Abstract
Measurements of sediment–water fluxes of O2, NO23, NH4, and PO4 and water column and sediment variables were conducted at 348 sites in Chesapeake Bay and Maryland Coastal Bays with most (~ 76%) of the 1746 sets of measurements collected during warm seasons when these processes were most active. We performed a system-wide synthesis of these spatially extensive, long-term data to identify the primary controlling factors on sediment–water fluxes over seasonal and interannual time periods and assess the relative contribution of sediment–water fluxes to nutrient cycling across distinct regions of Chesapeake Bay and the Maryland Coastal Bays. Bay-wide spatial patterns revealed hotspots for sediment–water fluxes, and statistical models were able to explain 46% (O2), 23% (NH4), 25% (NO23), and 38% (PO4) of variability in fluxes, with solute-specific controlling variables including temperature, bottom water oxygen and nutrient concentration, and sediment organic matter. An analysis of long-term variations in fluxes at six locations in the Bay (12–17 year time series) exhibited only weak evidence of long-term trends, but interannual variability was related to both water column and sediment variables, depending on the solute of interest. Finally, we compared external loads of “new” total nitrogen and phosphorus (TN and TP) to system-wide sediment–water fluxes of NH4 and PO4 at 22 Bay tributary and Coastal Bays sites, finding that ~ 64% of sites had annual sediment recycling rates that exceeded annual external loading rates, revealing the importance of recycled nutrients in these shallow systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediment–water exchanges of elements such as oxygen, nitrogen, phosphorus, silica, carbon, and others have been recognized as an important component of metabolism and nutrient cycling in estuarine and coastal marine waters (e.g., Boynton et al. 2018). Further, in these relatively shallow systems, many of which are less than 10 m in depth (Bricker et al. 2007), primary production in illuminated surface waters is immediately adjacent to aphotic deeper waters and the sediment surface where decomposition processes predominate. These adjacent zones of primary production and remineralization interact with each other, and these often complex interactions have been termed pelagic-benthic (P-B) coupling (Jensen et al. 1990; Kemp et al. 2005). P-B coupling promotes elevated and sustained levels of primary and secondary production (e.g., Nixon 1988).
In recent years, there have been several global-scale reviews of basic rate processes in estuarine and coastal marine systems. Rate measurements are important because they quantify the underlying processes which determine—and respond to—concentrations of commonly measured nutrients in the water column. Compared to the treasure trove of concentration measurements now available for analysis in the world’s coastal waters, rate measurements remain relatively rare even though techniques for making such measurements have been available for the past half century or more. Reviews of phytoplankton production rates (Cloern et al. 2014) and sediment–water oxygen and nutrient fluxes (Boynton et al. 2018) both indicated limited global coverage and serious lack of time-series measurements at specific locations as well as some methodological issues. The lack of such rate processes is a major constraint on the development and refinement of numerical models that are increasingly used in both science and management applications in these shallow environments. In addition, in the few cases where sediment–water flux time-series measurements were available, such measurements have been useful in evaluating eutrophication abatement (e.g., Tucker et al. 2014) and other environmental trends (Fulweiler and Nixon 2009). We believe the sediment–water oxygen and nutrient flux data set developed for the Chesapeake Bay and tributaries represents the largest such data set currently available for any coastal or estuarine system.
During the 1970s, it became increasingly clear that many estuaries and coastal marine ecosystems were experiencing early to advanced signs of eutrophication, including Chesapeake Bay. Beginning in the late 1970s, the US Environmental Protection Agency initiated a 5-year program to assess, among other things, the status and trends in water and habitat quality in the Bay (Environmental Protection Agency (EPA) 1982). These efforts directly led to the Chesapeake Bay Program, a sustained, coordinated, multi-state, and federal program aimed at restoration of the Bay and its many tributary rivers. That program is now in its 38th year of operation. One of the major findings of the initial EPA program was the need for a comprehensive and sustained monitoring program for the Bay and tributary rivers. EPA, the relevant states, academic groups, and other stakeholders designed a monitoring program that included water quality, food web, and habitat metrics and, importantly, included both the measurement of conventional water column and sediment concentrations, but also a limited number of process rate measurements. Such an emphasis on rates was unusual for a monitoring program at that time, and the program included estimates of external freshwater; nutrient and sediment loading rates from diffuse, point, and atmospheric sources; rates of algal primary production; deposition rates of particulate organic matter from the water column to estuarine sediments; and measurements of sediment–water exchanges of oxygen and nutrients. These rate measurements were supported, in addition to more traditional measurements, because it was clear that understanding the magnitude, seasonality, and geographic distribution of these processes would be essential in the initial development and later refinement of water quality models which have been intensively used to assess current and future water quality and habitat conditions as restoration efforts progress (Cerco and Noel 2004; Brady et al. 2013; Testa et al. 2013).
Analyses of portions of the Bay sediment–water flux data set have previously been completed and often focused on factors thought to influence sediment rate processes such as hypoxia or anoxia (e.g., Cerco 1985), depth (Kemp et al. 1992), macrofaunal effects (Bosch et al. 2015), organic matter availability (Cowan and Boynton 1996), bottom water nutrient and temperature conditions (Cornwell et al. 2016), pH (Gao et al. 2012), salinity (Jordan et al. 2008), and sediment resuspension effects (e.g., Porter et al. 2010). However, analyses of the multi-decade Chesapeake Bay sediment–water oxygen and nutrient flux data set aimed at developing an ecosystem-scale understanding have yet to be accomplished. We had several goals in developing this synthesis, including (1) to assemble a database of sediment–water flux measurements (O2, NH4, NO23 (NO2 + NO3), and PO4) and associated water column and sediment variables that have been made during the past four decades and make the database available to all interested researchers, particularly those involved with developing the next generation of estuarine water quality models; (2) to characterize the spatial distribution, magnitude, and seasonality of sediment–water fluxes among portions of this large estuarine system having strong gradients in physical, chemical, and biological properties; and (3) to use a comparative approach to examine interannual variability in sediment–water fluxes and to evaluate the relative importance of “new” (i.e., watershed loads) versus nutrients recycled from sediments in this system with large differences in nutrient loading rates among tributary rivers. In this latter approach, we did not analyze water column processes (e.g., water column recycling).
Study Area and Sampling Locations
Many descriptions of the Chesapeake Bay system and associated drainage basins are available in the literature (e.g., Pritchard 1952, 1967; Goodrich et al. 1987; Boicourt 1992; Brush et al. 1980; Brush and Brush 1994). In brief, the Chesapeake Bay system is a large, coastal plain, river-dominated estuary located in the USA mid-Atlantic region. The mainstem of the Bay is approximately 270 km in length, 8 to 40 km in width, and has a mean depth of about 9 m. The volume of the entire Bay system, including tidal tributaries, is approximately 74.4 × 109 m3. About 50% of the system surface area is in the tributary rivers and adjacent bays, while about 70% of the volume is in the mainstem Bay. The upper and lower thirds of the mainstem Bay are shallower (5 m and 9 m, respectively) than the middle portion (12 m; Cronin and Pritchard 1975).
Approximately 60 tributaries, many quite small, enter the mainstem Bay (Fig. 1) and together produce a mean freshwater discharge of 7 × 1010 m3 year−l which results in a freshwater fill-time of about 1 year (Boynton et al. 1995). The Susquehanna River at the head of the mainstem Bay accounts for approximately 50% of the freshwater flow, and the Potomac, James, Rappahannock, and York Rivers supply most of the remaining flow (Moyer and Blomquist 2017). The highest river flows normally occur in the late winter-early spring and lowest flows during late summer-fall, but unusually high and low flows have occurred in all months of the year. Freshwater inputs drive elevated seasonal stratification in summer (e.g., Murphy et al. 2011), which is a prerequisite condition for seasonal-scale hypoxia and anoxia to develop in most regions of the estuarine complex. In fact, interannual variations in river flow and associated nutrient load are the primary drivers of the extent and duration of hypoxia in Chesapeake Bay (Hagy et al. 2004; Scavia et al. 2021), where hypoxia typically occupies the deep channel of the mainstem Bay (> 10 m deep) from just north of the Chester River to just south of the Potomac River (Fig. 1). Hypoxia can extend as far south as the Rappahannock River mouth in high-flow years, and substantial hypoxic volumes occupy the Chester, Patuxent, and Potomac Rivers.
Maps of the Chesapeake Bay, tributary rivers, and the Maryland Coastal Bays showing the approximate location of sediment–water oxygen and nutrient flux measurement sites. Sites where measurements were conducted for 12–17 years are indicated by larger green circles, and the six locations in Fig. 5 are labeled, including St. Leonard Creek (STLC), Broomes Island (BRIS), and Marsh Point (MRPT) in the Patuxent River estuary, Ragged Point (RGPT) in the Potomac estuary, and Point No Point (PNPT) and R-64 in the mainstem Chesapeake Bay. Latitude and longitude for each sampling site are provided in the data set available at www.gonzo.cbl.umces.edu and Boynton and Ceballos (2019). Site descriptions are provided in Supplementary Table 1
The ratio of drainage basin area to estuarine surface area for the entire Chesapeake Bay system is 14:l indicating a large potential terrestrial influence on the estuary (EPA 1982). Total nitrogen (TN) and total phosphorus (TP) loading rates for the entire Chesapeake Bay system were approximately 10.8 × 106 kmol N year−1 and 0.36 × 106 kmol P year−1 during the mid to late 1980s (Boynton et al. 1995), but system-wide TN and TP loads have declined by 27% and 23%, respectively, during the most recent two decades (Testa et al. 2018). Interannual variability in loading rates to the estuary is large and is primarily controlled by interannual changes in river flow rather than interannual nutrient concentration changes. The range in areal loading rates among Bay systems is also large, ranging, for example, from about 0.07 mol N m−2 year−1 in portions of the Maryland Coastal Bays to over 7 mol N m−2 yr−1 in the Back River estuary, a small tributary in the upper Bay receiving substantial waste water treatment plant discharge (Testa et al. 2022).
Sediment–water oxygen and nutrient flux measurements were also conducted in the Maryland Coastal Bays. Detailed descriptions of these systems have been reported elsewhere (Dennison et al. 2009). In brief, these systems (Fig. 1) are uniformly shallow (~ 2-m mean depth), vertically well mixed, rarely exhibit severe hypoxia, have relatively long water residence times (Pritchard 1960), low freshwater inflows from mainland creeks, and exhibit high, or even hypersaline, conditions during dry years. Data from the Maryland Coastal Bays provided information from high salinity and low nutrient load sites, which was generally missing from other Chesapeake Bay sites.
