Abstract
Inclusion complexation of repaglinide (RPG) with hydroxypropyl-β-cyclodextrin (HPβCD) in presence and absence of auxiliary substances such as polyvinyl pyrrolidone-K30 (PVP) and l-arginine (ARG) was formulated in an attempt to improve its physicochemical properties by using three different techniques viz. physical mixing—by geometric mixing of individual components, kneading method—by incorporating binder solution in physical mixture and co-evaporation method—by using rotary evaporator. Phase solubility studies showed formation of complex in 1:1 M ratio with AL type of curve in presence and absence of both auxiliary substances. The rise in stability constant was observed in presence of auxiliary substances suggests positive effect on complex formation. As compared to the complexes with PVP, tremendous improvement in aqueous solubility of RPG in supramolecular inclusion complex with ARG was observed. The solid state characterization of inclusion complexes prepared by different methods were carried out by differential scanning calorimetry (DSC), powder X-ray diffraction (PX-RD), fourier transformation-infrared spectroscopy (FT-IR), scanning electron microscopy (SEM) and in vitro dissolution studies. Among all complexes, ternary complex with ARG prepared by co-evaporation method showed improvement in dissolution profile of RPG indicating complete amorphization and entrapment of pure drug in HPβCD which is evident by DSC and PX-RD. Investigation concludes that ARG and PVP can show synergistic effect in inclusion complex formation of RPG with HPβCD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In today’s pharmaceutical drug delivery pipeline, approximately 70 % of new chemical entity experienced the poor aqueous solubility. This is due to maximum use of combinatorial chemistry and high-throughput screening in drug discovery which has emerged in poorly water soluble drug candidates with high lipophilicity and poor dissolution rate [1]. Currently, approximately 40–60 % of the marketed immediate- release (IR) oral drugs are categorized as practically insoluble (<100 µg/ml) compound [2, 3]. According to the biopharmaceutics classification system (BCS), poorly soluble but highly permeable compound falls under BCS class II category. These poorly water soluble drugs exhibit slow drug absorption leading to inadequate and variable bioavailability and finally causes gastrointestinal mucosal toxicity. Limited solubility, poor dissolution rate and compromised oral absorption are the major problems of BCS Class II drugs and hence improving solubility of such actives is a major challenge [4].
Rigorous studies on cyclodextrins (CD) and cyclodextrin complexes gave immense knowledge of information related to the structural requirements for complex formation and the forces involved [5, 6]. By forming a reversible inclusion complex with the hydrophobic drug molecule CDs has gained an insight in the pharmaceutical field because of their potential to modify physical, chemical and biological properties of drug molecule [7]. This results into appropriate changes in the physicochemical properties of the drug, such as solubility, dissolution rate, stability and bioavailability [8] which in turn contributes them as an apt candidate for oral drug delivery. It is observed that a large amount of CDs are required to solubilize small amount of a poorly water-soluble drug because of their lower complexation efficiency [9] and hence larger amount of CD is required in oral solid dosage form [10]. It is an important challenge to enhance the complexation efficiency and solubilisation property of CD in pharmaceutical formulation. Among the various methods, to achieve this aim, investigator found that by adding a small amount of auxiliary hydrophilic substance such as polymer [11, 12], hydroxyl acids [13] and amino acids [7, 14] to the CD complex can improve the complexation efficiency [15]. It is reported that polymer as an auxiliary agent, in CD complex, decreases the drug crystallinity and enhances solubility as well as dissolution rate of poorly water soluble drug effectively through synergistic effect [16]. In current era, investigator are formulating ternary CD complex by using innovative approaches such as utilizing phospholipid [17], pluronics [18] bile salts [19] and meglumine [20].
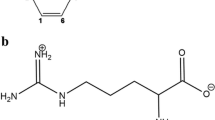
Repaglinide (RPG), is a novel carbamoylmethylbenzoic acid derivative which belongs to the meglitinide class of blood glucose lowering drugs (Fig. 1). It is a new prandial glucose regulator for the treatment of type 2 diabetes mellitus. It decreases the blood glucose level by stimulating the release of insulin from the pancreas. RPG, an acid–base ampholyte (pKa1 = 4.2; pKa2 = 6.0) exhibits very low water solubility (34 mcg/ml at 37 °C) and high lipophilicity (log P = 3.97). RPG is rapidly absorbed from the gastrointestinal tract after oral administration [21]. Being a potent molecule, RPG belongs to BCS class II (i.e., poorly water soluble and highly permeable) drug and also shows variable absolute oral bioavailability ranging from 56 to 63 % [22]. Clinical trial suggests that RPG exhibit high inter-individual variability in plasma concentrations. Investigator documented many approaches to enhance the solubility by upgrading the physicochemical properties of RPG such as nanostructured particles [23], ultra-rapid freezing technology [24], CD complex [25], forming co-amorphous system with saccharin [26], solid dispersion [27], co-solvent solubilisation [28], self-nanoemulsifying pellets [29].
