Abstract
Understanding the range of habitats needed to complete life-cycles is essential for the effective conservation and management of species. We combined otolith microchemistry, acoustic tracking, and underwater video to determine patterns of seascape use by an assemblage of tropical snappers, including two little-known species of high economic importance, the Papuan black bass (Lutjanus goldiei) and spot-tail snapper (Lutjanus fuscescens). All species appeared to have marine larval phases, and post-settlement distributions broadly overlapped across the coastal seascape. However, species and life stages were distributed along a gradient from freshwater to coastal waters. Lutjanus fuscescens is primarily a freshwater species post-settlement, but larger individuals move into brackish estuaries and even coastal waters at times. Lutjanus goldiei appear to recruit to low salinity or freshwater areas. Larger individuals tend to have home-ranges centred on brackish estuaries, while making regular movements into both coastal waters and freshwater. Lutjanus argentimaculatus also ranged widely from fresh to coastal waters, but juveniles were most common in the saline parts of estuaries. Ontogenetic shifts by L. argentimaculatus were similar to those reported from other regions, despite vast differences in the spatial proximity of seascape components. The wide-ranging seascape movements of our target species highlight the importance of maintaining effective connectivity between marine, estuarine, and freshwaters in the region to maintain ecosystem function and support sustainable sport fisheries. The combined approaches resolved some of the ambiguities of individual methods and provide a powerful approach to understanding seascape use by coastal fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many aquatic species use a wide range of habitats to complete their lifecycles (Beck et al. 2001). Ontogenetic habitat shifts may help to maximise fitness by allowing individuals to access resources and conditions optimal for their growth and survival (Dahlgren and Eggleston 2000; Snover 2008), and may involve extensive movements among seascape components or between ecosystems (Nagelkerken et al. 2000; Russell and McDougall 2005). Where multiple functionally similar species coexist within a seascape, coexistence may be facilitated by niche partitioning whereby different species or life-stages differentially use habitat mosaics, food, and other resources, thereby minimising competitive interactions despite overlapping distributions (Matich et al. 2017). A clear understanding of species movement patterns is essential for effective conservation and management (Crook et al. 2017).
Tropical snappers (Lutjanidae) are a circumglobal group of fishes that support important commercial, recreational, and subsistence fisheries throughout their range. Many lutjanids make substantial ontogenetic shifts through coastal, inshore, and offshore seascapes during their lives (Sheaves 1995; Nagelkerken et al. 2000; Russell and McDougall 2005; Nakamura et al. 2008; Mateo et al. 2010). Through these movements, individuals make use of a broad range of habitats and resources (Dorenbosch et al. 2004; Luo et al. 2009; Hammerschlag-Peyer and Layman 2010; Nagelkerken et al. 2015), act as vectors of nutrient transport among ecosystems (Sheaves and Molony 2000), and link systems through their functional roles in each of the ecosystems they occupy (Sheaves et al. 2015). Adult lutjanids are mostly coral reef-associated (Allen 1985), while juvenile life stages of different species occupy a variety of ecosystems spanning from coastal freshwater streams (Russell and McDougall 2005), through estuarine and coastal mangroves and seagrass (Sheaves 1995; Cocheret de la Moriniere et al. 2002), to inshore and offshore marine habitats (Allen 1985).
The Papuan black bass Lutjanus goldiei and spot-tail snapper Lutjanus fuscescens co-occur along the northern New Guinea coastline and the islands of New Britain and New Ireland in Papua New Guinea (PNG) (Allen 1985, 2004). Although almost nothing is known of their biology or ecology (Sheaves et al. 2016; Froese and Pauly 2017), these two large tropical snappers appear to represent the extreme of freshwater utilisation among the Lutjanidae (Allen 1985). Sportfishers targeting these species encounter individuals of > 20 kg in freshwater rivers (R. Reimann pers. comm). Until recently, L. goldiei was considered to be almost exclusively a freshwater fish (Allen 2004), but a review by Sheaves et al. (2016) confirmed small juveniles to large adults also occur in the brackish and saline parts of estuaries. There are also anecdotal reports of L. goldiei in coastal marine waters. Less is known about L. fuscescens (Froese and Pauly 2017). Guides and anglers report that it is the dominant species in the faster-flowing freshwater reaches of coastal streams and rivers and is rarely captured in brackish or saline waters. The extent of movement of individuals among components of the coastal seascape during their lives, the location of juvenile nursery habitats, adult spawning sites, and critical connectivities remain unknown.
Lutjanus goldiei and L. fuscescens, together with co-occurring snappers mangrove jack L. argentimaculatus and fingermark L. johnii, form the basis of a small but growing and potentially highly valuable sport fishing industry in PNG (Wood et al. 2013; Sheaves et al. 2016). Understanding key life-history movements required to complete their life-cycle is critical for their management (Barnett et al. 2016); however, seascape and ontogenetic movements of highly mobile organisms create a complex web of utilizations that can be difficult to resolve. Combining multiple sampling techniques that elucidate different aspects of movement and habitat occupancy offers opportunities to describe patterns of seascape use, advance our understanding of the ecological drivers, and identify essential habitats and connectivities that sustain populations (Gillanders et al. 2003; Sheaves et al. 2015). The aim of this study was to determine patterns of seascape use by the four co-occurring snappers, with a focus on L. goldiei and L. fuscescens which are the main targets of the fishery, and about which very little is known. We were particularly interested in defining patterns of movement across the freshwater-marine gradient at various life-cycle stages, and combined otolith microchemistry, acoustic telemetry, and underwater video census to describe these patterns.
Study Sites
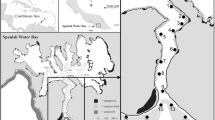
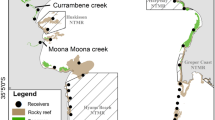
Fishes were collected from the rivers and coastal waters in the Pandi River-Open Bay region of West New Britain Province, PNG (Fig. 1). Coral reefs fringe parts of the coastline, with seagrass (mainly Enhalus acoroides) forming meadows on protected reef flats. Rhizophora spp. mangroves fringe sheltered coasts. The seafloor drops away rapidly offshore from the fringing coral reefs, reaching depths of over 500 m within 2 km of the coast (www.gpsnauticalcharts.com). Tides are diurnal with a range of 1 m. Annual rainfall for Open Bay is around 3700 mm (www.Climate-Data.org, 2017), with a distinct wet season from December to March.
a Map of coastal seascape study sites in the Pandi-Open Bay region of West New Britain, Papua New Guinea, and location of acoustic receiver arrays at b the Langa Langa-Pandi and c Toriu rivers. To visually interpret movements of acoustically tagged fish (Fig. 5), receivers were grouped to represent seascape zones, and the groupings are indicated
Specimens were collected from the Pandi, Langa Langa, Palè, Sei, Tagio, and Toriu Rivers, as well as from coastal coral reefs and fringing mangroves (Fig. 1). The Pandi is a larger (150 m wide at mouth, ≤ 5 m deep), fast-flowing river with scattered mangroves (mainly Sonneratia spp.) extending 1 km upstream from the mouth and interspersed among terrestrial forest vegetation. Salinity profiling revealed minimal salt intrusion at any time during the study period. Surface waters at the mouth were fresh on each sampling occasion, while bottom salinity ranged from 0 to 20 PSU. The maximum distance upstream where salinity > 0 was recorded (4 PSU) was 0.8 km upstream from the mouth during a low rainfall period in the mid dry season (July 2015).