Sampling was conducted in a total of 43 areas/tributaries of Chesapeake Bay and Maryland Coastal Bays for a total number of 348 different sediment–water flux sites. More specifically, sediment–water flux measurements were conducted in the upper, mid, and lower sections of the mainstem Bay, in most of the major tributary rivers (especially the Patuxent, Potomac, Choptank, Chester, and York Rivers), in three major sounds (Pocomoke, Tangier, and Eastern Bay), in twenty five small tributaries, and in the Maryland portion of the Coastal Bays. To provide descriptions of study areas, a summary of selected quantitative metrics have been organized for all locations where sediment–water oxygen and nutrient flux measurements have been conducted (Supplemental Table S1) and for the time periods when these measurements were conducted (Supplemental Table S2). General sampling locations are depicted in Fig. 1, and sites with longer time series of measurements are shown as color-coded larger circles. The exact coordinates for all 348 sites are provided in the full data set (www.gonzo.cbl.umces.edu and Boynton and Ceballos 2019).
Methods and Materials
Sediment–Water Oxygen and Nutrient Flux Measurements
Measurements of sediment–water oxygen (dissolved O2) and nutrient (ammonium, NH4; nitrite plus nitrate, NO23; soluble reactive phosphate, PO4) fluxes were conducted at a total of 348 unique sites (most sites sampled multiple times) in Chesapeake Bay and tributary rivers and the Maryland Coastal Bays between May 1977 and August 2018 (Fig. 1; Supplemental Tables S1 and S2). There are a total of 1746 sediment–water flux measurements in the data set where all fluxes (O2, NH4, NO23, and PO4) and water column and sediment variables were measured.
Detailed descriptions of sediment sampling and incubation techniques have been described in a number of reports and publications. In situ, diver placed chamber methods were used during the early years as described by Cerco (1985). More recent shipboard or laboratory incubation approaches were described in detail by Cowan and Boynton (1996) and further refined by Testa et al. (2022). This approach was by far the most frequently used technique (~ 95%). In brief, undisturbed sediment cores (~ 30 cm) were obtained using a box corer in deeper areas (> 3 m) or a pole corer in shallower areas (< 3 m). A Plexiglas® liner served as the incubation chamber for sediment cores collected with either coring device. Plexiglas® bottom and top plates with gaskets were attached to each core chamber with bungee cords to obtain a gas tight seal of the chamber. The top plate has portals for an oxygen and temperature probe equipped with a stirring motor and rod and the other for tubing used to sample and replace the water removed by sampling. In some cases, mixing of water in the chambers was accomplished using a stirring magnet suspended above the sediment surface in the incubation chamber. In all cases, stirring effectively mixed the water in the chambers but did not induce sediment re-suspension (Boynton et al. 2018). At most sites, an additional incubation chamber was filled with ambient bottom water and used as a water column control. All chambers were maintained at ambient temperature conditions. In most cases, cores were incubated on ship board, while in a few cases, sediment cores were transported from the field to a laboratory site for incubation. In these cases, air was bubbled into the water overlying the cores to avoid O2 depletion, and ambient temperature conditions were maintained. The time delay between sampling and initiation of incubation ranged from 2 to 6 h. In all cases, just prior to beginning sediment–water flux measurements, overlying water in each core was replaced multiple times with ambient bottom water to insure that water quality conditions in the cores closely resembled in situ conditions. All cores were placed in a darkened, water-filled incubator held at ambient temperature.
A total of four to five water samples were withdrawn from incubation chambers at about 1-h intervals during a 3- to 4-h incubation period. At hypoxic or anoxic stations, the replacement water was bubbled with N2 gas to prevent re-oxygenation. Water samples from the incubation cores were filtered (Whatman GF/F 2.5-cm diameter, 0.7-μm pore size glass-fiber filters) and the filtrate frozen for later laboratory analysis. Sediment–water fluxes of O2 and dissolved nutrients were computed based on the volumetric rate of change in concentrations in sediment cores (corrected for blank rates of change) and then converted to areal units (mass per area per time) using the volume and area dimensions of the sediment cores used in the incubations. Oxygen and nutrient fluxes directed from overlying water to sediments were reported as negative values; fluxes from sediments to overlying waters were reported as positive values.
The following methods were used to determine dissolved and particulate nutrient concentrations. Ammonium (NH4), nitrite plus nitrate (NO23), and soluble reactive phosphorus (PO4) were measured using the automated method of EPA (1979). Particulate carbon (PC) and particulate nitrogen (PN) samples were analyzed using a model 240B Perkin-Elmer Elemental Analyzer, while particulate phosphorus (PP) concentration was obtained by acid digestion of dried samples (Aspila et al. 1976). The methods of Strickland and Parsons (1972) and Parsons et al. (1984) were followed for chlorophyll-a analysis. Prior measurements have indicated that particulate nitrogen and carbon pools are almost completely organic in Chesapeake Bay water and sediments (Keefe 1994), thus PN/PC effectively equal PON/POC.
Surficial Sediment and Water Column Measurements
At all sampling sites, an additional intact sediment core was acquired using one of the sediment coring methods as above and was sub-cored to a depth of 1 cm using modified 60-ml centrifuge tubes to obtain material for analyses of sediment variables. Samples were transferred to pre-washed sample containers, frozen on shipboard or at the laboratory, and later analyzed for PC, PN, PP, and total and active chlorophyll-a concentration using methods listed above. Bottom water (and in some cases water column profiles) was sampled for temperature, salinity, dissolved oxygen, and the dissolved nutrients indicated earlier. During some years, and at some sites, PC, PN, PP, and total and active chlorophyll-a concentration were also measured in bottom, surface, or at additional locations in the water column. In all cases, temperature, salinity, and dissolved oxygen concentrations were measured using various types of water quality instruments (e.g., YSI Model 6920 or 6600 multi-parameter water quality instruments) which were calibrated prior to and after sampling cruises.
Statistical Analyses
Correlation Analyses
Spearman correlation analyses of net sediment–water fluxes were conducted versus a selection of physical, chemical, and biological variables (Table 1). Two-tailed p-values were computed using an asymptotic t-approximation. The analyses were conducted using Hmisc package in R (Harrell et al. 2016).
Spatial Analyses
Sediment–water flux and water quality measurements collected from all areas of the Bay and tributary rivers were interpolated using block co-Kriging (Pebesma 2004) to estimate spatial patterns of long-term summer (June–August) sediment–water flux magnitude between 1977 and 2016. Water quality measurements were extracted from multiple sources including the Chesapeake Bay Program long-term monitoring stations (www.chesapeakebay.net). A correlative tree analysis was conducted to examine associations between each flux measurement and environmental covariates (Therneau and Atkinson 2019). Oxygen flux was found to be associated with bottom oxygen concentration and salinity. Ammonium, nitrite plus nitrate, and phosphorus fluxes were associated with the corresponding bottom water concentrations of those compounds. Each flux measurement was co-located with the identified covariates to estimate their joint spatial distributions. Specifically, a cross-variogram was estimated using the Linear Model of Coregionalization (Webster and Oliver 2007). Block Kriging was then conducted to interpolate the flux measurements along the mainstem of the Bay and tributary rivers where data were available. Kriging standard errors were mapped to assess the reliability of the flux estimates (data not shown). Areas with sparse sampling were not mapped to reduce uncertainty.
General Additive Model Analysis
Generalized additive models (GAM) were built to generate a predictive framework for each sediment–water flux variable using a selection of physical, chemical, and biological variables (Table 2) as environmental covariates. All environmental covariates were included initially as predictors. Variance inflation factors (VIF) were calculated for each environmental covariate. Multi-collinearity was not detected among the covariates with maximum VIFs less than 10 (Fox 1997). Thin-plate regression splines were built for each covariate, with degrees of freedom estimated using the penalized restricted maximum likelihood method (Wood 2017). Statistical significance was estimated using approximate likelihood ratio tests based on the χ2 distribution. Backward selection was applied to identify a parsimonious model for each flux. Terms that were not statistically significant were removed until no additional terms could be dropped. The model’s predictive performance was measured using adjusted r and residual mean square errors. The scaled t-family was used to model the heavy-tailed distributions of the flux measurements. Model diagnostics were conducted using standardized residuals and fitted values of the GAM models. Model results and associated analyses are provided in Supplemental Figs. S6 and S7 and Table S3. We also conducted regression trees (CART) and random forests (RF) analyses and found that the predictive performances of GAM were comparable to those of random forests and exceeded regression tree performance (Supplemental Figs. S8 and S9). A brief description of CART and RF methods is presented in the Supplemental Materials Section D.
Analyses of “New” Versus Recycled N and P
The extensive spatial distribution of sediment–water oxygen and nutrient flux sites and the availability of TN and TP loading data for several tributaries of the Bay and the Maryland Coastal Bays made it possible to examine these data sets for patterns involving the relative importance of “new” (i.e., watershed loads) versus recycled nutrients (from sediments) in these systems. Total N and P loads used in this analysis were based on Chesapeake Bay Program watershed model simulations (Shenk et al. 2012) averaged for the years 1985–2020 and included both point and diffuse TN and TP sources. For the Maryland Coastal Bays, TN loading estimates were derived from the NLM model (Valiela et al. 1997, Brush unpublished data), but no TP estimates were available. The EPA Chesapeake Bay Program has defined 92 Bay and Coastal Bay segments (Chesapeake Bay Program 2005), and areas of these segments were used to transform tributary scale TN and TP loads to an average square meter of estuary. Summer N and P sediment flux estimates were transformed to annual flux estimates using ratios derived from a synthesis of 10 stations with sufficient measurements to estimate such values (Supplemental Section A; Supplemental Figs. S2, S3 and S4).