The objective of the present investigation was to enhance the solubility of RPG as well as to explore the potential of 2-hydroxypropyl-β-cyclodedextrin (HPβCD) in improving the oral delivery of RPG in presence and absence of auxiliary substances. Author formulated Binary and ternary complexes by using different methods followed by characterization of the same. Solubility and dissolution tests were carried out to evaluate the effectiveness of the CD and auxiliary agents for RPG. In addition, complexes were characterized by differential scanning calorimetry (DSC), X-ray powder diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM) to evaluate the state of the solid dispersions and possible interactions of drug-carrier.
Experimental
Materials
Repaglinide was obtained as gift sample from Ajanta Pharma. (Mumbai, India), of 99.7 % purity, was evaluated using HPLC. HPβCD was kindly provided by Gangwal chemicals, (Mumbai, India) (an average molecular weight ~1377 with 0.66M substitution, hydroxypropyl moiety). l-Arginine (ARG) and PVP K-30 (PVP) used in research work were purchased from S.D.Fine (Mumbai, India). A Milli-Q Water and Analytical grade reagents were used throughout of research project.
Phase solubility studies and saturation solubility studies
Phase solubility studies of RPG were conducted in distilled water according to the method illustrated in Higuchi and Conners [30]. An excess amount of RPG was added to 15 mL of various concentrations (0–15 mM) of HPβCD solutions in conical flask and shaken on an orbital shaker at 25 °C for 72 h. After equilibrium was achieved, the samples were filtered through 0.45 μ syringe filter and appropriately diluted. The concentration of RPG was determined against blanks prepared in the same concentration of HPβCD solutions by the developed UV analytical method at 278 nm. Phase solubility was also performed with or without auxiliary agents PVP and/or ARG. In case of hydrophilic polymer PVP, 0.7 % w/v solution was added to the solution containing HPβCD. While with amino acid, equimolar mixture of RPG-ARG was prepared and excess amount of this combination was added in series of HPβCD solutions. The apparent stability constants (Ks) and complexation efficiencies of the binary and ternary complexes were calculated from phase solubility diagram according to the following equation:
where S0 is the solubility of RPG in the absence of HPβCD and Slope obtained from phase solubility curve.
The complexation efficiencies of CDs were also calculated from the following equation [16].
where D0 is the intrinsic solubility of drug and K1:1 is stability constant.
Saturation Solubility of RPG was investigated in distilled water, pH 1.2 and pH 6.8.
An indication of process of transfer of RPG from pure water to aqueous solution of HPβCD was obtained from the values of Gibbs free energy of transfer (ΔGtr°) and it was calculated using the following equation:
where Sc is molar solubility of RPG in aqueous solution of HPβCD with or without auxiliary substance and S0 is molar solubility of RPG in distilled water in absence of HPβCD.
Preparation of cyclodextrin complexes
Equimolar binary (RPG–HPβCD) and ternary (RPG–HPβCD–ARG; RPG–HPβCD–PVP) systems were prepared from previously sieved components. The stoichiometric ratio used for binary system was 1:1 M. Ternary system with ARG was prepared in equimolar ratio with binary system 1:1:1 M, while with hydrophilic polymer, optimized concentration of 0.7 % w/w of PVP was added in equimolar binary system.
Physical mixture (PM)
The physical mixture (PM) was prepared by geometric mixing of RPG with HPβCD for binary system. This mixture was passed through an (80#) sieve prior to use. A ternary system was prepared by adding ARG and PVP.
Kneading method (KM)
For binary system, RPG and HPβCD in 1:1 M ratio were accurately weighed, mixed geometrically and transferred to mortar. Similar step was followed for ternary system in presence of ARG and PVP. Small portion of hydroalcoholic solution of water:ethanol (1:1 v/v) solution was added to these mixtures and triturated for 20–25 min. to form a homogenous paste. Paste was dried at 45 °C in an oven. The dried mass was pulverised and pass through sieve no. 80.
Co-evaporation method (CE)
RPG was dissolved in minimum quantity of ethanol and HPβCD was dissolved in water. For ternary system, ARG and PVP were dissolved with HPβCD in aqueous phase separately. Aqueous phase was added to organic phase and mixture evaporated at 45 °C on rotary evaporator. The clear solution was heated till a dried mass was formed which was then sieved through 80#.
All complexes were prepared in triplicate and stored in a desiccator which were then characterised and evaluated.
Evaluation and characterization of complexes
Saturation solubility studies
Excess amount of binary, ternary complexes were added into conical flask containing 10 ml of water. Flask was then shaken on an orbital shaker at 25 °C for 72 h. After equilibrium was achieved, the samples were filtered through 0.45 µ syringe filter. The concentration of RPG in the samples was determined using developed analytical method.