The Langa Langa is a low-flow mangrove complex dominated by Nypa fruticans with some Rhizophora spp., Bruguiera gymnorhiza, and Sonneratia spp. It has inputs of freshwater primarily through the eastern arm of the system, and through a small connecting channel to the Pandi River to the west (Fig. 1). Bottom salinities were typically above 10 PSU throughout the navigable length of the system, while surface salinity at the mouth ranged from 6 to 35 PSU. The Sei and Toriu Rivers are intermediate in physical conditions between the fast-flowing Pandi and the mangrove swamp of the Langa Langa, having freshwater inputs throughout the year and brackish estuaries extending ~ 4 km upstream in each system. The estuarine reaches have patches of fringing mangrove (N. fruticans, Avicennia spp. and Sonneratia spp.) and some broader mangrove forests in the lower 2 km. The Tagio is a short (< 1 km navigable length) Rhizophora-lined creek with minimal freshwater inflow except during wet-season rains, while the Palè is a fast-flowing stream that flows fresh to the mouth during normal flow conditions.
Methods
Otolith microchemical analysis (OMA), acoustic telemetry, and underwater video census (UVC) were combined to investigate patterns of seascape use. Each technique has strengths and limitations, and when combined, the three approaches provide complementary data to describe patterns of seascape use. OMA provides a continuous history of environmental conditions experienced throughout the life of an individual, but seascape movements can be difficult to distinguish from changing conditions around a stationary fish (Walther and Limburg 2012). Acoustic telemetry precisely tracks the seascape movements of individually tagged fish, but only within the spatial limits of the receiver array and the temporal limits of the tag battery life. UVC provides a snapshot of the distribution patterns at the population level, but spatial patterns in the distribution of different life-stages do not necessarily equate to ontogenetic movements of individuals (Gillanders et al. 2003). By combining the three approaches, we were able to overcome many of the limitations of interpreting results from individual approaches alone.
OMA focused on strontium and barium, as these elements typically provide the greatest resolution to detect movements across the freshwater-estuarine-marine seascape where sharp gradients in salinity and sediment load occur (Gillanders 2005; Elsdon et al. 2008; Walther and Limburg 2012). To identify life-history movements among different salinity zones in the coastal seascape, we compared otolith microchemical profiles of the four target snapper species to those obtained from locally sampled reef-resident and freshwater-resident reference species (Table 1). Concurrent UVC and acoustic tagging data provided complementary information on the distribution and movement patterns of the target species across the coastal seascape.
Fish Collections
Specimens were collected over six sampling trips between May 2013 and November 2015. A total of 54 Lutjanus fuscescens, 57 L. goldiei, 44 L. argentimaculatus, and 7 L. johnii (the four target species) were sampled for OMA (Table 1). Most of these were sampled from the estuaries and lower freshwater reaches (< 8 km from mouth) of rivers around Baia (Fig. 1, Online Resource 1). Additionally, five L. argentimaculatus were collected from the fringing mangroves along the sheltered coast near Baia Village, and two from coastal reefs adjacent to the village (Fig. 1). Two L. goldiei were collected from the mouth of the Barema River, about 60 km SW of Baia. Marine reference fish were collected from fringing and inshore coral reefs around Baia Village. All fish were caught by hook and line or cast net.
Otolith Preparation and Analysis
Both sagittal otoliths were removed from the fish and one randomly selected for sectioning. Otoliths were sectioned transversely through the core by either grinding on a Gemmasta Faceting Machine (Model GF4) using 1500 and 3000 grit grinding discs (small otoliths) or embedding in epoxy resin and cutting with a Buehler Isomet low speed saw (larger otoliths) before mounting and polishing the sawn sections as per small otoliths. Fish were aged by counting apparent annuli in the sectioned otoliths. Although the formation of annuli has not been confirmed for L. goldiei or L. fuscescens (Baker et al. 2018), annuli have been verified as the dominant visible increments for many other lutjanids (Cappo et al. 2000; Piddocke et al. 2015), including at least seven species considered here (Sheaves 1995; Cappo et al. 2000). We therefore assumed the similar growth increments in non-validated species to represent annuli (Choat et al. 2009; Piddocke et al. 2015; Baker et al. 2018). Ageing was undertaken to indicate the approximate timing of any substantial life-history seascape movements indicated by OMA profiles. The increment widths along the laser ablation transect were measured for a subset of individuals (13 L. fuscescens and 21 L. goldiei) to provide a more detailed chronological history of movements through the freshwater-marine seascape.
Sections were mounted on slides using crystal bond and sonicated in milli-Q water for 5 min before laser ablation. Microchemical analysis was conducted via LA-ICPMS at JCU’s Advanced Analytical Centre using a MICRO-LAS laser coupled to a Varian 820-MS mass spectrometer with He as a carrier gas. Prior to the analytical pass, a cleaning pass of the laser was performed to remove any remaining contaminants from the surface of the sectioned otoliths. The cleaning pass used the laser at 1 Hz with a 40-μm ablation spot size and a scan speed of 40 μm/s. The analytical pass was conducted at 10 Hz with a 32-μm ablation spot size and scan speed of 32 μm/s. NIST612 was used as a standard, and was ablated every 20 samples, and/or every 3 h. The ablation transects were analysed from core to edge for the May 2013 samples, and from edge-to-edge for all subsequent samples, aiming to pass through the inner-most visible growth ring exposed during sectioning (i.e., the presumed core of each section).
Otolith Microchemistry to Describe Seascape Movements
We used microchemical profiles from marine-resident and freshwater-resident reference fish to test that the expected differences in Sr:Ca ratios between marine and freshwaters were indeed reflected in OMA profiles of fish occupying these waters (Walther & Limburg 2012). A total of 12 individuals from eight species of reef-resident lutjanids and seven individuals from two species of freshwater-resident fish were analysed to define microchemical profiles expected in fish occupying marine and freshwater areas (Table 1). Although both freshwater reference species are diadromous (Allen 1985; Oka and Tachihara 2008), each was sampled from upstream freshwater areas where they were likely to have been resident for long enough that the outer stable portion of the microchemical profiles should reflect freshwater residence. Since our reef-resident fish complete their lifecycles in marine waters (Allen 1985), we considered their entire Sr:Ca profiles to reflect residence in saline marine environments. Using multiple species of reference fish helps to account for any species-specific differences in elemental uptake rates and discrimination coefficients between our reference and target species (Walther and Limburg 2012).
We defined endmembers reflecting the occupation of freshwater as Sr:Ca values below the 95th percentile of all Sr:Ca values from the pooled freshwater reference profiles, while the occupation of saline waters was defined by Sr:Ca values above the 5th percentile of all Sr:Ca values from marine reference fish. Mixing curves of Sr:Ca versus salinity indicate that the greatest change in Sr:Ca ratios typically occurs between 0 and 10–15 PSU (Brown and Severin 2009). As such, our definitions of freshwater and saline residents were interpreted to reflect the occupation of salinities below 0 and above 15 PSU, respectively. Intermediate values could reflect either outliers from fish occupying fresh or saline waters, or fish occupying estuarine areas of intermediate salinity (> 0–15 PSU).
Fish from the Toriu River tended to show ambiguous Sr:Ca profiles regardless of their location of capture. Detailed investigations of the spatio-temporal patterns in water chemistry across our study seascape could provide better resolution for defining transitions across salinity gradients in each of our study rivers (Elsdon et al. 2008), but such sampling was beyond the scope of the present study. Where atypical water chemistry produces ambiguous Sr:Ca profiles, and detailed water chemistry data are lacking, Sr:Ba profiles may provide better resolution to detect transitions from marine to freshwaters (McCulloch et al. 2005; Hamer et al. 2015). Hence, we also examined Sr:Ba profiles to classify movement patterns for fish with ambiguous Sr:Ca profiles.
It is conventional to interpret element-to-Ca ratios with Ca serving as an internal standard to compensate for any variation in yield during LA-ICPMS that may bias or confound the elemental profiles (Campana 1999). Therefore, before interpreting Sr:Ba profiles, the slope of raw Ca profiles were tested for deviation from zero that would indicate systematic variation in yield. Eight of the 178 profiles examined had a significant slope, and these trends were considered when interpreting Sr:Ba profiles from these fish. We calculated endmember values reflecting residence in marine and freshwater based on Sr:Ba profiles of our reference fish following the methods described above for Sr:Ca. Because both freshwater and reef resident fish showed variable Ba profiles, we considered only the outer stable portions of the Sr:Ba profiles as reflecting residence in each respective environment.