Results
Environmental Conditions at Sampling Sites
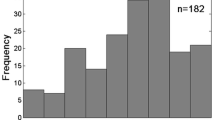
Surficial sediment and bottom water conditions were examined to provide context for later analyses of factors influencing sediment–water oxygen and nutrient fluxes. Sediment chlorophyll-a (Chl-a) and sediment particulate carbon (PC), phosphorus (PP), and nitrogen (PN) concentrations measured across all sites reveal large ranges but a clear central tendency for each constituent. Sediment Chl-a content in the top 1 cm of sediments ranged from 4 to 540 mg m−2. About 13% of measurements exceeded 60 mg m−2, and most of these were observed in low to moderate salinity areas (Fig. 2a). However, the majority of sediment chlorophyll-a measurements ranged from 4 to 40 mg m−2 (75%), similar to those reported for other shallow water estuarine and coastal systems (Boynton et al. 2018). Sediment PC concentrations ranged from 0.03 to 21.7% of sediment dry weight, but 72% of all measurements were between 2 and 5% of dry sediment weight (Fig. 2b). The extreme value (21.7%) was from an area adjacent to tidal marshes. Low PC values (< 1%) were associated with higher salinity sites, and high PC values (> 5%) were co-located with low salinity (< 5) sites. The distribution of sediment PP values differed from both PC and PN distributions, where a large percentage of PP measurements in lower salinity sites were among the highest values recorded (Fig. 2c). About 51% of all PP measurements were between 0.08 and 0.12% of sediment dry weight (the three largest bins), while the highest three bins for PC and PN represented only 10% of all measured values. The distribution of sediment PN values was similar to the PC values but generally lower by an order of magnitude with lowest values (< 0.1%) in the saltier sites and highest values (> 0.5%) in the tidal fresh and low salinity sites (Fig. 2d). About 76% of all sediment PN values ranged between 0.2 and 0.5% of dry sediment weight.
Histograms of sampling station surficial sediment characteristics including (a) total chlorophyll-a (mg m−2) and (b) particulate carbon (PC), (c) particulate phosphorus (PP), and (d) particulate nitrogen (PN) as % dry weight. All samples were collected from the top 1 cm of the sediment column. The salinity zones in which measurements were collected are color-coded in each histogram bar. Most measurements (> 50%) were collected at sites with salinities between 1 and 15. The number of samples is indicated above each bar
Water column measurements were made over a broad range of bottom water temperature, salinity, O2, and dissolved nutrient conditions (not shown; Supplemental Fig. 1). Bottom water temperatures ranged from 1.0 to 31.4 °C, but most measurements (76%) were between 15 and 25 °C and in waters having salinities ranging from 0 to 20. A small percentage of sediment–water flux measurements were made at salinities greater than 20 (~ 9%). Just over 10% of all flux measurements were made at bottom water temperatures less than 10 °C, so there is a clear emphasis on fluxes occurring at higher temperatures (> 15 °C). Bottom water O2 concentrations ranged between 0.0 and 519 μM. About 15% of all sediment–water flux measurements were made under anaerobic or extreme hypoxic conditions (< 31 μM), while an additional 23% were conducted at sites experiencing moderate hypoxia (O2 < 150 μM). Bottom water concentrations of NH4, NO23, and PO4 ranged between 0.02 and 112 μM, 0.01 and 207 μM, and 0.02 and 12.8 μM, respectively. Not surprisingly, between 20 and 30% of nutrient concentrations were very high (e.g., NO23 > 100 μM), reflecting the fact that many sampling sites were located in tidal fresh or lower salinity zones adjacent to riverine nutrient sources (Fig. 1) as well as point sources associated with waste water treatment plant (WWTP) discharges. The Chesapeake Bay and tributaries and the Maryland Coastal Bays are quite shallow, and this is reflected in the depth distribution of sediment flux measurements. Approximately 70% of flux measurements were conducted using samples from depths of less than 10 m, while 15% were made in waters greater than 15-m depth and less than 2% in waters greater than 20 m, generally following Bay hypsographic depth distributions (Kemp et al. 2005).
Annual Cycle of Sediment–Water Fluxes
Strong seasonal patterns of sediment–water fluxes emerged even when sediment–water flux data from all Chesapeake Bay and Maryland Coastal Bay sites were combined in salinity bins (Fig. 3). In all salinity bins, the magnitude of three of the four sediment–water fluxes (PO4, NH4, and O2) was greater during the warm portions of the year (Figs. 3a, b, and d and Supplemental Fig. 5). Sediment–water fluxes of NO23 differed from the other fluxes in several distinctive ways (Fig. 3c). First, NO23 fluxes were generally small, being about an order of magnitude lower than NH4 fluxes. Second, NO23 fluxes were positive (e.g., directed from sediments to water) primarily during the cooler portions of the year at higher salinity zones of the Bay during which time bottom waters were well oxygenated (> 200 μM). Finally, NO23 fluxes at low salinity sites were directed from overlying waters into sediments (plotted as negative fluxes in Fig. 3) and were generally the largest of the NO23 fluxes measured.
Box plots of monthly sediment–water fluxes of (clockwise from top left) O2, NH4, PO4, and NO23 organized by salinity zones (refer to box color to indicate salinity grouping). Data from all sites and sampling dates were used in developing these plots, where the top and bottom of the boxes are the 75th and 25th percentiles, respectively, horizontal red lines within boxes are medians, and black circles are outliers. Vertical dashed lines represent ± 2.7 sigma for a normal distribution
Overall, the magnitudes of sediment–water fluxes of oxygen and nutrients reported here were larger, or much larger, than the mean sediment–water fluxes of O2 (− 1370 μmol O2 m−2 h −1), NH4 (91 μmol N m−2 h−1), NO23 (− 7 μmol N m−2 h−1), and PO4 (11 μmol P m−2 h−1) reported for a global-scale synthesis of sediment–water oxygen and nutrient exchanges (Boynton et al. 2018) and emphasize the general eutrophic condition of many Chesapeake Bay zones and tributary rivers.
Spatial Distribution of Sediment–Water Fluxes
Broad and detailed spatial patterns of sediment–water fluxes have not been generally reported for coastal marine and estuarine systems because of severely limited spatial data (Boynton et al. 2018). There were sufficient sediment–water flux data collected in the Chesapeake system to develop both coarse seasonal-scale distributions based on salinity zones (Fig. 3) and more detailed spatial maps of summer season sediment–water fluxes (Fig. 4). We compared the monthly sediment–water nutrient and oxygen fluxes in each salinity region (via Kruskal–Wallis tests) and observed differences in the magnitude of monthly mean sediment–water fluxes between salinity bins from June to August, and for some variables, differences were also observed in February (PO4), April–May (NO23, NH4), and September–November (O2, NO23, PO4). Sediment–water fluxes of O2, NO23, and NH4 were also higher in the tidal fresh regions (salinity < 0.5) than regions with salinity greater than 15, while PO4 fluxes were highest in the mesohaline regions (salinity = 5–20).
Long term mean summer (June–August) spatial distribution of sediment–water fluxes of O2, NH4, NO23, and PO4 in the Chesapeake Bay mainstem and several large tributary rivers and bays. A divergent color ramp was used to highlight positive and negative flux values, where negative fluxes are those directed into sediments. Color classification was based on the natural break (Jenks) algorithm in ArcMap 10.4 (ESRI)
More spatially detailed distributions of summer season (June–August) sediment–water flux magnitude for O2, NH4, NO23, and PO4 were developed for the mainstem Bay and several major tributary rivers (Fig. 4). All sediment–water flux variables exhibited strong spatial patterns. In the case of O2 flux, summer values were high in zones of the Bay that did not experience hypoxia or anoxia (upper Bay and upper tributary rivers) and much lower in hypoxic and anoxic areas (e.g., mid-mainstem Bay). NH4 and PO4 flux also tended to be large in tributary rivers, as well as in mesohaline regions of the Bay where hypoxic or anoxic conditions were often present near the sediment surface. The spatial distribution of NO23 flux was clearly influenced by the concentration of NO23 in the water column with the highest fluxes directed into sediments in upper portions of tributary rivers and the upper mainstem Bay that are typically NO23-rich. NO23 fluxes directed from sediments to the water were almost always small during the summer seasons and limited to areas with non-hypoxic dissolved oxygen conditions in bottom waters (e.g., lower Mainstem Bay, eastern shore). The spatially weighted mean summer (June–August) values for the combined Bay and tributary system for O2, NH4, NO23, and PO4 fluxes were − 960, 255, 3.9, and 20.6 μmol O2, N, or P m−2 h−1, respectively.
Sediment–Water Flux Relationships with Environmental Variables
A quantitative exploration of relationships between single controlling variables and sediment–water fluxes was conducted by developing a correlation matrix (Table 1). The large number of observations in the data set resulted in many statistically significant results, but virtually all explained relatively small fractions of the observed variability. The highest Spearman’s ρ values were about 0.7, and others were in the range of 0.3 to 0.4. There were some consistent results among sediment–water fluxes where, for example, most sediment–water solute fluxes were significantly correlated with bottom water O2 concentration. Sediment–water NH4 and PO4 fluxes were larger when bottom water O2 concentrations were lower. Sediment consumption of O2 decreased as bottom water O2 concentration decreased (Marvin-DiPasquale and Capone 1998), and sediment–water fluxes of NO23 directed from sediments to overlying water tended to increase as bottom water O2 concentrations increased. Finally, a strong temperature correlation was observed for O2 and NH4 fluxes, but was less so for PO4 and NO23 fluxes.
We applied General Additive Models (GAM) to quantify the predictive power of key controlling variables on sediment–water fluxes, as well as several other multivariate, non-parametric procedures including CART (or Recursive Partitioning and Regression Trees; RPART) and Random Forest (CART and Random Forest results are summarized in Supplemental Figs. 7 and 8). GAM analyses of all four sediment–water fluxes indicated that commonly measured environmental variables such as water depth, temperature, sediment particulate nutrient content, and bottom water dissolved nutrient concentrations were frequent predictors of sediment–water flux in the GAM models (Table 2 and Supplemental Table 3). In some cases, the variables selected were probably indices of more causative variables, while others had direct effects on sediment–water fluxes. Results of GAM analyses are summarized as partial effects plots and scatter plots relating to predicted versus observed flux values in Supplemental Figs. 6 and 7, respectively. The resultant adjusted r2 values for the GAM models of the four fluxes were 0.46, 0.23, 0.25, and 0.38 for O2, NH4, NO23, and PO4 fluxes, respectively. Results for CART and Random Forest models generally had comparable or lower r2 values (Supplemental Figures S8 and S9).