Percentage drug content study
A UV spectrophotometric method was developed into estimate the concentration of RPG in prepared complexes. Drug content in complexes was examined by dissolving the complex equivalent to 4 mg of RPG in 10 ml Methanol. Appropriate dilution was made in methanol and then solution was filtered through 0.45 μ membrane filter. The RPG concentration was determined by developed UV analytical method and percent drug content was calculated.
Fourier transformation-infrared spectroscopy (FTIR)
Sample preparation involved mixing the sample with KBr, triturating in Agate mortar. Compact disc was formed by using hydraulic press. The obtained disc was then placed in sample holder and then into FT-IR instrument (Perkin Elmer). The spectra were recorded over a frequency range 4000–400 cm−1.
Differential scanning calorimetry thermal (DSC)
Thermal characteristic of RPG and sampled complexes were investigated by DSC (Seiko Instruments Inc. EXSTAR6000 series DSC-6200 with Muse measurement 6.9U). Approximately 2–5 mg of each sample was heated in a pierced aluminium pan from 30 to 300 °C at a heating rate of 10 °C/min.
Powder X-ray diffractometry (PX-RD)
Powder X-ray diffraction patterns of RPG and complexes of RPG were recorded using Phillips P Analytical X’Pert PRO powder X-ray diffractometer, using tube anode Cu over the interval 10–60°/2θ. The operational data were as follows: Generator tension (voltage) 40 kV, Generator tube current 30 mA, and scanning speed 2°/min.
Scanning electron microscopy (SEM)
The surface morphology of samples was determined using analytical scanning electron microscope. The samples were lightly sprinkled on a double adhesive tape stuck to an aluminium stub. The stub containing samples was placed in the scanning electron microscope chamber and bombarded with a focused beam of high energy electrons. The signals derived from electron-sample interactions over a selected area of surface display spatial variations in the external morphology of the sample.
Dissolution studies
Complex equivalent to 4 mg of pure RPG was taken for in vitro dissolution studies. Dissolution studies on the formulations were performed in triplicate in 900 ml of different media i.e. pH 6.8 phosphate buffer using USP type II paddle type dissolution apparatus at 37 ± 0.5 °C and stirred at 75 rpm. Aliquots (5 ml) were withdrawn at specified time intervals and filtered through Whatman filter paper no. 41. An equal volume of fresh dissolution medium was replaced to maintain the sink condition. The filtered samples were analysed by UV spectrophotometer. The graph was plotted, % drug release of RPG versus time (min). Dissolution efficiency at 5 min (DE5 min), described as the area under the dissolution curve up to 5 min, expressed as a percentage of the area of the rectangle described by 100 % dissolution in the same time [31, 32] was calculated according to following equation:
where Y is the percent drug dissolved at time t.
Statistical analysis
The values obtained were compared statistically using one-way ANOVA, followed by Tukey multiple comparison test.
Results and discussion
Phase solubility studies
Phase solubility analysis is the first mandatory step which involves effectiveness of CD towards the optimization of the development into inclusion complexes of the drugs to make it solubilize. The phase solubility curve of RPG in aqueous HPβCDs solutions with and without the addition of auxiliary substances is shown in Fig. 2 The solubility of RPG increased in a linear fashion as a function of HPβCDs concentration, showing the AL-type of phase solubility diagrams, indicating first order dependency of the interaction on the HPβCD concentration and the formation of water soluble complexes without precipitation in both binary and ternary systems. No change in the type of phase solubility diagram for ternary system was observed when two different auxiliary substances were used. The slope in each systems were found to be less than unity, because of straight line which suggest formation of soluble complex in stoichiometry ratio 1:1 in solution with HPβCD in presence and absence of auxiliary substances.
The parameters calculated from Phase solubility studies are given in Table 1. It could be seen that an addition of ARG and PVP to the complexation media increased slope, stability constant and complexation efficiency of the HPβCD toward RPG. No change in the type of phase solubility diagram for ternary system was observed when two different auxiliary substances were used. For many drug/CD complexes, binding constant values are in the range of 100–20,000 M−1 [33]. The solubilising efficiency data showed that HPβCD alone can improve solubility of RPG by 5.38 fold, whereas in the presence of auxiliary substances ARG and PVP it gave 95.74- and 35.35-fold rise in solubility of RPG respectively. Thus, it clearly indicates that solubilising efficiency of HPβCD prominently enhanced by adding water soluble auxiliary substances. In comparison with PVP, the results obtained from phase solubility data are clearly indicative of the positive effect of the addition of an auxiliary substance ARG in terms of improving solubility constant (p < 0.001), solubilising efficiency, slope as well as complexation efficiency of HPβCD towards RPG.