For each target fish, we plotted Sr:Ca, Ba:Ca, and Sr:Ba profiles to identify major transitions through the coastal seascape during their lifecycle. In addition, Mn:Ca profiles were examined to identify if the larval core was bisected by the laser, since Mn tends to peak in the core of fish otoliths independently of ambient concentrations (Brophy et al. 2004; Ruttenberg et al. 2005). Sr:Ca and Sr:Ba profiles were overlayed with the boundaries defining saline and freshwater residence derived from the reference fish profiles, and each fish was allocated into one of five categories based on these profiles (excluding the larval core). The Sr:Ca profiles were examined first, and where these were ambiguous, the Sr:Ba profiles were used to assist in categorising those fish. The categories were as follows: Freshwater Resident—profiles that entirely overlap with those of the freshwater reference fish; Freshwater-Brackish Transient—profiles that span both freshwater endmember values and values intermediate between freshwater and marine endmembers, providing evidence of transition between freshwater and brackish waters, or occupation of intermediate waters which could indicate residence in areas of fluctuating but low salinity; Seascape Migrant—transition between and occupation of different seascape components indicated by stepped profiles, or profiles ranging from FW to saline extremes; Saline-Brackish Transient—evidence of transition between brackish and saline waters, or occupation or intermediate waters that could reflect residence in a single area of fluctuating but high salinity; Saline Resident—profiles overlapping those of the reef-resident reference fish.
Based on the salinity profiles, the distribution of mangroves, and the geomorphological nature of each river, we considered a saline signature to reflect the occupation of coastal waters, the mouth of the Toriu or Sei Rivers, or throughout the lower parts of the Tagio or Langa Langa, intermediate values to reflect occupation of the brackish mixing zones indicated in Fig. 1, and freshwater values to indicate occupation of the freshwater reaches above the brackish mixing zones. Although the boundaries of these zones shift through time based on rainfall, river flows, and tides, our aims were to describe broad movements between freshwater, brackish, and marine environments, not to pinpoint the past locations of individual fish.
Underwater Video Census
Underwater video census (UVC) was conducted across a range of freshwater, estuarine, and coastal habitats, on three trips between September 2015 and November 2016. Surveys utilised Oregon Scientific ATK HD video cameras mounted on weighted bases and deployed for a minimum of 15 min, to collect replicate 15-min point census of fish presence in each habitat, as per the methods of Bradley et al. (2017). UVC targeted structured habitats including rock, coral, macroalgae, seagrass, mangrove edge, submerged riparian vegetation, and woody debris, since the vast majority of lutjanid occurrences recorded by UVC are in structured habitats (Bradley unpubl. data). A total of 412 replicate UVC samples were collected across these habitats in fresh (n = 86), estuarine (n = 169), and coastal (n = 157) waters. Each riverine video replicate was defined as being freshwater or estuarine based on surface and bottom salinity readings at each sampling site. If either reading showed salinity > 0, the replicate was considered estuarine. Coastal samples were those collected from coastal waters outside rivers. Detailed analyses of the fish-habitat relationships observed in the UVC survey are to be published separately. For the present study, we used UVC to examine patterns of distribution across the coastal seascape of the target species included in the otolith microchemical analyses. Based on colour patterns, we were able to distinguish early juvenile from late juvenile/adult phases for each species, and the distribution patterns for these life stages were examined separately.
Acoustic Tracking
Acoustic receivers (Vemco VR2w) were deployed in the Pandi, Langa Langa, and Toriu Rivers (Fig. 1) from June to November 2015. The in-river receiver arrays were removed for the wet season (Dec–Apr) to avoid receiver loss during wet season flows which typically involve water levels rising by several metres, very fast flows, and extensive debris including whole large trees. A total of 23 receivers were deployed across the three rivers: four in the Pandi, 12 in the Langa Langa, and seven in the Toriu (Fig. 1b, c). In each system, we focused receivers into the estuarine and lower freshwaters from the river mouth to a maximum of 9 km upstream. Range-testing indicated reliable detection of transmitters 200 m from receivers within the rivers. Since the widest section in these rivers did not exceed 170 m (and mostly much narrower), individual receivers effectively gated sections of the river. In each system, the upstream limit of the array and the distribution and number of receivers within the array was limited by water depth (needing at least 2.5 m for secure deployment) and river flow rate.
An additional seven receivers were deployed in coastal waters outside the river mouths to detect any fish moving outside of the rivers (Fig. 1). In July 2015, three receivers were deployed ca. 200–400 m offshore from the mouth of the Langa Langa, and two receivers 200–500 m offshore from the mouth of the Pandi River (Fig. 1b), and two receivers were deployed on coral reefs ca. 1 km in front of the mouth of the Toriu River in October 2015 (Fig. 1c). Coastal receivers were left in place through the wet season. Each of these rivers has extensive shallow sand banks in front of the mouth, meaning any detections on the coastal receivers could not be from a fish remaining inside the rivers, and would reflect a tagged fish having moved across these banks into coastal waters.
In June–July 2015, 21 L. goldiei and five L. fuscescens were tagged in the Pandi/Langa Langa, and 12 L. goldiei and 11 L. fuscescens in the Toriu. Fish were captured with rod and reel and immediately placed into an aerated holding tank with diluted anaesthetic (Aquis ms-222). Fish were held until loss of equilibrium indicated effective anaesthesia, about 5 min. Once anaesthetised, acoustic transmitters were surgically implanted into the body cavity, and this procedure lasted < 5 min during which time ambient water was continuously flushed across the gills. After surgery, fish were transferred to a recovery pen attached to the side of the boat and allowed to regain equilibrium and rest for a few minutes before release. Four L. goldiei were tagged with Vemco V13AP Accelerometer Pressure tags, with delays between 80 and 120 s and ca. 7 months battery life. The rest of the fish were tagged with V13 low power tags with delays of 50–110 s and ca. 3.3-year battery life. V13AP tags transmit 3D acceleration and depth data as well as tag identity. Detailed analyses of the acoustic tracking data are being undertaken for publication separately. For the current study, we restricted our analyses to describe general seascape movements of tagged individuals to complement OMA and UVC data on movement and distribution.
To visually interpret movement patterns within the array, receivers were grouped based on their distribution and our salinity profiling data to represent relevant zones of the coastal-estuarine-freshwater seascape, and we plotted the days each fish was detected at each receiver group on a timeline. The four Pandi receivers (P1–P4) were considered individually with P1 located inside the river mouth through to P3 in the junction of the Langa-Langa connector channel 1 km upstream, and P4 a further 3.5 km upstream (Fig. 1b). The Langa Langa receivers were grouped into east- and west-branch receivers, with the west-branch including nine receivers through the brackish/saline mangrove portion of the system between the mouth and the Pandi River, and three receivers up the eastern arm of the system (Fig. 1b). In the Toriu, the three receivers in the lower reach were grouped to represent the lower estuary, two receivers were grouped as mid-estuary, the receiver at a rockbar at the top of the estuary represented the upstream boundary of brackish water, and a receiver was located in the only accessible deep pool in the freshwater portion of the river, 9 km upstream from the mouth, and 4 km above the rockbar at the top of the estuary (Fig. 1c).
Results
Collections of Fish for LA-ICPMS
Lutjanus fuscescens were captured mainly in freshwater reaches of the coastal rivers, but some individuals were captured in the upstream parts of the brackish mixing zones. Lutjanus goldiei were captured primarily in the brackish estuarine reaches of each river, although some individuals were captured in freshwater. No individuals of either species were captured in coastal waters. Lutjanus johnii were captured in the lower part of the Toriu River estuary, while L. argentimaculatus were captured at sites ranging from freshwater to coastal fringing mangroves and reefs, although most were captured in the lower parts of estuaries.
