in binary complexes and their corresponding physical mixtures. The solubility enhancement of NAT in binary and ternary complexes was found to be 2–20 and 50–70-folds, respectively, with respect to saturation solubility of pure NAT in water (0.036 mg/mL) in various methods employed for preparation of IC. The solubility enhancement of NAT in binary and ternary complexes was found to be 4 to 53 and 18 to 198-folds, respectively, with respect to saturation solubility of pure NAT in pH 1.2 buffer (i.e., 0.0278 mg/mL). The higher solubility of NAT in ternary system compared to binary system can be attributed to enhanced complexation efficiency of HP–β–CD due to addition of ARG [Table 3]. The percent drug content in compexes prepared by all methods was found in the range of 97.3–100%
Saturation solubility of complexes in a water. b pH 1.2 buffer (where, BPM- binary physical mixture, BKN- binary kneading method, BCO- binary co-evaporation, BSD- binary spray drying, TPM- ternary physical mixture, TKN- ternary kneading method, TCV- ternary co-evaporation, TSD- ternary spray drying) (Data were evaluated using one way analysis of variance (ANOVA), and means were compared with Dunnet test (p < 0.05), where ** indicates p < 0.01, * indicates p < 0.05)
Dissolution Studies of Drug, Binary and Ternary Complexes in pH 1.2 Buffer
The in vitro dissolution of NAT and IC is shown in Fig. 4 . The percentage of drug release and dissolution efficiency (DE5) was determined. The dissolution study carried out in buffer of pH 1.2 showed 13.22 ± 2.24% release of pure drug NAT for first 5 min, and DE5 was identified as 6.61 ± 2.97%. At 60 min, the amount of drug dissolved in buffer of pH 1.2 was 26.79 ± 2.74%. Comparatively significant improvement in dissolution was observed between pure drug and drug binary complex, i.e., NAT and HP–β–CD. The order of dissolution rate among the binary complexes with respect to preparation methods was BPM< BCE< BKM<BSD. The complexes prepared by methods, viz., BPM, BCE, BKM, and BSD, exhibited dissolution rate of 15.34 ± 2.83, 21.64 ± 2.56, 24.78 ± 2.60, and 26.45 ± 1.82 in 5 min, and their corresponding DE5 was found to be 7.67 ± 0.96, 10.82 ± 1.92, 12.39 ± 1.69%, and 13.22 ± 2.32%, respectively. Better dissolution profile for the complex prepared by BSD method was observed which might be because of formation of soluble complex with HP–β–CD and improved hydrophilicity of drug NAT at pH 1.2 buffer. The release rate of drug from ternary complexes prepared by different methods, i.e., TPM, TCV, TKN, and TSD in 5 min exhibited 24.17 ± 3.92, 44.32 ± 1.69, 45.40 ± 2.16, and 46.34 ± 0.45%, respectively, and their corresponding DE5 displayed release rate of 12.08 ± 2.16, 22.16 ± 1.36, 22.70 ± 1.82%, and 23.17%, respectively. This experimental observation identified that addition of ARG enhanced the dissolution rate of drug. Interestingly, the complexes prepared by spray drying method exhibited better dissolution profile compared to other methods like physical mixing, co-evaporation, and kneading method which might be due to reduced crystallinity of drug NAT, and the same was identified and confirmed by XRD studies. The present investigation identified that ternary complex with ARG exhibited better and faster dissolution profile than binary complex of NAT with HP–β–CD and pure drug. This improved and enhanced dissolution efficiency of drug clearly indicates the complete conversion of drug from crystalline to amorphous state, increased hydrophilicity, hydrogen bond formation and other forces, wetting properties of carrier, etc. The statistical analysis (ANOVA) of dissolution efficiency of pure drug NAT and its binary and ternary complexes with HP–β–CD have exhibited remarkable difference (p < 0.001). Based on the method of preparation of binary and ternary complexes of NAT, spray drying method was identified as better one with experimental observations like improved dissolution profile (p < 0.001). In comparison, ternary systems of drug have displayed good dissolution parameters than binary. The study reveals that dissolution properties of drug is dependent on method of preparation of complex. The slope and Cmax values of linear portion of the dissolution graphs are depicted in Table 4 .
Characterization of Complexes
The results of saturation solubility and in vitro dissolution studies of NAT inclusion complexes prepared by various methods revealed that the complexes prepared by spray drying method has more improvement in solubility and dissolution of NAT among all binary and ternary system. Hence, the ICs of spray drying method were selected for characterization by FTIR, DSC, PXRD, and H-NMR studies. To demonstrate the formation of inclusion complexes, the spray drying products were compared with pure NAT and physical mixtures in all characterization techniques.
Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR is an important technique to predict a possible interaction of guest (drug) with host (cyclodextrin) molecules in solid state. The characteristic peaks due to various functional groups in guest molecule are affected due to an interaction between guest and host molecule [48]. In all binary and ternary systems, modifications in frequency, shifts and/or appearance, attenuation, broadening in characteristic bands of the NAT and CDs were noted. Most of the peaks of NAT were smoothened indicating strong physical interaction of NAT with HP–β–CD [Table 5].
In the binary physical mixture, the N–H stretch was observed at 3359.32 cm−1 similar to that of pure drug with slight broadening and the C–H stretch was observed at 2935.86.cm −1. The C = O stretch was observed at 1741.70 cm−1. In ternary physical mixture, the N–H stretch was observed at 3337.39 cm−1 with more broadening, the C–H stretch was observed at 2933.33 cm−1 and the C–O stretch was observed at 1644.90 cm−1. Ternary complex formed by physical mixing showed less interaction among the all three raw components because peaks were found to be sharp and prominent as compared to complexes formed by SD method.
In the spray drying binary complex, the N–H stretch was observed at 3389.29 cm−1; the C–H stretch was observed at 2930.46 cm−1 with slight shift, and rest of the peaks were flattened while in the ternary system, a broader N–H peak was observed at 3383.80 cm−1, C–H stretch at 2929.13 cm−1, C = O amide stretch was observed at 1644.07 cm−1, and rest of the peaks were flattened. The peaks of ARG have been disappeared in complexes formed by spray drying method.
These changes occurred in IR spectra of samples indicated that aromatic ring of guest has been entrapped in the hydrophobic cavity of host molecule and formation of inclusion complex in solid state. The intensity of the characteristic peaks of NAT was strongly reduced in complexed form. This suggested that, NAT could form inclusion complex with HP–β–CD in solid state. The FT-IR spectra of NAT, HP–β–CD, ARG, PM, and IC are depicted in Fig. 5 .
Differential Scanning Calorimetry Studies (DSC)
In the assessment of solid phases, thermal methods are widely used. In this study, DSC was used to characterize NAT complexes in the solid state and to obtain further supporting evidence of complex formation. The thermal curve of pure NAT was typical of a crystalline anhydrous substance with a sharp endothermic peak at 135 °C corresponding to the melting point of the drug. A broad endothermic band at 122 °C was observed for the amorphous HP–β–CD, which was related to the loss of water molecule, i.e., dehydration process. [49]
ARG showed three distinct endotherms peaking at 102.7, 178.4, and 231.3 °C, attributable to water loss from a small portion of ARG.2H2O, melting with decomposition of anhydrous arginine, and total decomposition of the melt, respectively. For binary complex (NAT: HP–β–CD), a shift in thermogram was observed as compared to thermogram of pure NAT. This indicated a partial loss of crystallinity and partial amorphization and/or complexation within the HP–β–CD matrix. The intensity of the peak was reduced in the physical mixture.
The DSC profile of the ternary physical mixture showed in succession the dehydration endotherm of ARG around 121.3 °C, a broad melting phenomenon (100–130 °C), an endothermic effect at 133.7 °C, attributed to salt formation. For the ternary complex with ARG, the peak disappeared, which could be explained on the basis of a major interaction between the drug and HP–β–CD in the presence of ARG. In ternary spray drying products, these melting effects disappeared, and only the endothermic effects due to dehydration (40–180 °C) and decomposition (200–300 °C) were observed, indicating complete interaction between the components.
The disappearance of the NAT endothermic peak in the ternary spray drying products is a strong evidence of the formation of amorphous entities and/or inclusion complexes. These results suggest that the spray drying products can be considered as true inclusion complexes, differing from simple physical mixtures Fig. 6.
Powder X-Ray Diffraction
Powder XRD is a useful method for the detection of CD complexation on powder or microcrystalline states. The diffraction pattern of the complex should be clearly distinct from those of the superposition of each component if a true inclusion complex has been formed. The XRD patterns of pure drug, HP–β–CD, ARG, binary, and the ternary system were taken and calculated the RDC values and peak intensities of NAT, and the complexes. X-ray diffraction patterns revealed that pure NAT was clearly in crystalline state as it showed sharp distinct peaks notably at 2θ diffraction angles of 10.5θ, 12.2θ, 15θ, and 16θ, 19.8θ. A typical hollow-pattern was recorded for HP–β–CD which showed peaks at 770, 763.9, 712.8, and 720.4 indicating its amorphous nature. The peak intensities for ARG were found to be 1160.3, 1466.4, 2564.9, and 2492.2 Table 6.