For the concentration of HPβCD: 0.002, 0.004, 0.006, 0.008, and 0.010 M the values of ΔGtr° (KJ/mol) in aqueous solution of binary system were found to be −0.91, −1.73, −2.57, −3.36, −3.72 respectively while in presence of arginine were found to be −9.92, −9.51, −9.91, −10.19, −10.30 respectively and in presence of PVP values were found to be −5.80, −6.84, −7.25, −7.57, −8.00 respectively. This is a clear indication of transfer of RPG from pure water to the aqueous solution of HPβCD. The hypothesis about spontaneous nature of RPG solubilisation is clarified by the negative ΔGtr° values for CDs at various concentrations. Negative value of ΔGtr° illustrates favourable condition. The ΔGtr° values demonstrate a decrease with an increase in concentration of HPβCDs which indicates that reaction becomes more favourable with increasing concentration of HPβCD [34]. Ternary system having ARG released immense energy upon complexation as compared to PVP ternary system.
Evaluation and characterization of complexes
Saturation solubility studies
RPG exhibits extremely poor solubility of 35 ± 1.0 µg/ml in distilled water. Solubility of RPG in pH 1.2 HCl buffer was found to be 3.15 ± 0.17 mg/ml whereas in pH 6.8 phosphate buffer RPG showed nominal increase in solubility of 51 ± 1.8 µg/ml. RPG showed strong pH dependent solubility, with maximum solubility was found in acidic pH and minimum at higher pH. Saturation solubility of prepared complexes was performed in Distilled water and the values are given in Table 2 along with percent drug content results.
The binary and ternary systems of RPG showed enhancement in the solubility as compared to pure drug alone Table 2. The enhancement in the solubility of complex is mainly attributed to the formation of stable inclusion complex of RPG with HPβCD. The ternary co-evaporation complex showed almost 475 times increase in solubility as compared to an increase of 6.2 times of a binary co-evaporation complex. From all complexes formed by three different methods, co-evaporation method showed maximum increase in solubility of drug.
Many literatures documented that hydrophilic polymer like PVP improves the aqueous solubility by interacting with the external surface of drug: CD complexes thereby constructing into soluble aggregates. Moreover, hydrogen bond formation, van der waal forces, hydrophobic interactions and/or dipole–dipole electrostatic bond between drug and polymer plays vital role in solubility enhancement of RPG [9, 35]. Many scientific papers have reported that basic amino acid l-arginine forms the ternary complex in solid state by simultaneously interacting with both the CD via hydrogen bond as well as with acidic drug via electrostatic interactions and salt formation [7, 36, 37]. The mechanism behind prominent increase in solubility of the acidic drug with ARG is attributed to the development of an amphiphilic structure of RPG characterized by strong hydrophobic portion and hydrophilic polar head in presence of counter-ion of ARG. Further, formation of supramolecular ternary complex could be the reason of complimentary interaction between the components in presence of HPβCD. The hydrophobic portion of this amphiphilic structure is assumed to interact with the hydrophobic CD cavity, whereas, at the same time, the hydrophilic portion can act as a surfactant towards the CD complex, subsequently lowering the aqueous surface tension, thereby favouring its wettability and dissolution [38].
Fourier transformation-infrared spectroscopy
The possible interaction between drug and carrier was studied by FTIR spectroscopy. The principle absorption peaks of RPG were observed at 3305.32 cm−1 (N–H, stretch, amine), 2930.25 cm−1 (C–H, stretch), 1675.59 cm−1 (C=O stretch, CONH), 1370.77 cm−1 (C–N stretch, aromatic), 1597.29 cm−1 (ring stretching vibrations), 1714.74 cm−1 (C=O stretch, COOH), 747.13 cm−1 (C–H bending), 1555.34 cm−1 (C=C stretch), 1203.8 cm−1 (C–O–C stretch). The absorption bands of HPβCD were found at 3398.24 cm−1 (O–H, stretch), 2924.85 cm−1 (C–H, Stretch), 1021.19 cm−1 (C–O–C stretch). FTIR spectrum of ARG shows prominent peaks at 3296.3 and 3068 cm−1 (broad OH carboxylic or NH guanidine due to intramolecular hydrogen bonding), 2864.29 (C–H stretch), 1678.0 cm−1 (C=O, amide), 1319.08 cm−1 (C–N stretch) and 1620 cm−1 (C=O carbonyl COOH). In the spectrum of PVP K30, a broad strong band due to O–H stretching vibration at 3442.61 cm−1 and a strong band due to the C=O stretching vibration at 1649.90 cm−1 was observed (Fig. 3).
The sharp absorption peak of RPG at 3305.32 cm−1 (N–H stretch) was not observed in the spectrum of complexes prepared, instead broad peak appeared. These indicate host–guest interactions through intermolecular hydrogen bonding between hydroxyl group of host cavity and the carbonyl group of RPG forming an inclusion complex in solid state. The intensity of the characteristic peaks of RPG was strongly reduced in complexed form. This suggested that, RPG could form inclusion complex with HPβCD in solid state.