Interannual Variability at Selected Long-Term Sediment–Water Flux Sites
A subset of the locations we analyzed included 12–17 consecutive years of sediment–water flux measurements during summer (June to August), allowing for investigations into interannual controls on summer season dissolved nitrogen and phosphorus fluxes. We focused on six of these locations, including three stations in the Patuxent River estuary (i.e., St. Leonard Creek, Broomes Island, Marsh Point) and three stations in the mainstem Chesapeake Bay or the lower Potomac estuary (Ragged Point, Point No Point, R-64; Fig. 5). Interannual variability was substantial at these locations, where NH4 fluxes ranged from 100 to 525 μmol N m−2 h−1 in the Patuxent and from 100 to 620 μmol N m−2 h−1 in the Potomac, NO23 fluxes ranged from 35 to − 90 μmol N m−2 h−1 in the Patuxent and from 10 to − 95 μmol N m−2 h−1 in the Potomac, and PO4 fluxes ranged from ~ 0–115 μmol P m−2 h−1 in the Patuxent and from ~ 0 to 82 μmol P m−2 h−1 in the Potomac (Fig. 5). We did not find any clear long-term temporal trends in the fluxes at these sites, despite a tendency for higher NH4 and PO4 fluxes in the late 1990s and early 2000s in the upper Patuxent (Marsh Point). Correlations of summer (June–August) mean fluxes and local conditions suggested variable-specific controlling factors on interannual variability in sediment–water fluxes, where NH4 fluxes were highly correlated with sediment particulate nitrogen (PN) concentrations (r = 0.48, n = 77), NO23 fluxes were highly correlated with overlying water NO23 concentrations (r = − 0.67, n = 77), and PO4 fluxes were highly correlated with overlying water O2 concentrations (r = − 0.5, n = 77). The variables influencing sediment–water fluxes in the interannual analysis were similar to those identified in the correlation matrix involving the entire data set.
Time-series plots (12 to 17 years) of sediment–water flux measurements (NH4, NO23, and PO4) during summer (June to August), at six Bay locations, including three stations in the Patuxent River estuary (i.e., St. Leonard Creek, Broomes Island, Marsh Point) and three stations in the mainstem Chesapeake Bay or the lower Potomac estuary (Ragged Point, Point No Point, R-64; Fig. 1). Scatter plots of summer (June–August) mean fluxes versus key water and sediment quality conditions are also shown in panels on the right side of the figure, where the solid line represents the best-fit regression line and the dashed lines are the 95% confidence intervals
We also considered the correlation between summer mean (June–August) sediment–water fluxes and both chlorophyll-a in the overlying water and freshwater inputs from major rivers (Patuxent, Susquehanna, Potomac). We considered chlorophyll-a and freshwater inputs both averaged over the annual cycle and during several periods in spring and summer (Supplemental Tables S4a, b). In general, we did not find many correlations with a p-value less than 0.05, but sediment–water PO43− fluxes were moderately correlated to February to March chlorophyll-a at Marsh Point (r = 0.58, n = 14) and Broomes Island (r = 0.56, n = 14) in the Patuxent River, and sediment–water O2 fluxes were correlated to annual (r = 0.59, n = 11) and January to May (r = 0.54, n = 11) chlorophyll-a at Point No Point. Seasonal metrics of river flow were correlated to mean summer fluxes, with differences across stations and constituents. Riverine inflows during winter-spring were correlated with O2 fluxes at R-64 and Point No Point in the mainstem Chesapeake, Ragged Point in the Potomac, and Marsh Point and Broomes Island in the Patuxent River (see Fig. 1). PO4 and NO23− fluxes were significantly correlated to riverine inflows at Broomes Island, while PO4 and NH4 fluxes were significantly correlated to riverine inflows at Point No Point and Marsh Point.
“New” Versus N and P Recycled from Sediments
Our analysis of the relative importance of “new” (i.e., watershed loads) nutrients versus those recycled from sediments across 22 tributaries of the Chesapeake Bay revealed (a) differences between N and P, (b) the tendency for sediment recycling to exceed watershed loads, and (3) long-term changes in the relationship between load and sediment recycling. TN loads varied more than two orders of magnitude from 8.4 to 1482 μmol N m−2 h−1 in the Sinepuxent Bay and the Patapsco estuary, respectively (Fig. 6). In a similar fashion, whole system sediment–water N fluxes varied from 10.19 to 591 μmol N m−2 h−1 in Sinepuxent Bay and the Patapsco estuary, respectively. TP loads and sediment–water P fluxes also exhibited a large range among tributaries (Fig. 6). For nitrogen, sediment N recycling was roughly comparable to watershed loads in the Coastal Bays, but recycling was lower than watershed load in the four low-salinity tributaries of the northern Bay (Bohemia, Elk, Northeast, Sassafras; see Fig. 1). For all other tributaries except the most heavily loaded systems (e.g., Back, Anacostia), sediment N recycling exceeded watershed nutrient loads (Fig. 6). In the Back River, where substantial wastewater nitrogen load reductions occurred (Testa et al. 2022), sediment N recycling declined in tandem with reduced loads. For phosphorus, sediment P recycling was more often higher than watershed load, including within 6 of the 8 systems with low summer dissolved oxygen (Fig. 6). In contrast, sediment P recycling was lower than watershed load for ~ 7 systems, including the low-salinity Anacostia and Northeast Rivers and high-load Patapsco (Fig. 6). Despite substantial P load reductions in the Back River, sediment P recycling exceeded loads in 4 of the 6 periods where data were available.
Scatter plots relating watershed-model-derived mean annual TN and TP loads (± SD of long-term data) in selected Bay tributaries versus estimates of annual sediment–water NH4 or PO4 fluxes (± SE of spatially weighted means). The color of symbols represents the minimum summer dissolved oxygen concentration measured at the site. The Back River estuary is highlighted, where six independent years (1994, 1995, 1997, 2014–2015, 2018) of data are included over a period when substantial point source load reductions occurred. The Maryland Coastal Bays are only included in the top panel (TN), as no TP loads are available for these systems. The “Low-Salinity, North Bay” sites have low NH4 fluxes per TN load, and these sites are located in eastern tributaries of the north part of the Bay. Note the x-axis is on a log scale to aid in illustration of the data
Discussion
Our synthesis of sediment–water nutrient and oxygen exchanges in Chesapeake Bay and the Maryland Coastal Bays builds upon recent global syntheses (Boynton et al. 2018) and assessments of long-term changes in sediment–water fluxes in response to watershed nutrient load reductions (Taylor et al. 2011; Testa et al. 2022). Few, if any, comparable sediment–water flux data sets exist for a single estuary globally, and our analysis reveals that high spatial variability in sediment–water fluxes results from spatial and seasonal differences in water column conditions and local rates of organic matter accumulation in sediments. Assessments of multi-decade time series at select stations reinforce the interaction between sediment organic matter and water column chemistry and emphasize that sediment–water fluxes and the conditions that control them can vary substantially in estuaries over time. Sediment–water fluxes of PO4 and O2 (and to a lesser extent NH4) were positively correlated to interannual variability in external inputs of freshwater (Supplemental Table 4a), but perhaps more importantly, the magnitude of sediment–water nutrient regeneration relative to external nutrient loads is substantial, varied across subsystems, and was different for nitrogen versus phosphorus (Fig. 6). Below, we discuss these primary conclusions of our synthesis in more detail.
Decade-Scale Trends in Sediment–Water Fluxes at Selected Locations
Estuarine and lake ecosystems are typically responsive to interannual variations in freshwater inputs and the nutrient loads associated with these freshwater inputs (e.g., Vollenweider 1976; Nixon 1988). As a consequence, water column nutrient concentrations and phytoplankton biomass vary substantially from year to year, supporting a rich literature associated with natural climatic fluctuations that influence riverine discharges and phytoplankton communities (Harding et al. 2015; Cloern et al. 2014), changes in watershed nutrient inputs associated with watershed management (Kubo et al. 2019), and dramatic alterations to grazer communities (Petersen et al. 2008). In contrast, studies investigating interannual variations in sediment–water nutrient and oxygen exchanges are relatively rare, leading to gaps in our understanding of this process. This gap is particularly relevant for eutrophication science, given assertions that sediments preserve a legacy of past nutrient enrichment (e.g., Walve et al. 2018; Kubo et al 2019), that could dampen interannual variability by releasing nutrients accumulated over several years or decades.
In the few cases where long-enough time series of sediment–water nutrient flux measurements were made to assess long-term changes, a background of interannual variability was evident within clear long-term declines in sediment oxygen uptake and nutrient release (Tucker et al. 2014; Foster and Fulweiler 2014; Testa et al. 2022). Tucker et al. (2014) reported a strong and apparently linear reduction of sediment–water NH4 flux magnitude in Boston Harbor in response to large nitrogen load reductions from wastewater treatment plants (WWTPs). Testa et al. (2022) also reported a fivefold reduction in TN loading from a major WWTP in Baltimore, Maryland, USA, and this load decline was associated with a fivefold reduction in sediment–water NH4 flux during the 1994–2017 period (see also Fig. 6). In the Back River, the magnitude of NH4 flux per unit nitrogen load was smaller than what we found for the subset of Chesapeake Bay systems (Fig. 6), which may be related to the fact that in the Back River (and in Boston Harbor), bottom waters were not subjected to hypoxic conditions because of tidal-induced mixing (Boston Harbor) or because of very shallow depths and effective wind and tidal mixing (Back River). While Tucker et al. (2014) and Testa et al. (2022) reported very little temporal lag between nutrient load reductions and subsequent reductions in sediment–water NH4 flux in Boston Harbor and the Back River (respectively), other studies have reported continued internal phosphorus generation for several years to a decade after major nutrient reductions (Conley et al. 2002, 2009; Walve et al. 2018; Kubo et al. 2019).