Location of Larval Phases
Where the larval core was represented in the OMA transect, the chemical profiles were consistent with a marine larval phase for all individuals analysed. Cores were characterised by elevated Sr:Ca and very low Ba:Ca, comparable to the marine reference fish values, and for otoliths ablated edge to edge, followed by sharp but “symmetrical” variation along the rest of the transect (Fig. 2, Online Resource 3). The larval core was considered to be represented in the OMA profiles (40% of otoliths analysed) when post-ablation examination of the sectioned otolith revealed the laser track had bisected the inner-most visible growth ring (the putative larval core), and the Mn:Ca profile showed a distinct peak at the position along the transect corresponding to the putative core (Fig. 2d; Online Resource 3).
Example of otolith microchemical profiles from otolith where the laser ablation transect bisected the larval core. X-axis represents distance along the transect which ran from edge to edge through the otolith core (indicated by arrow). The fish was a 455-mm TL, 12-year-old spot-tail snapper (Lutjanus fuscescens) collected from the Pandi River. Elevated Mn:Ca (2d) is characteristic of the larval core of many marine fishes (Ruttenberg et al. 2005). Sr:Ca in the core matching marine reference values (a), and very low Ba:Ca (c) (and therefore high Sr:Ba—b) are consistent with a marine larval phase. Horizontal dotted lines indicate boundaries of values of marine and freshwater reference fish as defined in Online Resource 2
Seascape Movements Based on OMA
As expected, Sr:Ca values were considerably higher during all life stages in marine reference fish (5th percentile = 3.54 mmol/mol) than in freshwater fish (95th percentile = 1.81 mmol/mol), and any species-specific variation among multiple reference species within an environment was small relative to the large and distinct differences in signatures between freshwater and marine environments (Online Resource 2). Similarly, Sr:Ba values were well separated between freshwater and reef resident fish (Sr:Ba 95th percentile FW resident = 97; 5th reef resident = 305; Online Resource 2), providing good resolution to define seascape transitions when Sr:Ca results were ambiguous (i.e., fish collected from the Toriu River).
The otolith chemistry profiles revealed a gradient in post-settlement seascape use by the co-occurring riverine snappers (Table 1, Online Resource 3), that was broadly consistent with the distribution of fish captures during sampling described above. Almost half of the L. fuscescens appeared to be freshwater residents (Fig. 3a) since recruiting to the rivers, a quarter showed evidence of having been resident in the upper brackish reaches of the estuaries (Freshwater-Brackish Transients, Fig. 3b), while another quarter showed wider-ranging movements along rivers during their lives, thereby classifying them as seascape migrants (Fig. 3c, Table 1, Online Resource 3). Seascape migrants showed consistent life-history movement patterns; individuals apparently spent most of their time in fresh or low-salinity brackish waters, with no individuals showing profiles consistent with any periods of extended occupation of saline waters (Table 1). Following extended occupation of fresh/low salinity waters, all individuals moved downstream into more saline waters (Online Resource 3). For the ten seascape migrant L. fuscescens with otolith increments overlayed onto the microchemical profiles, this downstream movement from fresh to more saline waters occurred at between 3 and 10 years of age (Online Resource 3). The average age of the 38 freshwater resident and freshwater-brackish transients was 7 years (range 0+ to 12 yr), while the 14 seascape migrants tended to be older fish, with an average age of 10 years (6–14 yr).
Representative examples of otolith microchemical profiles from fish classified into each of the five life-cycle seascape movement categories: a Freshwater Residents; b Freshwater-Brackish Transients; c Seascape Migrants; d Saline-Brackish Transients; e Saline Residents. Categorizations for these fish were based primarily on the Sr:Ca values (left column), while Sr:Ba profiles (right column) were used to assist categorizations when Sr:Ca profiles were ambiguous. All categorizations were based on the portion of the profile excluding the larval core, which is identified by the arrow in each panel. X-axes represent distance along the laser transect, which ran from core to edge for May 2013 fish, and from edge to edge for all others. Horizontal dotted lines indicate the boundaries of values obtained from marine (upper line) and freshwater (lower line) reference fish as defined in Online Resource 2. Values above the upper line were interpreted as the occupation of saline waters, below the lower line indicate occupation of freshwaters, and intermediate values indicate brackish water occupation
Two-thirds of the L. goldiei were classified as seascape migrants, based on apparent wide-ranging movements across the seascape during their lives (Table 1). Another 30% were classified as saline-brackish transients (e.g., Fig. 3d). The seascape migrants spent most of their time in brackish waters, and while no individuals appeared to have spent their entire lives post-settlement in either fresh or fully saline waters (i.e., none classified as either freshwater or saline residents), some of seascape migrants spent at least part of their lives occupying these areas. Only four individuals (7%) were classified as freshwater-brackish transients, so these results must be interpreted with caution, but these were younger fish (average age 6 year), while the seascape migrants and saline-brackish transients tended to be older (av. 9 year).
Lutjanus argentimaculatus showed the broadest seascape use based on their microchemical profiles, with almost 2/3 of individuals occupying brackish to saline waters (saline-brackish transients and saline residents, e.g., Fig. 3e), and another 1/3 ranging more widely across the seascape (seascape migrants, Table 1). Among the seascape migrants, fish tended to occupy more saline waters, but at least some individuals resided in freshwater for parts of their lives. The two fish classified as freshwater-brackish transients were both young (2 and 4 year), the 14 seascape migrants had an average age of 10 year (6–17 year), while the saline-brackish transients and saline residents fish averaged 6 years old (1–12). Lutjanus argentimaculatus was the only target species captured outside of rivers in coastal waters, with five collected from coastal fringing mangroves, and two from adjacent coral reefs. One 4-year-old individual collected from coastal fringing mangroves was classified as a freshwater-brackish transient based on profiles reflecting residence in low salinity waters (Online Resource 3), suggesting an atypical OMA profile or recent migration to coastal waters from lower-salinity estuarine areas. The other six fish collected from coastal waters were all classified as seascape migrants, and all showed profiles consistent with having occupied fresh-brackish waters earlier in their lives before making distinct transitions to marine saline waters (Online Resource 3).
Distribution: Underwater Video Census
The distribution patterns across the coastal seascape observed in the underwater video census (Fig. 4) were broadly consistent with the distributions indicated by otolith chemistry profiles and fish collections. Lutjanus fuscescens were widespread in freshwater areas, and were observed exclusively in freshwater (Fig. 4a). Early juveniles were observed in 19 of the 86 freshwater videos, and late juveniles/adults in 21 videos. Consistent with the microchemistry profiles, L. goldiei showed a distribution intermediate between L. fuscescens and L. argentimaculatus, being present in both fresh and estuarine waters (Fig. 4b). Lutjanus argentimaculatus were more widespread, being observed from freshwaters to coastal fringing mangroves (Fig. 4c). As for L. goldiei, there is evidence of downstream ontogenetic movements when the early juvenile and late juvenile/adult distributions are compared (Fig. 4). No L. johnii were observed during the UVC surveys.