The ternary systems of RPG have shown somewhat different pattern of IR spectra as compared to the binary systems. The absorption peaks of ARG have been disappeared in complexes formed by kneading and co-evaporation methods. This explains interaction of ARG with RPG and HPβCD forming ternary complex formation. Ternary complex formed by physical mixing with ARG showed less interaction among the all three raw components because peaks were found to be sharp and prominent as compared to other complexes formed by different methods. FTIR spectra of ternary complexes prepared with PVP showed complete complex formation because absorption peak of C=O stretching vibration was found to disappear [39].
Differential scanning calorimetry
The interaction between the drug and excipients in formulation can also be investigated by DSC technique. When guest molecules are included in CD cavities, their melting, boiling and sublimation points shift to different temperature or disappear. The DSC thermogram of RPG and its complexes along with its individual components are shown in Fig. 4.
The DSC thermogram of RPG showed a sharp endothermic peak at 135 °C corresponding to its melting point. The DSC thermogram of HPβCD was characterized by a broad endothermic band at 122 °C, indicating loss of water molecule due to dehydration process. ARG showed three distinct endotherms peaking at 102.7, 178.4 and 231.3 °C, attributed to water loss from a small portion of ARG. 2H2O, melting with decomposition of anhydrous arginine, and total decomposition of the melt.
In binary complex (RPG:HPβCD), phase transition identified in thermogram of RPG with gradual dropping in the onset temperature as well as concomitant peak shift to lower temperature and fusion of peak observed below 130 °C. This demonstrated a partial loss in crystallinity and/or partial amorphization of surface modification of drug within the HPβCD matrix.
In ternary co-evaporated and kneading products, showed melting of salt in the range of 120–155 °C recognized by marked broadening of peak followed by decomposition in the range of 200–250 °C, indicating full amorphization of RPG. For the DSC profile of the ternary complexes with ARG, the peak of RPG completely disappeared, which could be explained on the basis of a strong interaction between the drug and HPβCD in the presence of ARG and formation of stable inclusion complex.
Powder X-ray diffractometry
Powder X-ray diffractometry is a useful technique to detect crystallinity of sample from diffraction pattern containing representative peak height to confirm formation of new solid state. PXRD diffraction patterns of all the components and complexes are presented in (Fig. 5). The diffractogram of the pure drug RPG showed its highly crystalline nature, indicated by numerous distinctive peaks at diffraction angles of 2θ [10.16°, 13.08°, 13.84°, 18.68°, 20.56°, 23°] throughout the scanning range with intensities 919.9, 992.6, 812.7, 881.1, 641. A typical hollow-pattern was recorded for HPβCD which showed peaks at 770, 763.9, 712.8, and 720.4 indicating its amorphous nature. The peak intensities for ARG were found to be 1160.3, 1466.4, 2564.9, and 2492.2 whereas for PVP peak intensities were found to be 939.6, 861.7, 909.0, and 957.7 indicating their crystalline nature.
Powder X-ray diffraction (PX-RD) patterns of pure components and inclusion complexes prepared by different methods. a RPG; b HPβCD; c ARG; d PVP; e KM: RPG–HPβCD; f PM: RPG–HPβCD; g CE: RPG–HPβCD; h PM: RPG–HPβCD–ARG; i KM: RPG–HPβCD–ARG; j CE: RPG–HPβCD–ARG; k CE: RPG–HPβCD–PVP; l KM: RPG–HPβCD–PVP; m PM: RPG–HPβCD–PVP
In binary complex, HPβCD was partially bound to RPG, indicating a significant reduction in the crystallinity. This may be due to partial loss of crystalline nature of drug during the process of complex preparation.
A complete amorphous state was achieved in the case of ternary complexes with ARG as indicated by their diffraction patterns with the intensities lower than the pure drug RPG which might have contributed for improved solubility and dissolution rate during physicochemical studies. This confirms that RPG is completely entrapped within the CD structure and also interacted with ARG. On the other hand, ternary complexes prepared by CE in presence of PVP showed decrease in crystallinity of the RPG and peaks of pure drug were almost disappeared which indicates participation of PVP in inclusion complex formation. However, complexes formulated by PM and KM showed less amorphism as compared to CE method and peak were visible though it was less intense corresponding to pure drug.
Scanning electron microscopy
SEM of selected preparations including RPG, HPβCD, and ARG are shown in (Fig. 6). SEM images showed that RPG existed in a long needle-shaped crystal habit; pure drug was characterized by the presence of rough, uneven, broken particles of larger size and irregular flat plate surface while some crystals were shown to have rectangular shape. HPβCD appeared somewhat spherical particle. ARG exhibited long thick needle shaped crystal structure.
Ternary PM showed presence of the entire three components but in aggregate form. Irregular particles shapes were found in ternary kneading and co-evaporation complex. These micrographs support the formation of complexes of RPG with HPβCD and ARG in the solid state. Thus, altered particle shape, reduced particle size and close contact between HPβCD and ARG in complexes might be responsible for the enhanced drug solubility and dissolution rate of RPG [40].