The complexation products were identified by comparing their diffractograms with those of pure drug and the complexes (Fig. 7 ). The XRD pattern of the PM contains the principal diffraction peaks of NAT and CD with a marked reduction in the intensity of the peaks. A decrease in RDC of IC was higher than PM. This can be attributed to the reduction in particle size as a consequence of the preparation method and to the dilution of the drug in the PM. A reduction in the number of signals were noticeable in the complexes, with a markedly reduced intensity, demonstrating the nature of the inclusion compounds, compared with the free molecules. This observation was also in agreement with the results of the IR and DSC studies. The degree of amorphous entity formation in the various ternary systems can be ranked in the following order: Drug ˂ PM < SD products.
Nuclear Magnetic Resonance (1HNMR)
The formation of a NAT, HP–β-CD, and ARG complex was evidenced by comparing the 1HNMR spectra of NAT and binary and ternary complexes under the same experimental conditions. Chemical shift values of binary and ternary complexes have been observed and compared with the pure drug (Table 7). These shifts represent a downfield shift as the Δ shift values are positive. Thus, it can be concluded that the protons H-2```, H-3```, H-2``, H-1, and H-3`, have undergone changes during complexation and may have contributed in the enhancement of solubility. The NMR spectra of NAT and IC of spray drying method are depicted in Fig. 8 .
Molecular Docking
To provide structural insights and to identify the favorable role of ARG in improving the solubility of NAT with HP–β–CD, molecular modeling were performed. Studies were carried out by using Gold software following standard protocol.
To rationalize the experimental results described above, we performed molecular docking studies using the Genetic Algorithm (GA). Docking is a technique that can reliably predict the preferred configuration of one molecule relative to another molecule when they are bound to each other to form a stable complex. Evaluation of generated poses was mainly based on the number of interactions they formed with the residues of active site upon binding and ChemPLP score. The optimized geometry with the lowest binding energy of the inclusion binary, and ternary complexes is displayed in Fig. 9. ChemPLP score is one of the accurate and default empirical scoring function to identify the optimized predicted pose. It uses the ChemScore hydrogen bonding term and multiple linear potentials to model van der Waals and repulsive terms; additionally, it considers the distance and angle dependent hydrogen and metal bonding terms. ChemScore identifies the optimized pose by considering the hydrophobic–hydrophobic contact area, hydrogen bonding, flexibility of ligand, and interactions of metal. Docking results demonstrate that the ARG is completely embedded into the HP–β–CD cavity. The amine groups and carboxylic groups of the ARG is making hydrogen bond interactions with the hydroxyl groups and bridged oxygen atoms within 2.3–3 Å and also making vast number of close van der wall interactions, these interactions shows the stability of the complex (Table 8).
The ARG and HP–β–CD ternary complex was taken for the further docking with the NAT. The results reveal that Nateglinide amine and carboxylic acid groups have formed essential interactions with hydroxypropyl groups and of glucopyranoside oxygen atom of HP–β–CD in ternary complex. Based on the scores and interactions of binary and ternary complex, a thoughtful idea about the favorable virtual mode of interactions of ligands with the drug can be presumed. The most identified mode of binding involves the hydrophobic portion is inserted inside the cavity of HP–β–CD and hydrophilic portion towards the outside portion of cavity and exposed to solvent molecules. The optimized structure of HP–β–CD, docked pose of L-arginine and HP–β–CD, and docked pose of L-arginine, NAT, and HP–β–CD are depicted in Fig. 9 .
Conclusion
In the present investigation, significant improvement in solubility and dissolution profile of NAT by its complexation with HP–β–CD in the presence of an auxiliary substance, ARG, using spray drying and other methods was achieved. The aqueous solubility of the ternary system by spray drying method showed maximum increase. This increment in solubility is mainly attributed to the formation of stable complex of NAT with cyclodextrin in the presence of auxiliary substance. Ternary complex prepared by spray drying method was found to be an effective approach for solubility enhancement of poorly soluble drugs with pH dependent solubility. The results were also supported by various characterization studies. There was tremendous change in crystallinity of NAT that was confirmed by P-XRD and DSC which is one of the reasons of improvement in solubility of NAT. Experimental and molecular modeling studies clearly identify that ARG and NAT make closely strong hydrogen bond and van der wall interactions with HP–β–CD. Molecular docking study supports that ARG interactions played an essential role in increasing the stability and solubility of the complex.
References
Aungst B. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci. 1993;82:979–87.