Distribution of target species, aLutjanus fuscescens; bL. goldiei; cL. argentimaculatus, observed in during underwatwer video census of structured/complex habitats in rivers and coastal waters of the Pandi-Open Bay region, West New Britain, Papua New Guinea. Data represent the proportion of videos from each zone (x-axis) in which the species/life stage was observed. Sample size for each sampling zone is provided in parenthesis on x-axis. Total number of replicate videos in which the species/life stage was observed is in parenthesis in each figure caption, (n = early juveniles, late juveniles/adults). Early juveniles were distinguished from late juveniles and adults based on coloration. Total sample size is 412 replicate 15-min video drops
Movement Patterns of Acoustically Tagged Fish
All 49 fish surgically implanted with acoustic transmitters in June–July 2015 were detected repeatedly during the subsequent 4-month season. Tagged L. goldiei and L. fuscescens showed distributions and movements spanning the full spatial scale of movements inferred from the OMA data (Fig. 5, Online Resource 4). Lutjanus fuscescens spent most of their time in freshwaters or the upper parts of estuaries, while L. goldiei mainly occupied the lower parts of estuaries (Online Resource 4). Importantly, tracking revealed that individuals of both species made extensive movements across the seascape from outside the river mouths to the uppermost freshwater receivers in each river (Fig. 5, Online Resource 4), confirming the scale of seascape movement inferred from OMA was undertaken by individual tagged fish.
Representative timelines of detections of acoustically tagged fish: aLutjanus goldiei in the Toriu River; bL. fuscescens in the Toriu River; cL. goldiei in the Pandi-Langa Langa Rivers; dL. fuscescens in the Pandi-Langa Langa Rivers, West New Britain, Papua New Guinea. Detections indicate at least one detection of the tagged individual on the relevant receivers during each day. Receivers were grouped based on their distribution and our salinity profiling data, to represent relevant zones of the coastal-estuarine-freshwater seascape. The location of Individual receivers and the groupings that represent seascape zones are shown in Fig. 1b, c
The Pandi and Langa Langa systems are connected by a small channel around 1 km upstream from the mouth of the Pandi River (Fig. 1). Our acoustic data revealed regular movements of fish between these rivers through the channel (Online Resource 4b), and so the two rivers are treated as one system. Eleven of the 21 L. goldiei tagged in the Langa Langa-Pandi (LL-P) moved from the mouth to the upstream end of the array within the Pandi, or between the freshwater of the Pandi and the brackish/saline waters of the Langa Langa through the small connecting creek, equivalent to movements of seascape migrants (Fig. 5c, Online Resource 4b). Eight of the L. goldiei were only detected within the Langa Langa, and two were only detected in the lower reach of the Pandi between the mouth and the Langa Langa connector creek. One of the 12 L. goldiei tagged in the Toriu was detected only by receivers within the estuary, while the other 11 moved between the lower estuary and the uppermost receiver in freshwater 4 km above the top of the estuary and 9 km upstream from the mouth (Fig. 5a, Online Resource 4a).
During July–Nov 2015, ten of the 11 L. fuscescens tagged in the Toriu were detected no further downstream than the rockbar at the top of the estuary (Online Resource 4c). The other fish spent most of its time between the mid estuary and freshwater, but was also detected in the lower estuary (Fig. 5b). Four of the five L. fuscescens tagged in the Pandi moved between the most downstream and upstream receivers (Fig. 5d), while the 5th fish was detected only on the 3 upstream receivers (Online Resource 4d).
Sixteen of the 21 L. goldiei tagged in the LL-P were detected by the coastal receivers in front of those rivers, and many of those fish made regular (ca. monthly) movements into coastal waters between July 2015 and September 2016 (Online Resource 4e). Similarly, five L. goldiei and two L. fuscescens tagged in the Toriu were detected by the receivers at the coral reefs 1 km offshore from the mouth, between January and July 2016 (Online Resource 4f). The L. goldiei made regular movements, with individuals being detected on two to five separate occasions at about monthly intervals around the new moon. The two L. fuscescens detected had both remained at or above the rockbar during the previous river season (i.e., prior to Nov 2015 when the river receivers were removed), while the five L. goldiei had all made extensive in-river movements between the lower estuary and upper freshwater during the previous season.
Discussion
Lifecycle and Seascape Movement Patterns
The environment occupied by larval L. goldiei and L. fuscescens was previously unknown (Sheaves et al. 2016). Our OMA profiles indicate that both species have marine larval phases, similar to all other known members of the Lutjanidae (Allen 1985). The microchemical profiles from otoliths where the core was successfully ablated showed consistently low Ba:Ca values through the core, and elevated Sr:Ca values equivalent to those of the marine-resident reference fish, consistent with marine larval phases (Elsdon and Gillanders 2003; Gillanders 2005). Although some of the chemical signal recorded in the otolith primordium can result from maternal transfer (Thorrold et al. 2006), any such transfer would act to mask rather than enhance the apparent offshore marine signals observed in the cores of our OMA profiles. This is because our combined approaches indicate that adults of L. goldiei and L. fuscescens are largely riverine, and the movements into coastal waters recorded in acoustically tracked fish are too brief to equilibrate otoliths with ambient conditions (Walther and Limburg 2012). Therefore, any maternal transfer would serve to impart a riverine signature to the primordium of larval otoliths.
The combined approaches of otolith microchemical analysis (OMA), acoustic tracking, and underwater video census (UVC) provided complementary data that revealed contrasting patterns of post-settlement seascape use by the co-occurring riverine snappers. Although three of the target species ranged widely across the seascape from fresh to coastal waters, species were broadly distributed along a gradient from freshwaters, through estuaries, and into coastal waters suggesting niche partitioning within the assemblage. Lutjanus fuscescens appears to recruit to freshwaters where they reside until around the age of sexual maturity (about 4–6 years; authors unpubl. data). The seascape migrant L. fuscescens identified by OMA tended to be older individuals than the freshwater residents, and the clear trend was for down-stream lifecycle movements bringing larger individuals from residence in freshwaters earlier in life, into brackish waters. These movements may relate to the onset of sexual maturity, and bring individuals closer to spawning sites, although the location of spawning remains unconfirmed. We have identified marine larval phases for L. fuscescens (and L. goldiei); however, this should not be assumed to reflect marine spawning. Adults occupy flowing rivers with short estuaries such that even freshwater spawning could deliver larvae into saline coastal waters within a few hours, and this early transition would be difficult to detect in OMA profiles (Walther and Limburg 2012). We have observed large aggregations (100 s) of mature-sized individuals on structured habitats in the upper estuary on three of our study rivers, during times when L. fuscescens are reproductively active (from October to April; authors unpbul. data). These fish showed no interest in feeding and we have been unable to assess the reproductive status of individuals within these aggregations; however, these could be spawning aggregations.
Acoustic tracking and OMA indicated some degree of variability in short-term (days to weeks) movement patterns of individual L. goldiei that may indicate individual specialisation in seascape use (e.g., Fodrie et al. 2015). However, the OMA profiles show relatively clear and consistent longer-term patterns of ontogenetic movements through the seascape. Early juveniles recruit from marine waters into low salinity or freshwater areas. Many then make stepped movements through the seascape, with a general trend for the centre of the home range for larger individuals to shift downstream into brackish or saline waters. Large individuals continue to make short-term wide-ranging seascape movements. Acoustic tracking revealed that individual fish made regular (ca. monthly) movements from estuaries into coastal and/or freshwaters, but most spent the majority of time in the lower brackish and saline parts of estuaries.
The timing and regularity of the movements of L. goldiei in the Toriu suggest two potential drivers: feeding or spawning. Some estuarine-resident sparids make regular spawning migrations to estuary mouths to spawn at night on peak ebb tides (Garratt 1993; Sheaves et al. 1999). We observed freshwater plumes during moderate river flows extending over the coral reefs where the coastal receivers were deployed, and presumably this would occur during much of the wet season. Similarly, during the dry season, the upstream extent of saline intrusion is typically at the rockbar. If the movements of L. goldiei to the offshore coral reefs, which we detected during the wet season, and the upstream rockbar, which we detected during the dry season, do not temporally overlap, then these patterns would reflect movements to large structured habitats in the freshwater-saline mixing zone. As such, movements to these areas on the new moon may ensure that spawning occurs in the preferred salinity and that eggs and larvae are dispersed offshore during the ebb-tide (Sheaves et al. 1999). Lutjanus goldiei were detected on the coastal receivers offshore from the Langa Langa-Pandi system during all months of the year. As for the Toriu, the greatest number of individuals tended to be detected around the new moon. We have not discovered any substantial structured habitat in these coastal waters, and with the different nature of these river systems, it is unclear if the movement patterns into coastal waters around the Langa Langa-Pandi are consistent with those observed in the Toriu.