Dissolution studies
The in vitro dissolution profiles of RPG through binary and ternary complexes are illustrated in a graph plotted percentage of drug dissolved versus time (Fig. 7). The %drug release and dissolution efficiency (DE5) was evaluated at 5 min.
Dissolution study was performed in pH 6.8 phosphate buffer where pure RPG showed 29.49 ± 3.43 % drug release in first 5 min and DE5 was found to be 14.75 ± 3.37 %. This may be due to less solubility of RPG in phosphate buffer and this was found in saturation solubility studies. At 60 min, 58.80 ± 2.99 % of drug dissolved in pH 6.8 phosphate buffer.
Binary complex with HPβCD showed limited improvement in dissolution rate as compared to pure RPG. The decreases in dissolution rate among binary complexes are in the order of complexes prepared by: KM > CE > PM. Complex prepared by physical mixing, kneading and co-evaporation method showed up 33.68 ± 3.93, 39.64 ± 1.66, 38.98 ± 1.70 % release of drug at 5 min and their corresponding DE5 were found to be 19.82 ± 2.24, 19.49 ± 1.30 and 16.84 ± 2.59 % respectively. The better dissolution properties of the PM as compared to pure RPG can be ascribe by formation of in situ soluble complex with HPβCD in the dissolution medium and/or predominant effect of HPβCD, in pH 6.8 phosphate buffer media, as the complexation is highly governed by the hydrophobicity of the RPG [41]. However, after 20 min moderate drug release was observed which may be due to formation of a crystal growth of RPG in the dissolution medium.
Continuing our investigation, ternary complex prepared in presence of PVP manifest significant improvement in dissolution profile of RPG from the complexes prepared by different method. The %drug release at 5 min from PM, kneading and co-evaporation complex was found to be 48.31 ± 4.87, 52.54 ± 3.57 and 65.70 ± 1.82 %, respectively and their corresponding DE5 appeared to be 24.15 ± 4.49, 26.27 ± 3.36 and 32.85 ± 2.82 % respectively. The rise in DE indicates the better ternary complex formation with PVP which in turn played significant role in enhancing dissolution of RPG by interacting with exterior part of the complex. The complex prepared by kneading method showed greater dissolution as compared to PM and CE. The enhanced dissolution rate of complex prepared by KM might be attributed to formation of inclusion complex in solid state with reduction in crystallinity of RPG which is evidenced by XRD studies.
Based on findings, ternary complex in presence of ARG demonstrated the faster dissolution as compared to binary system and pure RPG. The %drug release at 5 min form PM, KM and CE complex were found to be 50.89 ± 2.6, 97.43 ± 1.04 and 99.64 ± 2.33 % with corresponding DE5 25.44 ± 2.59, 48.72 ± 2.40 and 49.82 ± 4.98 respectively. The remarkable improvement in dissolution profile of pure drug demonstrates the complete change in crystallinity to amorphous nature. The synergistic effect ARG and CD was observed in ternary system and showed better performance as compared to binary complexes, lead to salt formation. The instant release of RPG might be attributed to better stability and complexing properties, increase in hydrophilicity, wetting properties of carrier as well as hyrodgen bonding and other forces [42–44]. The statistical analysis (ANOVA) of DE5 value for RPG and its complexes demonstrated a remarkable difference between the dissolution profile of pure RPG and all its binary and ternary system with HPβCD (p < 0.001). The complex prepared by CE method was found to be the best method with increase in solubility and dissolution propertied of RPG (p < 0.001). Moreover, all ternary systems showed significant elevation in drug dissolution parameter when compared with that of binary system (p < 0.001). From the above findings in dissolution profile of RPG, it could be concluded that extent of the increase in dissolution rate was found to be dependent on method used for the preparation of inclusion complex.
Conclusion
Our study demonstrated the inclusion complex formation of RPG with HPβCD in presence and absence of ARG and PVP by three different methods. ARG proves to be an excellent auxiliary substance as compared to PVP by remarkably enhancing an aqueous solubility and physicochemical properties of RPG. Phase solubility study demonstrated formation of more stable inclusion complex in ternary system as compared to binary system. Change in crystallinity of pure form of drug to complete amorphous nature in a solid state of ternary complex was unequivocally evident by special techniques such as DSC and XRD.
Use of co-evaporation method here provided better complexes for the drug having low dose with least expenditure of resources and time. Co-evaporation method should be an effective approach for solubility enhancement for poorly aqueous and pH dependent soluble drugs. Ternary complex RPG:HPβCD:ARG obtained by co-evaporation method could be utilized in modified drug delivery system for diabetic patient with prolonged therapeutic effect having better bioavailability. From this investigation, it is concluded that inclusion complex of RPG could be formulated with HPβCD in presence and absence of ARG and PVP with enhanced solubility and better dissolution rate which can improve its oral bioavailability.