We analyzed data from six stations where at least a decade of sediment–water flux observations were made and determined that high interannual variability was a function of solute-specific environmental controls. First, the fact that sediment–water NH4 fluxes were most strongly correlated with sediment organic nitrogen (Fig. 5) reinforces the understanding that remineralization is a primary control on potential NH4 flux, consistent with an understanding that organic matter deposition to sediments (a proxy for sediment N content) is a dominant control on sediment metabolism (Middelburg et al. 1993; Brady et al. 2013). High interannual variability in NH4 fluxes (twofold) and the tendency for substantial correlations between riverine inflows and sediment oxygen demand (Supplemental Table 4a; Boynton et al. 2018) support the notion that sediments are highly responsive to year to year variability in conditions. A similar conclusion regarding high variability in NO23 fluxes is evident from our analysis, but in contrast to NH4 fluxes, NO23 flux is clearly influenced by the availability of overlying-water NO23. This reveals a dominant control of diffusive inputs of NO23 driven by water–sediment concentration gradients and suggests that interannual variability in water column NO23 concentrations associated with watershed nutrient loading or riverine inflows will impact the magnitude of sediment NO23 uptake. A key consequence of this series of linked processes is that high nutrient loads, and thus concentrations, will drive NO23 diffusion into sediment to support high rates of denitrification (Stӕhr et al. 2017). Finally, the correlation between PO4 fluxes and water column dissolved oxygen at the stations we analyzed adds to the substantial literature relating oxygen depletion to enhance sediment–water P release (e.g., Sundby et al. 1992; Conley et al. 2009; Faganeli and Ogrinc 2009; Murrell and Lehrter 2011). The emergence of this relationship in a multi-decade time series reveals how freshwater inputs can indirectly support P recycling through the generation of low-oxygen conditions resulting from stratification and nutrient-induced phytoplankton production (Hagy et al. 2004; Laurent et al. 2016).
Statistical Modeling of Sediment–Water Oxygen and Nutrient Fluxes
We subjected the sediment flux data set to several types of statistical analyses in efforts to better evaluate the utility of using routinely measured environmental conditions to better understand the magnitude and direction of measured sediment fluxes. Many of the key environmental factors that explained variations in fluxes suggest both direct and indirect effects. For example, depth probably acted as an index of both (a) dissolved oxygen stress because persistent low O2 conditions were more common in deeper areas of the Bay and (b) organic matter availability which declines as depth increases (Kemp et al. 1992). Similarly, salinity acted to serve as a proxy for location along estuarine gradients (as opposed to a direct effect), where organic enrichment and nutrient concentrations were highest in low-salinity regions. Temperature and nutrient concentrations (e.g., NO23 concentration) had more direct biogeochemical effects on flux magnitude and, in some cases, flux direction. It appears, however, that the statistical models considered here did not explain large amounts of sediment–water flux variability, and we offer several reasons for that result here. First, several key environmental variables were simply not available for this analysis including the rate and quality of organic matter deposition to the sediment surface and infaunal biomass and composition, both of which likely play important roles in shaping sediment–water flux magnitude and temporal characteristics (Rysgaard et al. 1995; Bosch et al. 2015). All of the sediment–water nutrient flux variables we examined were correlated with one or more surficial sediment N or C content, while NH4 and PO4 fluxes were often correlated with early spring water column chlorophyll-a (Supplemental Table 4b). This highlights the importance of organic matter deposition in controlling sediment–water flux magnitude (Middelburg et al. 1993), but our data were either stock measures (e.g., sediment %N) or indirect measures of potential deposition (e.g., spring chlorophyll-a), limiting their potential to reflect integrated rates over longer time frames. Benthic infaunal organisms play an important role in the mixing of sediments and the processing of deposited organic material (Norkko et al. 2012), and our measurements did not account for the presence, abundance, or biomass of these organisms and thus any potential impacts on fluxes. High spatial heterogeneity in sediment–water fluxes has been associated with gradients of benthic invertebrates and diagenesis (e.g., Rafaelli et al. 2003; Mazur et al. 2021). Future monitoring programs should consider quantifying benthic invertebrate biomass and indices of organic matter input rates alongside sediment–water flux measurements.
This data set also did not lend itself to considering lag times between late spring sediment organic matter conditions and summer sediment–water fluxes. It is likely that summer sediment–water fluxes respond to previously deposited organic matter or that which is transported horizontally in response to lateral or axial advection (e.g., Wang 2020). It may be that improved understanding of complex sediment–water biogeochemical processes is best achieved using simulation models where multiple control factors and non-linear interactions can readily be captured and lag times can be addressed. Brady et al. (2013) and Testa et al. (2013) combined a subset of the sediment–water fluxes presented here with a sediment biogeochemical model and were able to derive rates of sediment organic matter deposition, revealing how deposition interacted with overlying oxygen conditions to modulate sediment–water N and P fluxes. Ratmaya et al. (2022) reported a strong link between organic matter deposition associated with an algal bloom and NH4 fluxes over relatively short periods (weeks), while Ait Ballagh et al. (2021) revealed spatially varying controls on phosphorus cycling and flux by combining benthic observations with a diagenetic model. Statistical models that include variables representing organic matter availability and overlying water conditions have been able to capture sediment–water fluxes in other marine environments (e.g., Serpetti et al. 2016), but these models cannot be used to test process-based hypotheses. This suggests that multiple factors were involved in regulating flux magnitude, that we could not capture non-linear interactions among the variables that influence fluxes, and that our data set was not resolved enough in space and time to measure all of the relevant dynamics.
Relative Importance of Sediment Nutrient Recycling Versus Inputs of “New” Nutrients
A great deal has been written concerning the general concept of recycling in ecosystems (e.g., Odum 1971; Valiela 1995; Christian and Thomas 2003). However, differences in the relative importance of “new” nutrients (nutrients entering a system from an external source; Dugdale and Goering 1967) versus recycled nutrients among systems with different nutrient loading histories are less well understood. In Chesapeake Bay and other river-dominated temperate systems, “new” nutrient loads are typically large during winter/spring and small during summer/fall, except when influenced by tropical storms or hurricanes (Davis and Laird 1976). In contrast, rates of phytoplankton primary production exhibit the opposite pattern, peaking during summer when “new” nutrient sources are small. Even on an annual basis, the importance of recycled nutrients in support of primary production rates is clear. For example, in the mainstem Chesapeake Bay annual rates of phytoplankton production have ranged from about 350 to almost 800 g C m−2 year−1 (Mihursky et al. 1977; Flemer 1970; Taft et al. 1980), and in more recent years, values of about 550 g C m −2 year−1 have been reported (Kemp et al. 1997; Harding et al. 2002). An estimate of both the N and P needed to support the latter level of production can be made using Redfield C:N:P proportions for phytoplankton growth (Redfield 1934; 106:16:1). Using this approach, about 96 g N m−2 year−1 and about 13 g P m−2 yr−1 would be needed, values 4 to 12 times larger than estimates of annual new N or P inputs to the mainstem Bay (24 ± 10.6 g N m−2 year−1 and 1.5 ± 0.6 g P m−2 year−1) and much higher than nutrient loads to most coastal and estuarine systems (Boynton and Kemp 2008). We are aware of the uncertainties and other limitations associated with this analysis related to interannual variations in external loads, use of an indirect method to estimate phytoplankton nutrient demand, and a focus on just the Chesapeake Bay mainstem. Thus, our approach here is broad brush rather than detailed and is not meant to be comprehensive. However, even with the uncertainties associated with this effort, the importance of nutrient recycling is clear, with annualized estimates of sediment–water N and P fluxes providing 14 g N m−2 year−1 and 2.3 g P m−2 year−1, which is insufficient to make up for the missing demand. Other potential external sources of N and P are either small or uncertain (direct atmospheric deposition; Castro et al. 2003, eroding marsh; Su et al. 2020). Thus, it is likely that the missing N and P may come from water column recycling, which has been reported at high rates in Chesapeake Bay (Glibert 1998) and is consistent with calculations where water column respiration is a large component of mainstem oxygen demand (Li et al. 2016).
We further explored the relative importance of “new” versus one source of recycled nutrients (sediments) for a subset of Chesapeake Bay subsystems and the Maryland Coastal Bays (for nitrogen) that share similar attributes including latitudinal location, generally shallow depths, modest tidal energies, and relatively long water residence times (e.g., Hagy et al. 2000). However, these systems span a substantial range in nutrient loading rates and degree of hypoxia/anoxia present during the warm periods of the year. Scatter plots of annual sediment NH4 and PO4 flux versus annual TN or TP loads to these systems clearly indicate the importance of sediment nutrient recycling relative to watershed inputs of new N and P (Fig. 6). In the case of nitrogen, 14 of the 23 sites exhibited annual sediment–water NH4 flux amounts that exceeded mean annual TN inputs. Of the remaining nine sites, annual NH4 recycling amounted to greater than 50% of new TN inputs. The four sites in the lower left of the diagram (Fig. 6) were all from the Maryland Coastal Bays, which are uniformly shallow, normoxic, and high salinity sites with low TN loading rates. It is not clear why sediment recycling plays a relatively small role in these systems, but these systems are shallow, and the sediments receive sufficient light for photosynthesis (Ganju et al. 2020), indicating that benthic algae may sequester nutrients within sediments (Sundbäck et al. 2000). Heavily loaded tributaries such as the Back and Patapsco Rivers exhibited relatively low (< 100%) N recycling versus new TN input signatures. Kelly et al. (1985) reported similar patterns based on experimental mesocosm nutrient loading experiments (University of Rhode Island MERL system) where new inputs dominated the nutrient dynamics at high loading rates. However, we are not aware of any previous work reporting this pattern based on a comparative analysis of estuarine sites.
The relationship between sediment recycling versus “new” TP inputs appeared more complex or at least less obvious. Of the 19 sites, 12 had sediment–water PO4 recycling rates greater than watershed inputs of TP. All but one of the sites with sediment–water PO4 fluxes greater than double the rate of watershed TP inputs experienced moderate to severe hypoxia (Fig. 6), which would tend to enhance sediment–water PO4 fluxes (Harris et al. 2015). In contrast, the sediment–water PO4 fluxes which were small or even negative relative to watershed TP inputs were located in low salinity to tidal freshwater zones, and the cause of this pattern was likely related to PO4 sorption onto iron-rich sediments (Jordan et al. 2008). This comparative analysis, while not comprehensive because it focused only on Chesapeake and Maryland Coastal Bays sites, indicates that both N and P recycling from sediments is a major feature in the nutrient dynamics of these systems, that sediment recycling of N and P alone often exceeds the magnitude of new inputs of TN and TP, that oxygen conditions in bottom waters can influence the magnitude of sediment recycling, especially for P, and that for both N and, to a lesser extent, for P, the relative importance of sediment–water recycling decreases as external inputs of TN and TP increase. However, future improvements in this analysis are certainly possible and might lead to further refinements and confidence in understanding of these processes. For example, we considered only the sediment recycling of NH4 rather than all forms of nitrogen. NO23 fluxes were generally small relative to NH4 fluxes, and thus ignoring these would not strongly influence our general conclusions. There is more uncertainty associated with sediment–water dissolved organic nitrogen (DON) fluxes which have been rarely measured in the Bay (Cowan and Boynton 1996; Boynton et al. 2018). The limited available DON data and model estimates suggest that DON fluxes are minor (< 5%) relative to inorganic nitrogen fluxes (Burdige and Zheng 1998; Clark et al. 2017).