We have observed subadult to large adult L. goldiei feeding heavily on recruiting post-larval sicydiine gobies which recruit to rivers around the new moon. These “whitebait” runs can be highly variable. We observed recruitment events that we estimated to number in the millions of fish, while on other new moons no whitebait were observed. The post-larval gobies move from marine waters where they spend their larval phase, through estuaries, and into freshwaters where the adults live and spawn (Keith et al. 2015). The movements of L. goldiei around the new moons from estuaries offshore into coastal waters and upstream into freshwaters could reflect fish tracking recruitment pulses of these gobies to maximise feeding on them during their migration.
For L. argentimaculatus, OMA and UVC revealed that while this species makes wide use of the coastal seascape from fresh to coastal waters, its distribution is more centred on the saline parts of estuaries. Despite the vast differences in the spatial scale of separation of seascape components (e.g., 10’s of m in our study area vs. 10’s of km in NE Australia), the life-cycle movement patterns inferred in the present study are consistent with those reported from elsewhere in the species range; juveniles recruit from marine waters into estuarine or lower freshwater areas, followed by an ontogenetic movement from estuaries into coastal sub-adult habitats before moving to deeper waters around coral reefs as adults (Sheaves 1995; Russell and McDougall 2005). Lutjanus johnii are a widespread species known to inhabit estuarine, coastal, and inshore waters (Travers et al. 2010; Tanaka et al. 2011; Cappo et al. 2013), and the few specimens we collected showed OMA profiles consistent with occupation of lower estuaries or coastal waters.
The short-term seascape use and longer-term ontogenetic movements observed in our study are broadly consistent with patterns described for other lutjanids (Nagelkerken et al. 2000; Verweij et al. 2007). Several lutjanid species undertake movements to form spawning aggregations (Sadovy 2016), and cyclical monthly movements similar to those observed in the current study are related to spawning events (Biggs and Nemeth 2014). However, much of our understanding about habitat use and ontogenetic movements of lutjanids and other coastal fishes comes from indirect evidence of size-structured distributions among habitats and ecosystems (Beck et al. 2001; Gillanders et al. 2003). The current study adds to a growing body of literature employing multiple approaches to clarify seascape use and lifecycle resource requirements of snappers (Verweij et al. 2007; Luo et al. 2009) and other species (Matich et al. 2017; Crook et al. 2017).
The patterns described above represent relatively consistent ontogenetic seascape movements among individuals within species, yet not all individuals adhered to these general patterns. For example, L. fuscescens generally appear to recruit to freshwaters before migrating downstream to brackish areas around the age of sexual maturity (4–6 years); however, individuals as old as 12 years were classified as freshwater residents. This suggests the possibility for multiple migratory contingents within the population (Secor 1999), where the older resident individuals could be skip spawners yet to undertake the migrations reflected in typical seascape migrants (e.g., Crook et al. 2017). However, based on the current data, we are unable to unambiguously identify migratory contingents. Although reproduction is likely to be an important driver of lifecycle movements (Crook et al. 2017), the location of spawning remains unknown, and individuals classified as residents from their OMA profiles could still have undertaken migrations across salinity gradients to participate in spawning events that are too brief to be recorded in their otoliths (Walther and Limburg 2012).
Interpretation of Movement Patterns from Multiple Methods
Although the three methods provided information on distribution and movement at different spatio-temporal scales, they showed broadly consistent patterns for individual species, and the multiple methods overcame some of the ambiguities associated with interpreting individual methods in isolation. For example, otolith microchemical profiles provide a record of environmental conditions experienced by a fish throughout its life, but the exact relationship between ambient water chemistry and otolith composition can be modified by a range of factors (Elsdon and Gillanders 2003), and changes in otolith chemical profiles do not necessarily reflect movements by the fish (Elsdon et al. 2008). As such, the seascape movements inferred from OMA in the present study would need to be interpreted cautiously in the absence of either detailed spatio-temporal data on water chemistry and complementary analyses, or the supporting information from other techniques. However, the patterns of movement inferred from OMA were reflected in the distribution of the population observed by UVC, and in the movements of individually tagged fish. Similarly, the spatial distributions of different life stages within a population as observed by UVC do not necessarily equate to ontogenetic movements of individual fish (Gillanders et al. 2003). However, the complementary information from OMA and acoustic tracking indicates movements of individuals rather than spatial patterns in growth and survival.
The Sr:Ba profiles recommended by McCulloch et al. (2005) and Hamer et al. (2015) proved effective in highlighting movements between marine and estuarine/freshwater for fish from the Toriu River, which presented atypical Sr:Ca profiles. However, the interpretation of in-river movements between estuarine and freshwater areas from Sr:Ba profiles was more complex. Elsdon and Gillanders (2005) found a negative relationship between ambient Ba:Ca and salinity in estuaries of southern Australia, but suggest the relationship was likely driven by higher loads of terrestrial sediments and greater bioavailability of Ba in freshwaters, rather than a direct link to salinity itself. The estuaries in our study systems tended to remain moderately turbid, while the freshwater reaches alternated between periods of very low and high turbidity. As a result, it is expected that fish moving from estuaries into freshwaters with variable sediment loads would give rise to a range of Sr:Ba profiles (Elsdon and Gillanders 2005; Elsdon et al. 2008). So, while Sr:Ba proved useful for detecting seascape movements in our target fish, detailed interpretation of in-river movements from these profiles alone should be made with caution.
Our study also yielded new information specific to individual techniques. Acoustic tracking provided insights into movement patterns over relatively short periods of an individual’s lifespan. While the scale of these movements was consistent with those inferred from OMA, acoustic tracking revealed potentially significant short-term movements between estuaries and coastal reefs that would be difficult to resolve from otolith chemistry or unlikely to be captured by UVC. UVC identified population-level distribution patterns consistent with the other techniques. It also provided detailed habitat-association information not available from the other techniques (e.g., Bradley et al. 2017) that will be published separately, and successfully pinpointed the whereabouts of smaller juveniles (< 10 cm) of each species.
Implications for Management and Directions for Further Research
The wide-ranging seascape movements of our target species highlight the importance of maintaining effective connectivity between marine, estuarine, and freshwaters in the region to maintain ecosystem function (Nagelkerken et al. 2015; Crook et al. 2017) and support sustainable sport fisheries (Barnett et al. 2016). Ensuring the target species have access to the resources needed to complete their life-cycles extends beyond the maintenance of physical connectivity. It includes ensuring high-quality habitat throughout their range, and good water quality to allow access to required habitats and resources (Arthington et al. 2010; Sheaves et al. 2015). While the construction of significant barriers to movement within rivers in remote parts of PNG may be unlikely at present, there is extensive mining, logging, and plantation agriculture in the region (Sheaves et al. 2016), and these can have a range of potential impacts on connectivity, habitat, and water quality (Swales et al. 2000; Caddy 2008; O’Connor et al. 2016).