References
Lipinski, C.A.: Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 44, 235–249 (2000)
Renukuntla, J., Vadlapudi, A.D., Patel, A., Boddu, S.H.S., Mitra, A.K.: Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 447, 75–93 (2013)
Kawabata, Y., Wada, K., Nakatani, M., Yamada, S., Onoue, S.: Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int. J. Pharm. 420, 1–10 (2011)
Savjani, K.T., Gajjar, A.K., Savjani, J.K.: Drug solubility: importance and enhancement techniques. ISRN Pharma. (2012). doi:10.5402/2012/195727
Li, L.E.I., Guo, Q.: The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 42, 1–14 (2002)
Loftsson, T., Hreinsdóttir, D., Másson, M.: Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 302, 18–28 (2005)
Mura, P., Maestrelli, F., Cirri, M.: Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and aminoacids. Int. J. Pharm. 260, 293–302 (2003)
Carrier, R.L., Miller, L.A., Ahmed, I.: The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 123, 78–99 (2007)
Ribeiro, L., Loftsson, T., Ferreira, D., Veiga, F.: Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta-cyclodextrin binary and ternary complexes. Chem. Pharm. Bull. 51, 914–922 (2003)
Jadhav, P., Petkar, B., Pore, Y., Kulkarni, A., Burade, K.: Physicochemical and molecular modeling studies of cefixime-l-arginine-cyclodextrin ternary inclusion compounds. Carbohydr. Polym. 98, 1317–1325 (2013)
Soares-Sobrinho, J.L., Santos, F.L.A., Lyra, M.M.A., Alves, L.D.S., Rolim, L.A., Lima, A.A.N., Nunes, L.C.C., Soares, M.F.R., Rolim-Neto, P.J., Torres-Labandeira, J.J.: Benznidazole drug delivery by binary and multicomponent inclusion complexes using cyclodextrins and polymers. Carbohydr. Polym. 89, 323–330 (2012)
Chadha, R., Gupta, S., Shukla, G., Jain, D.V.S., Pissurlenkar, R.R.S., Coutinho, E.C.: Interaction of artesunate with β-cyclodextrin: Characterization, thermodynamic parameters, molecular modeling, effect of PEG on complexation and antimalarial activity. Results Pharma Sci. 1, 38–48 (2011)
Pokharkar, V., Khanna, A., Venkatpurwar, V., Dhar, S., Mandpe, L.: Ternary complexation of carvedilol, beta-cyclodextrin and citric acid for mouth-dissolving tablet formulation. Acta Pharm. 59, 121–132 (2009)
Aiassa, V., Zoppi, A., Albesa, I., Longhi, M.R.: Inclusion complexes of chloramphenicol with β-cyclodextrin and aminoacids as a way to increase drug solubility and modulate ROS production. Carbohydr. Polym. 121, 320–327 (2015)
De Miranda, J.C., Elyan, T., Martins, A., Veiga, F., Ferraz, H.G.: Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 47, 665–681 (2011)
Kurkov, S.V., Loftsson, T.: Cyclodextrins. Int. J. Pharm. 453, 167–180 (2013)
Cirri, M., Maestrelli, F., Mennini, N., Mura, P.: Physical—chemical characterization of binary and ternary systems of ketoprofen with cyclodextrins and phospholipids. J. Pharm. Biomed. Anal. 50, 683–689 (2009)
Figueiras, A., Nunes, S.C.C., Simões, S., Pais, A.A.C.C., Veiga, F.: Molecular interaction governing solubility and release profiles in supramolecular systems containing fenbufen, pluronics and cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 81, 395–407 (2015)
Holm, R., Schönbeck, C., Somprasirt, P., Westh, P., Mu, H.: A study of salt effects on the complexation between β-cyclodextrins and bile salts based on the Hofmeister series. J. Incl. Phenom. Macrocycl. Chem. 80, 243–251 (2014)
Basavaraj, S., Sihorkar, V., Shantha Kumar, T.R., Sundaramurthi, P., Srinivas, N.R., Venkatesh, P., Ramesh, M., Kumar Singh, S.: Bioavailability enhancement of poorly water soluble and weakly acidic new chemical entity with 2-hydroxy propyl-β-cyclodextrin: selection of meglumine, a polyhydroxy base, as a novel ternary component. Pharm. Dev. Technol. 11, 443–451 (2006)
Mandić, Z., Gabelica, V.: Ionization, lipophilicity and solubility properties of repaglinide. J. Pharm. Biomed. Anal. 41, 866–871 (2006)
Culy, C.R., Jarvis, B., Marbury, T.C., Clinical, O., Mooradian, A.D.: Repaglinide a review of its therapeutic use in type 2 diabetes mellitus. Drugs 61, 1625–1660 (2001)
Sinswat, P., Matteucci, M.E., Johnston, K.P., Williams, R.O.: Dissolution rates and supersaturation behavior of amorphous repaglinide particles produced by controlled precipitation. J. Biomed. Nanotechnol. 3, 18–27 (2007)