Considerations for Future Monitoring and Management
Upon reflection on the sediment–water flux measurement program spanning 40 years, a number of insights, successes, and failures have become apparent that may serve as a lesson for those building or re-assessing similar monitoring or research programs. At a conceptual level, we were surprised that the US EPA Chesapeake Bay Program initially chose to support monitoring of sediment–water processes because most monitoring programs emphasized simpler and less expensive measurements of water column and sediment stocks or concentrations. Thus, the Chesapeake Bay Program was somewhat unique in its early recognition that the issue of estuarine cultural eutrophication had been defined as a rate process (Nixon 1995). Furthermore, the early development and application of simulation models in support of water quality restoration programs in the Chesapeake Bay clearly indicated the need for quantitative data on the magnitude, seasonality, and spatial distribution of sediment–water fluxes, and this was one of our central programmatic goals. During the development of the Chesapeake Bay water quality model (Cerco et al. 2010), one of the lead investigators referred to the database presented here as the “gold standard” against which models needed to be judged (D.M. Di Toro, pers comm. to W.R. Boynton). More recently, substantial improvements to that model again relied on this data set for guidance (Brady et al. 2013; Testa et al. 2013), and we expect additional use of this data set as the Bay water quality models continue to evolve.
A second programmatic goal was to improve our understanding of factors controlling sediment–water flux rates, and here we had successes but also some frustrations and failures. For example, most sediment–water flux measurements were made during the summer periods when rates were typically largest, but we had limited success in relating summer fluxes to important controlling factors such as organic matter supply rate to sediments (e.g., Cowan and Boynton 1996). More complete seasonal coverage would have been useful coupled with laboratory-based organic matter enrichment experiments. Examination of benthic infauna was not a regular part of these studies due to financial constraints, but limited analyses of Chesapeake Bay data and many other reports (e.g., Norkko et al. 2012) have indicated that benthic macrofauna effects on sediment–water fluxes are substantial and will only become more so if bottom water dissolved oxygen conditions continue to improve (Bosch et al. 2015).
There are exciting opportunities for continued measurements of sediment–water fluxes. Water quality managers continue to struggle with expectations concerning the timing and magnitude of estuarine responses to pollution reductions. Recent time-series analyses of sediment–water flux responses to substantial nutrient load reductions in a small tributary of the Bay indicted rapid (months to a year) and linear responses (Testa et al. 2022; see also Fig. 6). Continued time-series measurements in areas of the Bay or other estuarine systems exposed to different levels of oxygen stress and degrees of enrichment would add to basic understanding of both estuarine eutrophication and water quality restoration trajectories. During the early years of our work, we were limited to the number of variables that could reasonably be measured in a monitoring program, but now measurements of sediment denitrification, CO2 and organic nitrogen flux, and anaerobic processes can be routinely measured and would likely enhance interpretation of water quality trends and be useful in further improvements to water quality models.
Summary and Conclusions
We analyzed four decades of sediment–water flux measurements of dissolved nutrients and oxygen in a large and variable temperate estuarine system. Strong seasonal patterns were observed for all flux variables, but temperature only explained a portion of seasonal variations because overlying water O2 concentrations and sediment organic content were also significant drivers. We can infer from these patterns that eutrophication reduction (i.e., elevated oxygen, reduced organic matter deposition) will lead to lower sediment–water fluxes of all variables we considered. Multivariate statistical models based upon local water quality and surficial sediment conditions were only partially successful in predicting sediment flux magnitude, reflecting solute-specific environmental controls on sediment–water fluxes. Finally, a cross-system analysis of the ratio of system-wide sediment–water N and P fluxes and external loads underscores the importance of recycled nutrients in support of water column primary production. We hope that this analysis stimulates interest in the building of comprehensive time series of ecological rate processes, like sediment–water fluxes, that allow for mechanism-based assessments of eutrophication (and its abatement) and the validation of the ever-growing number of numerical models used to forecast and quantify processes in the coastal zone.
References
Ait Ballagh, F.E., C. Rabouille, F. Andrieux-Loyer, K. Soetaert, B. Lansard, B. Bombled, et al. 2021. Spatial variability of organic matter and phosphorus cycling in Rhône River prodelta sediments (NW Mediterranean Sea, France): A model-data approach. Estuaries and Coasts 44: 1765–1789.
Aspila, I., H. Agemian, and A.S.Y. Chau. 1976. A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. The Analyst 101: 187–197. https://doi.org/10.1039/an9760100187.
Boicourt W.C. 1992. Influences of circulation processes on dissolved oxygen in the Chesapeake Bay. In Oxygen dynamics in the Chesapeake Bay: a synthesis of recent research, eds. D. Smith, E. Leffler and G.M. Mackiernan, 7–59. College Park: Maryland Sea Grant.
Bosch, J.A., J.C. Cornwell, and W.M. Kemp. 2015. Short-term effects of nereid polychaete size and density on sediment inorganic nitrogen cycling under varying oxygen conditions. Marine Ecology Progress Series 524: 155–169. https://doi.org/10.3354/meps11185.
Boynton, W.R., J.H. Garber, R. Summers, and W.M. Kemp. 1995. Inputs, transformations and transport of nitrogen and phosphorus in Chesapeake Bay and selected tributaries. Estuaries 18: 285–314. https://doi.org/10.2307/1352640.
Boynton, W.R., and W.M. Kemp 2008. Nitrogen in estuaries. In: Nitrogen in the Marine Environment (Second Edition), eds. D.G. Capone, E.J. Carpenter, D.A. Bronk, M.R. Mulholland, 809–866. Amsterdam: Elsevier Science, Academic Press. 9780123725226.
Boynton, W.R., M.A.C. Ceballos, E.M. Bailey, C.L.S. Hodgkins, J.L. Humphrey, and J.M. Testa. 2018. Oxygen and nutrient exchanges at the sediment-water interface: A global synthesis and critique of estuarine and coastal data. Estuaries and Coasts 41: 301–333. https://doi.org/10.1007/s12237-017-0275-5.
Boynton, W.R., and M.A.C. Ceballos. 2019. Chesapeake Bay and Maryland Coastal Bays sediment-water oxygen and nutrient flux data set, Mendeley Data, v1. https://doi.org/10.17632/jpwvc5jytk.1.
Brady, D.C., J.M. Testa, D.M. Di Toro, W.R. Boynton, and W.M. Kemp. 2013. Sediment flux modeling: Calibration and application for coastal systems. Estuarine, Coastal and Shelf Science 117: 107–124. https://doi.org/10.1016/j.ecss.2012.11.003.
Bricker, S.B., B. Longstaff, W. Dennison, A. Jones, K. Boicourt, C. Wicks, and J. Woerner. 2007. Effects of nutrient enrichment in the nation’s estuaries: a decade of change. Silver Springs: NOAA Coastal Ocean Program Decision Analysis Series No. 26.
Brush, G.S., C. Lenk, and J. Smith. 1980. The natural forests of Maryland: An explanation of the vegetation map of Maryland. Ecological Monographs 50: 77–92. https://doi.org/10.2307/2937247.
Brush, G.S., and L.M. Brush. 1994. Transport and deposition of pollen in an estuary: signature of the landscape. In Sedimentation of Organic Particles, ed. A. Traverse, 33–46. Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511524875.004.
Burdige, D.J., and S. Zheng. 1998. The biogeochemical cycling of dissolved organic nitrogen in estuarine sediments. Limnology and Oceanography 43: 1796–1813.
Castro, M.S., C.T. Driscoll, T.E. Jordan, W.G. Reay, and W.R. Boynton. 2003. Sources of nitrogen to estuaries of the United States. Estuaries 26 (3): 803–814.
Cerco, C.F. 1985. Sediment-water column exchanges of nutrients and oxygen in the tidal James and Appomattox rivers. National Tech. Information Service, PB85–242915 XAB.
Cerco, C.F., and M.R. Noel. 2004. Process-based primary production modeling in Chesapeake Bay. Marine Ecology Progress Series 282: 45–58.
Cerco, C.F., S.-C. Kim and M.R. Noel. 2010. The 2010 Chesapeake Bay Eutrophication Model. A Report to the US Environmental Protection Agency Chesapeake Bay Program and US Army Engineer Baltimore District. Annapolis, MD. 228 pages, https://www.chesapeakebay.net/content/publications/cbp_55318.pdf.
Chesapeake Bay Program. 2005. Analytical segmentation scheme: revisions, decisions and rationales 1983–2003. Annapolis: Monitoring and analysis subcommittee, Tidewater monitoring and analysis workgroup.
Christian, R.R., and C.R. Thomas. 2003. Network analysis of nitrogen inputs and cycling in the Neuse River Estuary, North Carolina, USA. Estuaries 26 (3): 815–828.
Clark, J.B., W. Long, and R.R. Hood. 2017. Estuarine sediment dissolved organic matter dynamics in an enhanced sediment flux model. Journal of Geophysical Research: Biogeosciences 122: 2669–2682. https://doi.org/10.1002/2017JG003800.
Cloern, J.E., S.Q. Foster, and A.E. Kleckner. 2014. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11: 2477–2501.
Conley, D.J., C. Humborg, L. Rahm, O.P. Savchuk, and F. Wulff. 2002. Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environmental Science and Technology 36: 5315–5320. https://doi.org/10.1021/es025763w.
Conley, D.J., H.W. Paerl, R.W. Howarth, D.F. Boesch, S.P. Seitzinger, K.E. Havens, C. Lancelot, and G.E. Likens. 2009. Controlling eutrophication: Nitrogen and phosphorus. Science 323: 1014–1015. https://doi.org/10.1126/science.1167755.