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
Duchene D, Wouessidjewe D. Pharmaceutical and Medicinal Applications of Cyclodextrins, In: Dumi triu S, editors. Polysaccharides in Medical Applications. New York: 1996. pp. 572–602.
Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98:2045–76.
Rajewski R, Stella V. Pharmaceutical applications of cyclodextrins, II: in vivo drug delivery. J Pharm Sci. 1996;85:1142–69.
Del Valle EM. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–46.
Loftsson T, Fridriksdottir H, Sigurdadottir AM, Gudmundsdottir TK. The effect of water soluble polymers on the aqueous solubility of drugs. Int J Pharm. 1996;127:293–6.
Loftsson T. Increasing the cyclodextrin complexation of drugs and drug bioavailability through addition of water soluble polymers. Pharmazie. 1998;53:733–40.
Loftsson T, Hreinsdóttir D, Másson M. The complexation efficiency. J Inclusion Phenom Macrocycl Chem. 2007;57:545–52.
Mura P, Faucci T, Bettinetti P. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-beta-cyclodextrin. Eur J Pharm Sci. 2001;13:187–94.
Loftsson T, Fridriksdottir H. The effect of water soluble polymers on the aqueous solubility and complexing abilities of β-cyclodextrin. Int J Pharm. 1998;163:115–21.
Ribeiro L, Loftsson T, Ferreira D, Veiga F. Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta-cyclodextrin binary and ternary complexes. Chem Pharm Bull. 2003;51:914–22.
Alexanian C, Papademou H, Vertzoni M, Archontaki H, Valsami G. Effect of pH and water-soluble polymers on the aqueous solubility of nimesulide in the absence and presence of beta-cyclodextrin derivatives. J Pharm Pharmacol. 2008;60:1433–9.
Redenti E, Szente L, Szejtli J. Drug-cyclodextrin-hydroxy acid multicomponent systems: properties and pharmaceutical applications. J Pharm Sci. 2000;89:1–8.
Redenti E, Szente L, Szejtli J. Cyclodextrin complexes of salts of acidic drugs: thermodynamic properties, structural features, and pharmaceutical applications. J Pharm Sci. 2001;90:979–86.
Fauci T, Melani F, Mura P. 1H NMR and molecular modelling techniques for the investigation of the inclusion complex of econazole with β-cyclodextrin in the presence of malic acid. J Pharm Biomed Anal. 2000;23:25–31.
Hirayama F, Uekama K. Cyclodextrin-based controlled drug release system. Adv Drug Del Rev. 1999;36:125–41.
Loftsson T, Fridriksdottir H, Olafsdottir J. Solubilization and stabilization of drugs through cyclodextrin complexation. Acta Pharm Nord. 1991;3:215–7.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–25.
United State Pharmacopoeia 28, National Formulary 23, The Official compendia of Standards. United States Pharmacopoeial Convection Inc. Asian Edition: Rockville; 2005. p. 702.
American Diabetes Association, Standards of Medical Care in Diabetes-2008, Diabetes Care, Suppl. 2008.1, S12–S54.
Loftsson T, Frikdriksdottir H, Sigurkdardottir M, Ueda H. The effect of water-soluble polymers on drug-cyclodextrin complexation. Int J Pharm. 1994;110:169–77.
Asbahr C, Franco L, Barison A, Silva CW, Ferraz G, Rodrigues N. Binary and ternary inclusion complexes of finasteride in HPbetaCD and polymers: preparation and characterization. Bioorg Med Chem. 2009;17:2718–23.
Jug M, Mennini N, Kövér KE, Mura P. Comparative analysis of binary and ternary cyclodextrin complexes with econazole nitrate in solution and in solid state. J Pharm Biomed Anal. 2014;91:81–91.
Wang D, Li H, Gu J, Guo T, Yang S, Guo Z, Zhang J. Ternary system of dihydroartemisinin with hydroxypropyl-β-cyclodextrin and lecithin: simultaneous enhancement of drug solubility and stability in aqueous solutions. J Pharm Biomed Anal. 2013;83:141–8.
Anwer K, Jamil S, Ansari M, Al-Shdefat R, Ali E, Ganaie A, Shakeel F. Water soluble binary and ternary complexes of diosmin with β-cyclodextrin: spectroscopic characterization, release studies and anti-oxidant activity. J Mol Liq. 2014;199:35–41.
Grebogi H, Tibola O, Barison A, Grandizoli W, Ferraz G, Rodrigues N. Binary and ternary inclusion complexes of dapsone in cyclodextrins and polymers: preparation, characterization and evaluation. J Incl Phenom Macro. 2012;73:467–74.