Our study was conducted in just one part of the broader global distribution of these species, and in other parts of their ranges the nature and spatial extent of the coastal seascape can vary considerably. For example, L. goldiei also occur in the Gulf of Papua along the southern coast of mainland PNG (Allen 2004). They have been recorded around 1000 km upstream in the Fly River (Swales et al. 2000), and there are anecdotal reports of them on oil infrastructure over 25 km offshore in the Gulf of Papua. Despite substantial differences in the spatial separation of seascape components, we found similar patterns of ontogenetic life cycle movements for L. argentimaculatus in our study area where freshwaters and coral reefs are separated by 10’s of meters, to that previously reported for NE Australia where juvenile nurseries and adult habitats are separated by 10’s of km (Sheaves 1995; Russell and McDougall 2005). Further study of our target species elsewhere in their range could provide insights into the extent to which seascape and life cycle movement patterns are fixed throughout a species range, or if some species exhibit flexible life-history movement patterns depending on the seascape context (Kimirei et al. 2011). Research in larger river systems could also provide better resolution to distinguish different migratory contingents within the population (Secor 1999), each of which may have differing vulnerabilities to anthropogenic impacts (Crook et al. 2017).
References
Allen, G.R. 1985. An annotated and illustrated catalogue of lutjanid species known to date. FAO species catalogue, Vol. 6. Snappers of the World. FAO, Rome.
Allen, G.R. 2004. A review of the freshwater fish fauna of the trans Fly ecoregion. Report to World Wildlife Fund South Pacific program, Suva. In Fiji.
Arthington, A., R. Naiman, M. McClain, and C. Nilsson. 2010. Preserving biodiversity and ecological services of rivers: new challenges and research opportunities. Freshwater Biology 55: 1–16.
Baker, R., M. Bradley, S. Freddi, K. Abrantes, A. Barnett, and M. Sheaves. 2018. Non-lethal aging of tropical catch-and-release sport fishery species. Fisheries Research 207: 110–117.
Barnett, A., K.G. Abrantes, R. Baker, A. Diedrich, A. Kuilboer, T. Mahony, I. McLeod, G. Moscardo, M. Prideaux, A. van Luyn, and M. Sheaves. 2016. Sport fisheries, conservation, and sustainable livelihoods: a multidisciplinary assessment of best practice. Fish and Fisheries 17: 696–713.
Beck, M., K. Heck, K. Able, D. Childers, D. Eggleston, B. Gillanders, B. Halpern, C. Hays, K. Hoshino, T. Minello, R. Orth, P. Sheridan, and M. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51: 633–641.
Biggs, C.R., and R.S. Nemeth. 2014. Timing, size, and duration of a Dog (Lutjanus jocu) and Cubera Snapper (Lutjanus cyanopterus) spawning aggregation in the U.S. Virgin Islands. Proceedings of the Gulf and Caribbean Fisheries Institute 67: 240–245.
Bradley, M., R. Baker, and M. Sheaves. 2017. Hidden components in tropical seascapes: deep-estuary habitats support unique fish assemblages. Estuaries and Coasts 40: 1195–1206.
Brophy, D., T.E. Jeffries, and B.S. Danilowicz. 2004. Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins. Marine Biology 144: 779–786.
Brown, R., and K. Severin. 2009. Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Canadian Journal of Fisheries and Aquatic Science 66: 1790–1808.
Caddy, J. 2008. The importance of “cover” in the life histories of demersal and benthic marine resources: a neglected issue in fisheries assessment and management. Bulletin of Marine Science 83: 7–52.
Campana, S. 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188: 263–297.
Cappo, M., P. Eden, S.J. Newman, and S. Robertson. 2000. A new approach to validation of periodicity and timing of opaque zone formation in the otolith of eleven species of Lutjanus from the central Great Barrier Reef. Fishery Bulletin 98: 474–488.
Cappo, M., R. Marriott, and S. Newman. 2013. James’s rule and causes and consequences of a latitudinal cline in the demography of John’s Snapper (Lutjanus johnii) in coastal waters of Australia. Fishery Bulletin 111: 309–324.
Choat, J.H., J.P. Kritzer, and J.L. Ackerman. 2009. Aging in coral reef fishes: Do we need to validate the periodicity of increment formation of every species of fish for which we collect age-based demographic data? In: Green, B.S., Mapstone B.D., Carlos G., Begg G.A. (Eds) 2009. Tropical fish otoliths: information for assessment, management and ecology. Reviews: Methods and Technologies in Fish Biology and Fisheries, Vol. 11.
Cocheret de la Moriniere, E., B.I.A. Pollux, I. Nagelkerken, and G. van der Velde. 2002. Post-settlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries. Estuarine Coastal and Shelf Science 55: 309–321.
Crook, D.A., D.J. Buckle, Q. Allsop, W. Baldwin, T.M. Saunders, P.M. Kyne, J.D. Woodhead, R. Maas, B. Roberts, and M.M. Douglas. 2017. Use of otolith chemistry and acoustic telemetry to elucidate migratory contingents in barramundi Lates calcarifer. Marine and Freshwater Research 68: 1554–1566.
Dorenbosch, M., M.C. Verweij, I. Nagelkerken, N. Jiddawi, and G. van der Velde. 2004. Homing and daytime tidal movements of juvenile snappers (Lutjanidae) between shallow-water nursery habitats in Zanzibar, western Indian Ocean. Environmental Biology of Fishes 70: 203–209.
Dahlgren, C.P., and D.B. Eggleston. 2000. Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81: 2227–2240.
Elsdon, T., and B. Gillanders. 2003. Recontructing migratory patterns of fish based on environmental influences on otolith chemistry. Reviews in Fish Biology and Fisheries 13: 219–235.
Elsdon, T., and B. Gillanders. 2005. Consistency of patterns between laboratory experiments and field collected fish in otolith chemistry: an example and applications for salinity reconstructions. Marine and Freshwater Research 56: 609–617.
Elsdon, T., B. Wells, S. Campana, B. Gillanders, C. Jones, K. Limburg, D. Secor, S. Thorrold, and B. Walther. 2008. Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology: An Annual Review 46: 297–330.
Fodrie, F.J., L.A. Yeager, J.H. Grabowski, C.A. Layman, G.D. Sherwood, and M.D. Kenworthy. 2015. Measuring individuality in habitat use across complex landscapes: approaches, constraints, and implications for assessing resource specialization. Oecologia 178: 75–87.
Froese, R., and D. Pauly. 2017. Editors, FishBase. World Wide Web electronic publication. www.fishbase.org, version (06/2017).
Garratt, P.A. 1993. Spawning of riverbream, Acanthopagrus berda, in Kosi estuary. South African Journal of Zoology 28: 26–31.
Gillanders, B.M. 2005. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquatic Living Resources 18: 291–300.
Gillanders, B., K. Able, J. Brown, D. Eggleston, and P. Sheridan. 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247: 281–295.
Hamer, P., A. Henderson, M. Hutchinson, J. Kemp, C. Green, and P. Feutry. 2015. Atypical correlation of otolith strontium: Calcium and barium: calcium across a marine-freshwater life history transition of a diadromous fish. Marine and Freshwater Research 66: 411–419.
Hammerschlag-Peyer, C.M., and C.A. Layman. 2010. Intrapopulation variation in habitat use by two abundant coastal fish species. Marine Ecology Progress Series 415: 211–220.
Keith, P., C. Lord, and K. Maeda. 2015. Indo-Pacific Sicydiine Gobies. Biodiversity, life traits and conservation. 256p. Societe Francaise d’Ichtyologie, Paris, France.
Kimirei, I.A., I. Nagelkerken, B. Griffioen, C. Wagner, and Y.D. Mgaya. 2011. Ontogenetic habitat use by mangrove/seagrass-associated coral reef fishes shows flexibility in time and space. Estuarine Coastal and Shelf Science 92: 47–58.
Luo, J.G., J.E. Serafy, S. Sponaugle, P.B. Teare, and D. Kieckbusch. 2009. Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Marine Ecology Progress Series 380: 255–269.
Matich, P., J.S. Ault, R.E. Boucek, D.R. Bryan, K.R. Gastrich, C.L. Harvey, M.R. Heithaus, J.J. Kiszka, V. Paz, J.S. Rehage, and A.E. Rosenblatt. 2017. Ecological niche partitioning within a large predator guild in a nutrient-limited estuary. Limnology and Oceanography 62: 934–953.