Cornwell, J.C., M.S. Owens, W.R. Boynton, and L.A. Harris. 2016. Sediment-water nitrogen exchange along the Potomac River estuarine salinity gradient. Journal of Coastal Research 32: 776–787. https://doi.org/10.2112/JCOASTRES-D-15-00159.1.
Cowan, J.L.W., and W.R. Boynton. 1996. Sediment-water oxygen and nutrient exchanges along the longitudinal axis of Chesapeake Bay: Seasonal patterns, controlling factors and ecological significance. Estuaries 19: 562–580. https://doi.org/10.2307/1352518.
Cronin, W.B., and D.W. Pritchard. 1975. Additional statistics on the dimensions of the Chesapeake Bay and its tributaries: cross-section widths and segment volumes per meter depth. Special Report 42. Baltimore: Chesapeake Bay Institute, The Johns Hopkins University. Reference 75–3.
Davis, J., and B. Laird, eds. 1976. Effects of tropical storm Agnes on the Chesapeake Bay estuarine system, 54. Baltimore: The Johns Hopkins University Press. Chesapeake Research Consortium Publication No.
Dennison, W.C., J.E. Thomas, C.J. Cain, T.J.B. Carruthers, M.R. Hall, R.V. Jesien, C.E. Wazniak, and D.E. Wilson. 2009. Shifting sands: Environmental and cultural change in Maryland’s Coastal Bays. Cambridge: Ian Press.
Dugdale, R.C., and J.J. Goering. 1967. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnology and Oceanography 12: 196–206.
Environmental Protection Agency (EPA). 1979. Methods for chemical analysis of water and wastes. Cincinnati: Environmental Monitoring and Support Laboratory. USEPA-6000/4–79–020.
Environmental Protection Agency (EPA). 1982. Chesapeake Bay Program, technical studies: A synthesis. Washington, DC: United States Environmental Protection Agency.
Faganeli, J., and N. Ogrinc. 2009. Oxic–anoxic transition of benthic fluxes from the coastal marine environment (Gulf of Trieste, northern Adriatic Sea). Marine and Freshwater Research 60: 700–711. https://doi.org/10.1071/MF08065.
Flemer, D.A. 1970. Primary production in the Chesapeake Bay. Chesapeake Science 11: 117–129. https://doi.org/10.2307/1350486.
Foster, S.Q., and R. W. Fulweiler. 2014. Spatial and historic variability of benthic nitrogen cycling in an anthropogenically impacted estuary. Frontiers in Marine Science 1.
Fox, J. 1997. Applied regression analysis, linear models, and related methods. Thousand Oaks: Sage Publications.
Fulweiler, R.W., and S.W. Nixon. 2009. Responses of benthic-pelagic coupling to climate change in a temperate estuary. Hydrobiologia 629: 147–156.
Ganju, N.K., J.M. Testa, S.E. Suttles, and A.L. Aretxabaleta. 2020. Spatiotemporal variability of light attenuation and net ecosystem metabolism in a back-barrier estuary. Ocean Science 16: 593–614.
Gao, Y., J.C. Cornwell, D.K. Stoecker, and M.S. Owens. 2012. Effects of cyanobacterial-driven pH increases on sediment nutrient fluxes and coupled nitrification-denitrification in a shallow fresh water estuary. Biogeosciences 9: 2697–2710.
Glibert, P.M. 1998. Interaction of top-down and bottom-up control in plankton nitrogen cycling. Hydrobiologia 363: 1–12.
Goodrich, D.M., W.C. Boicourt, P. Hamilton, and D.W. Pritchard. 1987. Wind-induced destratification in Chesapeake Bay. Journal of Physical Oceanography 17: 2232–2240.
Hagy, J.D., W.R. Boynton, and L.P. Sanford. 2000. Estimation of net physical transport and hydraulic residence times for a coastal plain estuary using box models. Estuaries 23: 328–340. https://doi.org/10.2307/1353325.
Hagy, J.D., W.R. Boynton, C.W. Keefe, and K.V. Wood. 2004. Hypoxia in Chesapeake Bay, 1950–2001: Long-term change in relation to nutrient loading and river flow. Estuaries 27: 634–658.
Harding, L.W., Jr., M.E. Mallonee, and E.S. Perry. 2002. Toward a predictive understanding of primary productivity in a temperate, partially stratified estuary. Estuarine, Coastal and Shelf Science 55: 437–463.
Harding, L.W., C.L. Gallegos, E.S. Perry, W.D. Miller, J.E. Adolf, M.E. Mallonee, and H.W. Paerl. 2015. Long-term trends of nutrients and phytoplankton in Chesapeake Bay. Estuaries and Coasts 39: 664–681.
Harrell Jr., F.E., C. Dupont, and Many Others. 2016. Hmisc: Harrell Miscellaneous. R package version 3.17–4. https://CRAN.R-project.org/package=Hmisc.
Harris, L.A., C.L.S. Hodgkins, M.C. Day, D. Austin, J.M. Testa, W.R. Boynton, L. Van Der Tak, and N.W. Chen. 2015. Optimizing recovery of eutrophic estuaries: Impact of destratification and re-aeration on nutrient and dissolved oxygen dynamics. Ecological Engineering 75: 470–483. https://doi.org/10.1016/j.ecoleng.2014.11.028.
Jensen, M.H., E. Lomstein, and J. Sørensen. 1990. Benthic NH4+ and NO3- flux following sedimentation of a spring phytoplankton bloom in Aarhus Bight, Denmark. Marine Ecology Progress Series 61: 87–96. https://doi.org/10.3354/meps061087.
Jordan, T.E., J.C. Cornwell, W.R. Boynton, and J.T. Anderson. 2008. Changes in phosphorus biogeochemistry along an estuarine salinity gradient: The iron conveyer belt. Limnology and Oceanography 53: 172–184. https://doi.org/10.4319/lo.2008.53.1.0172.
Keefe, C.W. 1994. The contribution of inorganic compounds to the particulate carbon, nitrogen, and phosphorus in suspended matter and surface sediments of Chesapeake Bay. Estuaries 17: 122–130.
Kelly, J.R., V.M. Berounsky, S.W. Nixon, and C.A. Oviatt. 1985. Benthic-pelagic coupling and nutrient cycling across an experimental eutrophication gradient. Marine Ecology Progress Series 26: 207–219.
Kemp, W.M., P.A. Sampou, J.H. Garber, J. Tuttle, and W.R. Boynton. 1992. Seasonal depletion of oxygen from bottom waters of Chesapeake Bay: Roles of benthic and planktonic respiration and physical exchange processes. Marine Ecology Progress Series 85: 137–152.
Kemp, W.M., E.M. Smith, M. Marvin-DiPasquale, and W.R. Boynton. 1997. Organic carbon balance and net ecosystem metabolism in Chesapeake Bay. Marine Ecology Progress Series 150: 229–248.
Kemp, W.M., W.R. Boynton, J. Adolf, D.F. Boesch, W.C. Boicourt, G. Brush, J.C. Cornwell, T. Fisher, P. Glibert, J. Hagy, L.W. Harding, E. Houde, D. Kimmel, W.D. Miller, R. Newell, M. Roman, E. Smith, and J. Stevenson. 2005. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Marine Ecology Progress Series 303: 1–29. https://doi.org/10.3354/meps303001.
Kubo, A., F. Hashihama, J. Kanda, N. Horimoto-Miyazaki, and T. Ishimaru. 2019. Long-term variability of nutrient and dissolved organic matter concentrations in Tokyo Bay between 1989 and 2015. Limnology and Oceanography 64: S209–S222. https://doi.org/10.1002/lno.10796.
Laurent, A., K. Fennel, R. Wilson, J. Lehrter, and R. Devereux. 2016. Parameterization of biogeochemical sediment-water fluxes using in situ measurements and a diagenetic model. Biogeosciences 13: 77–94. https://doi.org/10.5194/bg-13-77-2016.
Li, M., Y.-J. Lee, J.M. Testa, Y. Li, W. Ni, W.M. Kemp, et al. 2016. What drives interannual variability of estuarine hypoxia: Climate forcing versus nutrient loading? Geophysical Research Letters 43: 2127–2134.
Marvin-DiPasquale, M.C., and D.G. Capone. 1998. Benthic sulfate reduction along the Chesapeake Bay central channel. I. Spatial trends and controls. Mar Ecol Progr Ser 168: 213–228.
Mazur, C.I., A.N. Al-Haj, N.E. Ray, I. Sanchez-Viruet, and R.W. Fulweiler. 2021. Low denitrification rates and variable benthic nutrient fluxes characterize Long Island Sound sediments. Biogeochemistry 154: 37–62.
Middelburg, J.J., T. Vlug, F. Jaco, and W.A. van der Nat. 1993. Organic matter mineralization in marine systems. Global and Planetary Change 8: 47–58. https://doi.org/10.1016/0921-8181(93)90062-S.
Mihursky, J.A., D.R. Heinle, and W.R. Boynton. 1977. Ecological effects of nuclear steam electric station operations on estuarine systems. Solomons: Chesapeake Biological Laboratory. Technical Report, UMCEES Ref. No. 77–28-CBL.
Moyer, D.L., and J.D. Blomquist. 2017. Summary of nitrogen, phosphorus, and suspended-sediment loads and trends measured at the Chesapeake Bay Nontidal Network Stations: Water Year 2016 Update. USGS Chesapeake Bay Trends. https://cbrim.er.usgs.gov/data/NTN%20Load%20and%20Trend%20Summary%202016_Combined.pdf. Accessed 23 June 2020.
Murphy, R.R., W.M. Kemp, and W.P. Ball. 2011. Long-term trends in Chesapeake Bay seasonal hypoxia, stratification, and nutrient loading. Estuaries and Coasts 34: 1293–1309. https://doi.org/10.1007/s12237-011-9413-7.
Murrell, M.C., and J.C. Lehrter. 2011. Sediment and lower water column oxygen consumption in the seasonally hypoxic region of the Louisiana continental shelf. Estuaries and Coasts 34: 912–924. https://doi.org/10.1007/s12237-010-9351-9.
Nixon, S.W. 1988. Physical energy inputs and the comparative ecology of lake and marine ecosystems. Limnology and Oceanography. https://doi.org/10.4319/lo.1988.33.4part2.1005.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199–219.