Purvis, T., Mattucci, M.E., Crisp, M.T., Johnston, K.P., Williams, R.O.: Rapidly dissolving repaglinide powders produced by the ultra-rapid freezing process. AAPS PharmSciTech 8, E1–E9 (2007)
Nicolescu, C., Aramǎ, C., Monciu, C.M.: Preparation and characterization of inclusion complexes between repaglinide and β-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin and randomly methylated β-cyclodextrin. Farmacia 58, 78–88 (2010)
Gao, Y., Liao, J., Qi, X., Zhang, J.: Coamorphous repaglinide-saccharin with enhanced dissolution. Int. J. Pharm. 450, 290–295 (2013)
Kavitha, R., Abdul Hasan Sathali, A.: Enhancement of solubility of repaglinide by solid dispersion technique. Int. J. Chem. Sci. 10, 377–390 (2012)
Seedher, N., Kanojia, M.: Co-solvent solubilization of some poorly-soluble antidiabetic drugs. Pharm. Dev. Technol. 14, 185–192 (2009)
Desai, N.S., Nagarsenker, M.S.: Design and evaluation of self-nanoemulsifying pellets of repaglinide. AAPS PharmSciTech 14, 994–1003 (2013)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Costa, P., Sousa Lobo, J.M.: Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 13, 123–133 (2001)
Khan, K.A.: The concept of dissolution efficiency. J. Pharm. Pharmacol. 27, 48–49 (1975)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery : an updated review. AAPS PharmSciTech 6, 329–357 (2005)
Patel, R.P., Patel, D.J., Bhimani, D.B., Patel, J.K.: Physicochemical characterization and dissolution study of solid dispersions of furosemide with polyethylene glycol 6000 and polyvinylpyrrolidone K30. Dissolut. Technol. 15, 17–25 (2008)
Loftsson, T., Másson, M.: The effects of organic salts on the cyclodextrin solubilization of drugs. J. Drug Deliv. Sci. Technol. 14, 35–43 (2004)
Laveneziana, D., Speranza, R., Raulli, P., Paredi, G.: Comparative efficacy of ibuprofen arginine and β-cyclodextrin piroxicam as treatment for tension-type headache. Clin. Drug Investig. 11, 1–7 (1996)
Berge, S., Bighley, L., Monkhouse, D.C.: Pharmaceutical salts. J. Pharm. Sci. 66, 1–19 (1977)
Mura, P., Bettinetti, G.P., Cirri, M., Maestrelli, F., Sorrenti, M., Catenacc, L.: Solid-state characterization and dissolution properties of naproxen-arginine-hydroxypropyl-beta-cyclodextrin ternary system. Eur. J. Pharm. Biopharm. 59, 99–106 (2005)
Mura, P., Faucci, M.T., Bettinetti, G.P.: The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 13, 187–194 (2001)
Yadav, D., Yadav, A., Karekar, P., Pore, Y., Gajare, P.: Enhanced solubility and dissolution rate of Olmesartan medoxomil using crystallo-co-agglomeration technique. Der Pharm. Sin. 3, 160–169 (2012)
Manca, M.L., Zaru, M., Ennas, G., Valenti, D., Sinico, C., Loy, G., Fadda, A.M.: Diclofenac-beta-cyclodextrin binary systems: physicochemical characterization and in vitro dissolution and diffusion studies. AAPS PharmSciTech 6, E464–E472 (2005)
Dollo, G., Le Corre, P., Chollet, M., Chevanne, F., Bertault, M., Burgot, J.L., Roger, L.V.: Improvement in solubility and dissolution rate of 1,2-dithiole-3-thiones upon complexation with β-cyclodextrin and its hydroxypropyl and sulfobutyl ether-7 derivatives. J. Pharm. Sci. 88, 889–895 (1999)
Lin, S.Y., Kao, Y.H.: Solid particulates of drug–cyclodextrin inclusion complexes directly prepared by a spray-drying technique. Int. J. Pharm. 56, 249–259 (1989)
Moyano, J.R., Arias-Blanco, M.J., Ginés, J.M., Giordano, F.: Solid-state characterization and dissolution characteristics of gliclazide-β-cyclodextrin inclusion complexes. Int. J. Pharm. 148, 211–217 (1997)
Acknowledgments
We are thankful to Ajanta Pharma for gift sample of Repaglinide. We are also grateful to Gangwal chemicals for providing HPβCD. A special thanks to Mr. Nilesh Kulkarni from TIFR, Mumbai for PX-RD analysis. We also express gratitude towards Dr. (Mrs.) Munira Momin, Dr. Kavita Singh and Dr. Darshana Jain for supporting in research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vakani, S.S., Kajwe, A., Suvarna, V. et al. Influence of auxiliary agents on solubility and dissolution profile of repaglinide with hydroxypropyl-β-cyclodextrin: inclusion complex formation and its solid-state characterization. J Incl Phenom Macrocycl Chem 83, 239–250 (2015). https://doi.org/10.1007/s10847-015-0559-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0559-y