Mateo, I., E.G. Durbin, R.S. Appeldoorn, A.J. Adams, F. Juanes, R. Kingsley, P. Swart, and D. Durant. 2010. Role of mangroves as nurseries for French Grunt Haemulon flavolineatum and schoolmaster Lutjanus apodus assessed by otolith elemental fingerprints. Marine Ecology Progress Series 402: 197–212.
McCulloch, M., M. Cappo, J. Aumend, and W. Muller. 2005. Tracing the life history of individual barramundi using laser ablation MC-ICP-MS Sr-isotopic and Sr/Ba ratios in otoliths. Marine and Freshwater Research 56: 637–644.
Nagelkerken, I., M. Sheaves, R. Baker, and R.M. Connolly. 2015. The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish and Fisheries 16: 362–371.
Nagelkerken, I., M. Dorenbosch, W. Verberk, E.C. de la Moriniere, and G. van der Velde. 2000. Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: patterns in biotope association, community structure and spatial distribution. Marine Ecology Progress Series 202: 175–192.
Nakamura, Y., M. Horinouchi, T. Shibuno, Y. Tanaka, T. Miyajima, I. Koike, H. Kurokura, and M. Sano. 2008. Evidence of ontogenetic migration from mangroves to coral reefs by black-tail snapper Lutjanus fulvus: stable isotope approach. Marine Ecology Progress Series 355: 257–266.
O’Connor, J.J., D. Lecchini, H.J. Beck, G. Cadiou, G. Lecellier, D.J. Booth, and Y. Nakamura. 2016. Sediment pollution impacts sensory ability and performance of settling coral-reef fish. Oecologia 180: 11–21.
Oka, S., and K. Tachihara. 2008. Migratory history of the spotted flagtail, Kuhlia marginata. Environmental Biology of Fishes 81: 321–327.
Piddocke, T., G. Butler, P. Butcher, S. Purcell, D. Bucher, and L. Christidis. 2015. Age validation in the Lutjanidae: a review. Fisheries Research 167: 48–63.
Russell, D.J., and A.J. McDougall. 2005. Movement and juvenile recruitment of mangrove jack, Lutjanus argentimaculatus (Forsskal), in northern Australia. Marine and Freshwater Research 56: 465–475.
Ruttenberg, B., S. Hamilton, M. Hickford, G. Paradis, M. Sheehy, J. Standish, O. Ben-Tzvi, and R. Warner. 2005. Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Marine Ecology Progress Series 297: 273–281.
Sadovy, Y. 2016. Mainstreaming fish spawning aggregations into fishery management calls for a precautionary approach. Bioscience 66: 295–306.
Secor, D.H. 1999. Specifying divergent migrations in the concept of stock: the contingent hypothesis. Fisheries Research 43 (1–3): 13–34.
Sheaves, M. 1995. Large lutjanid and serranid fishes in tropical estuaries: are they adults or juveniles? Marine Ecology Progress Series 129: 31–40.
Sheaves, M., and B. Molony. 2000. Short-circuit in the mangrove food chain. Marine Ecology Progress Series 199: 97–109.
Sheaves, M., B. Molony, and A. Tobin. 1999. Spawning migrations and local movements of a tropical sparid fish. Marine Biology 133: 123–128.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2015. True value of estuarine and coastal nurseries for fish: incorporating complexity and dynamics. Estuaries and Coasts 38: 401–414.
Sheaves, M., R. Baker, I. McLeod, K. Abrantes, J. Wani, and A. Barnett. 2016. The conservation status of Niugini black bass: a world-renowned sport fish with an uncertain future. Fisheries Management and Ecology 23: 243–252.
Snover, M.L. 2008. Ontogenetic habitat shifts in marine organisms: influencing factors and the impact of climate variability. Bulletin of Marine Science 83: 53–67.
Swales, S., A. Storey, and K. Bakowa. 2000. Temporal and spatial variations in fish catches in the Fly River system in Papua New Guinea and the possible effects of the Ok Tedi copper mine. Environmental Biology of Fishes 57: 75–95.
Tanaka, K., Y. Hanamura, V. Chong, S. Watanabe, A. Man, F. Kassim, M. Kodama, and T. Ichikawa. 2011. Stable isotope analysis reveals ontogenetic migration and the importance of a large mangrove estuary as a feeding ground for juvenile John’s snapper Lutjanus johnii. Fisheries Science 77: 809–816.
Thorrold, S.R., G.P. Jones, S. Planes, and J.A. Hare. 2006. Transgenerational marking of embryonic otoliths in marine fishes using barium stable isotopes. Canadian Journal of Fisheries and Aquatic Sciences 63: 1193–1197.
Travers, M., I. Potter, K. Clarke, S. Newman, and J. Hutchins. 2010. The inshore fish faunas over soft substrates and reefs on the tropical west coast of Australia differ and change with latitude and bioregion. Journal of Biogeography 37: 148–169.
Verweij, M.C., I. Nagelkerken, K.E.M. Hol, A.H.J.B. van den Beld, and G. van der Velde. 2007. Space use of Lutjanus apodus including movement between a putative nursery and a coral reef. Bulletin of Marine Science 81: 127–138.
Walther, B.D., and K.E. Limburg. 2012. The use of otolith chemistry to characterize diadromous migrations. Journal of Fish Biology 81: 796–825.
Wood, A., J. Butler, M. Sheaves, and J. Wani. 2013. Sport fisheries: opportunities and challenges for diversifying coastal livelihoods in the Pacific. Marine Policy 42: 305–314.
Acknowledgements
We thank Riccard and Nathalie Reimann of Baia Sportfishing and the people of Baia Village for their support of this research, Lina Pandihau of the Papua New Guinea National Fisheries Authority for assistance with field work and logistical planning, Dr. Yi Hu of the Advanced Analytical Centre at JCU for training and advice on LA-ICPMS, and Mark O’Callaghan for advice on the preparation of otoliths for analysis.
Funding
This research was funded by the Papua New Guinea National Fisheries Authority and the Australian Centre for International Agricultural Research under grant FIS-2013-015. RB was partly supported by a fellowship from the Tropical Landscapes Joint Venture between the Commonwealth Scientific and Industrial Research Oganisation and James Cook University.
Author information
Authors and Affiliations
Contributions
All authors designed the study and collected the samples; MB sectioned and aged otoliths, and conducted LA-ICPMS; RB analysed the otolith microchemistry data; AB analysed the acoustic tracking data; MB conducted and analysed the underwater video census; RB wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Field work was conducted in collaboration with and permission of the Papua New Guinea National Fisheries Authority. All applicable institutional and national guidelines for the care and use of animals were followed, and this work was completed in accordance with JCU’s animal ethics guidelines under JCU Ethics Permit A2308.
Conflict of Interest
The authors declare they have no conflict of interest.
Additional information
Communicated by Mark S. Peterson
Electronic Supplementary Material
Supplementary Material are provided in four appendices. Online Resource 1 provides information on the spatial distribution of samples collected for OMA. Online Resource 2 presents Sr:Ca and Sr:Ba profiles of marine-resident and freshwater-resident reference fish used to define otolith microchemical values expected in target species during occupation of saline or freshwaters. Online Resource 3 provides individual otolith microchemical profiles for every target fish, grouped by life-history movement categories summarised in Table 1. Online Resource 4 provides timeline figures of individual acoustically tagged fish, a subset of which are presented in Fig. 5.
ESM 1
(PDF 3897 kb)
Rights and permissions
About this article
Cite this article
Baker, R., Barnett, A., Bradley, M. et al. Contrasting Seascape Use by a Coastal Fish Assemblage: a Multi-methods Approach. Estuaries and Coasts 42, 292–307 (2019). https://doi.org/10.1007/s12237-018-0455-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0455-y