Norkko, J., D.C. Reed, K. Timmermann, A. Norkko, B.G. Gustafsson, E. Bonsdorff, et al. 2012. A welcome can of worms? Hypoxia mitigation by an invasive species. Global Change Biology 18: 422–434.
Odum, E.P. 1971. Fundamentals of ecology, 3rd ed. Philadelphia: Saunders.
Parsons, T.R., Y. Maita, and C.M. Lalli. 1984. Determination of chlorophylls and total carotenoids: spectrophotometric method. In A Manual of Chemical & Biological Methods for Seawater Analysis, eds. Parsons, T.R., Y. Maita and C.M. Lalli, 101–104. Oxford: Pergamon Press. https://doi.org/10.1016/B978-0-08-030287-4.50032-3.
Pebesma, E.J. 2004. Multivariable geostatistics in S: The gstat package. Computers and Geosciences 30: 683–691. https://doi.org/10.1016/j.cageo.2004.03.012.
Petersen, J.K., J.W. Hansen, M.B. Laursen, P. Clausen, J. Carstensen, and D.J. Conley. 2008. Regime shift in a coastal marine ecosystem. Ecological Applications 18: 497–510.
Porter, E.T., R.P. Mason, and L.P. Sanford. 2010. Effect of tidal resuspension on benthic-pelagic coupling in an experimental ecosystem study. Marine Ecology Progress Series 413: 33–53. https://doi.org/10.3354/meps08709.
Pritchard, D.W. 1952. Salinity distribution and circulation in the Chesapeake estuarine system. Journal of Marine Research 11: 106–123.
Pritchard, D.W. 1960. Salt balance and exchange rate for Chincoteague Bay. Chesapeake Science 1: 48–57. https://doi.org/10.2307/1350536.
Pritchard, D.W. 1967. Observations of circulation in coastal plain estuaries. In Estuaries, ed. G.H. Lauff, 37–44. Washington, DC: American Association for the Advancement of Science.
Raffaelli, D., E. Bell, G. Weithoff, A. Matsumoto, J.J. Cruz-Motta, P. Kershaw, et al. 2003. The ups and downs of benthic ecology: Considerations of scale, heterogeneity and surveillance for benthic–pelagic coupling. Journal of Experimental Marine Biology and Ecology 285–286: 191–203.
Ratmaya, W., A.M. Laverman, C. Rabouille, Z. Akbarzadeh, F. Andrieux-Loyer, L. Barillé, et al. 2022. Temporal and spatial variations in benthic nitrogen cycling in a temperate macro-tidal coastal ecosystem: Observation and modeling. Continental Shelf Research 235: 104649.
Redfield, A.C. 1934. On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In James Johnstone Memorial Volume, ed. R.J. Daniel, 176–192. Liverpool: University Press.
Rysgaard, S., P.B. Christensen, and L.P. Nielsen. 1995. Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna. Marine Ecology Progress Series 126: 111–121.
Scavia, D., I. Bertani, J.M. Testa, A.J. Bever, J.D. Blomquist, M.A.M. Friedrichs, L.C. Linker, B.D. Michael, R.R. Murphy, and G.W. Shenk. 2021. Advancing estuarine ecological forecasts: Seasonal hypoxia in Chesapeake Bay. Ecological Applications 31: e02384.
Serpetti, N., U.F.M. Witte, and M.R. Heath. 2016. Statistical modeling of variability in sediment-water nutrient and oxygen fluxes. Frontiers in Earth Science. https://doi.org/10.3389/feart.2016.00065.
Shenk, G.W., J. Wu, and L.C. Linker. 2012. Enhanced HSPF model structure for Chesapeake Bay watershed simulation. Journal of Environmental Engineering 138: 949–957.
Stæhr, P.A., J.M. Testa, and J. Carstensen. 2017. Decadal changes in water quality and net productivity of a shallow Danish estuary following significant nutrient reductions. Estuaries and Coasts 40: 63–79.
Strickland, J.D.H., and T.R. Parsons. 1972. A practical handbook of seawater analysis. Second Edition. Ottawa: Fisheries Research Board of Canada, Bulletin 167.
Su, J., W.-J. Cai, J. Brodeur, N. Hussain, B. Chen, J.M. Testa, et al. 2020. Source partitioning of oxygen-consuming organic matter in the hypoxic zone of the Chesapeake Bay. Limnology and Oceanography 65: 1801–1817.
Sundbäck, K., A. Miles, and E. Göransson. 2000. Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: An annual study. Marine Ecology Progress Series 200: 59–76.
Sundby, B., C. Gobeil, N. Silverberg, and A. Mucci. 1992. The phosphorus cycle in coastal marine sediments. Limnology and Oceanography 37: 1129–1145. https://doi.org/10.4319/lo.1992.37.6.1129.
Taft, J.L., W.R. Taylor, E.O. Hartwig, and R. Loftus. 1980. Seasonal oxygen depletion in Chesapeake Bay. Estuaries 3: 242–247. https://doi.org/10.2307/1352079.
Taylor, D.I., C.A. Oviatt, and D.G. Borkman. 2011. Non-linear responses of a coastal aquatic ecosystem to large decreases in nutrient and organic loadings. Estuaries and Coasts 34: 745–757. https://doi.org/10.1007/s12237-010-9312-3.
Testa, J.M., D.C. Brady, D.M. Di Toro, W.R. Boynton, J.C. Cornwell, and W.M. Kemp. 2013. Sediment flux modeling: Simulating nitrogen, phosphorus, and silica cycles. Estuarine Coastal and Shelf Science 131: 245–263. https://doi.org/10.1016/j.ecss.2013.06.014.
Testa, J.M., J. Blomqvist, W.R. Boynton, P. Bukaveckas, K. Eshleman, L. Harris, R. Karrh, J. Keisman, M. Lane, V. Lyubchich, and R. Murphy. 2018. Progress toward the restoration of Chesapeake Bay in time and space. A white paper report to the USEPA Chesapeake Bay Program. Chesapeake Biological Laboratory Technical Series TS-716–18.
Testa, J.M., W.R. Boynton, C.L.S. Hodgkins, A.L. Moore, E.M. Bailey, and J. Rambo. 2022. Biogeochemical states, rates, and exchanges exhibit linear responses to large nutrient load reductions in a shallow, eutrophic urban estuary. Limnology and Oceanography 67 (4): 739–752.
Therneau, T., and B. Atkinson. 2019. rpart: recursive partitioning and regression trees. R package version 4.1–15. https://CRAN.R-project.org/package=rpart.
Tucker, J., A.E. Giblin, C.S. Hopkinson, S.W. Kelsey, and B.L. Howes. 2014. Response of benthic metabolism and nutrient cycling to reductions in wastewater loading to Boston Harbor, USA. Estuarine, Coastal and Shelf Science 151: 54–68. https://doi.org/10.1016/j.ecss.2014.09.018.
Valiela, I. 1995. Marine ecological processes, 3rd ed. NY: Springer.
Valiela, I., G. Collins, J. Kremer, K. Lajtha, M. Geist, B. Seely, J. Brawley, and C.H. Sham. 1997. Nitrogen loading from coastal watersheds to receiving estuaries: New method and application. Ecological Applications 7 (2): 358–380.
Vollenweider, R.A. 1976. Advances in defining critical loading levels for phosphorus in lake eutrophication. Memorie Dell’istituto Italiano Di Idrobiologia 33: 53–83.
Walve, J., M. Sandberg, U. Larsson, and C. Lännergren. 2018. A Baltic Sea estuary as a phosphorus source and sink after drastic load reduction: Seasonal and long-term mass balances for the Stockholm inner archipelago for 1968–2015. Biogeosciences 15: 3003–3025. https://doi.org/10.5194/bg-15-3003-2018.
Wang, H. 2020. A numerical investigation of variability in particulate organic matter transport and fate, phytoplankton and primary production, and denitrification in a partially mixed estuary. PhD Dissertation, University of Maryland.
Webster, R., and M.A. Oliver. 2007. Geostatistics for environmental scientists. Hoboken: Wiley and Sons.
Wood, S.N. 2017. Generalized additive models: An introduction with R, 2nd ed. New York: CRC Press.
Acknowledgements
Review and synthesis work is not possible without the assistance of many colleagues, institutions, and agencies. We acknowledge the financial and staff support from the Maryland Department of Environment, the Maryland Department of Natural Resources, the EPA Chesapeake Bay Program, US Army Corp of Engineers, Washington, DC Council of Governments, the City of Baltimore, MD, the National Science Foundation, and the National Oceanographic and Atmospheric Administration. All of these agencies supported sediment flux investigations in Chesapeake Bay and the Maryland Coastal Bays for long or short periods of time between 1977 and 2018. Much of this work would not have been possible without the often intensive use of the UMCES research vessels RV Orion, RV Aquarius, RV Venus, and RV Rachel Carson and, in particular, the RV captains and crews who operated these vessels with remarkable skill including M. O’Berry, W. C. Keefe, B. Elder, R. Younger, J. Crane, M. J. Reusing, B. Cornwall, M. Hulme, and R. Nilsen. There were also a skilled group of Chesapeake Biological Laboratory (CBL) staff who developed techniques to make these measurements in the context of both research and monitoring programs and made all this equipment work in fair and foul weather. C. Osborne, K. Kaumeyer, L. Lubbers, J. Barnes, J. Garber, R. Stankelis, J. Frank, P. Smail, and E. M. Bailey all played leadership roles during portions of this four decade effort. We thank Qian Zhang for providing watershed loading estimates. Finally, we had the pleasure of working with the CBL Nutrient Analytical Services Laboratory for virtually all chemical analyses required in this work.
Funding
This synthesis manuscript effort was supported by grants or contracts from the National Science Foundation (1353766 DEB-1353766; 1360395 CBET-1360395), the EPA-Chesapeake Bay Program Office (075–239030), and the Chesapeake Biological Laboratory (07–2-93513). This is UMCES Publication number 6221 and CBL Reference Number UMCES CBL 2023-020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Charles Simenstad
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boynton, W.R., Ceballos, M.A.C., Hodgkins, C.L.S. et al. Large-Scale Spatial and Temporal Patterns and Importance of Sediment–Water Oxygen and Nutrient Fluxes in the Chesapeake Bay Region. Estuaries and Coasts 46, 356–375 (2023). https://doi.org/10.1007/s12237-022-01127-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01127-0