Jadhav P, Petkar B, Pore Y, Kulkarni A, Burade K. Physicochemical and molecular modeling studies of cefixime–l-arginine–cyclodextrin ternary inclusion compounds. Carbohyd Polym. 2013;98:1317–25.
Medarević D, Kachrimanis K, Djurić Z, Ibric S. Influence of hydrophilic polymers on the complexation of carbamazepine with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2015;78:273–85.
Singh SK, Srinivasan KK, Singare DS, Gowthamarajan K, Prakash D. Formulation of ternary complexes of glyburide with hydroxypropyl-β-cyclodextrin and other solubilizing agents and their effect on release behavior of glyburide in aqueous and buffered media at different agitation speeds. Drug Dev Ind Pharm. 2012;38:1328–36.
Srivalli K, Mishra B. Improved aqueous solubility and antihypercholesterolemic activity of ezetimibe on formulating with hydroxypropyl-β-cyclodextrin and hydrophilic auxiliary substances. AAPS PharmSciTech. 2016;17:272–83.
Vakani SS, Kajwe A, Suvarna V. Sherje A. Influence of auxiliary agents on solubility and dissolution profile of repaglinide with hydroxypropyl-b-cyclodextrin: inclusion complex formation and its solid-state characterization. J Incl Phenom Macrocycl Chem. 2015;83:239–50.
Sherje A, Vaishali L. Inclusion complexes of hydroxy propyl-β-cyclodextrin and paliperidone: preparation and characterization. Curr Drug Discov Technol. 2014;11:271–8.
Sherje A, Vaishali L. Ternary inclusion complex of paliperidone with β-cyclodextrin and hydrophilic polymer for solubility and dissolution enhancement. J Pharm Innov. 2015;10:324–34.
Baka E, Comer JEA, Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal. 2008;46:335–41.
Higuchi T, Connors KA. Phase solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212.
Magnusdottir A, Masson M, Loftsson T. Self-association and cyclodextrin solubilization of NSAIDs. J Incl Phenom Macro. 2002;44:213–8.
Ashok CI, Yogesh AS, Bonnie AA, Mitchell AA, Christy MW. Interaction of artemisinin and its related compounds with hydroxypropyl-β-cyclodextrin in solution state: Experimental and molecular-modeling studies. J Pharm Sci. 2003;92:649–55.
Mura P, Giampiero B, Fabrizio M, Alfredo M. Solid-state characterization and dissolution properties of naproxen-arginine-hydroxypropyl-beta-cyclodextrin ternary system. Eur J Pharm Sci. 1995;3:347–55.
Sybyl Molecular Modeling Software Packages, ver. X.2.1., Tripos Inc., St Louis, MO, 2013.
ACD/ChemSketch, ver. 2.5, Advanced Chemistry Development, Inc., Toronto, Ontario, Canada, 2015.
GOLD 5.2, CCDC, Cambridge, UK (2013)
De Miranda C, Azevedo Martins E, Veiga F, Humberto F. Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Brazilian J Pharm Sci. 2011;47:665–81.
Loftsson T, Hreinsdóttir D, Másson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302:18–28.
Brewster ME, Loftsson T. Cyclodextrin as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–66.
Granero E, Maitre M, Garnero C, Longhi R. Synthesis, characterization and in vitro release studies of a new acetazolamide-HP-beta-CD-TEA inclusion complex. Eur J Med Chem. 2008;43:464–70.
Mura P, Bettinetti G, Cirri M, Maestrelli F, Sorrenti M, Catenacci L. Solid-state characterization and dissolution properties of naproxen-arginine-hydroxypropyl-beta-cyclodextrin ternary system. Eur J Pharm Biopharm. 2005;59:99–106.
Amareshwar K, Rai DK. Spectroscopic studies of some antidiabetic drugs. Spectrochim Acta A. 2003;59:1673–80.
Tenreiro CR, Carmen A, Concheiro A, Torres L. Characterization of cyclodextrin carbopol interactions by DSC and FTIR. J Therm Anal Calorim. 2004;77:403–11.
Acknowledgements
Authors are thankful to Biocon Ltd., Bangalore, India and Gangwal Chemicals, Mumbai, India for providing gift samples of Nateglinide and HP–β–CD, respectively. We are also thankful to Tata Institute of Fundamental Research (TIFR), Mumbai, India for performing XRD and DSC studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Suvarna, V., Kajwe, A., Murahari, M. et al. Inclusion Complexes of Nateglinide with HP–β–CD and L-Arginine for Solubility and Dissolution Enhancement: Preparation, Characterization, and Molecular Docking Study. J Pharm Innov 12, 168–181 (2017). https://doi.org/10.1007/s12247-017-9275-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-017-9275-z