Abstract
Total antioxidant activity was evaluated in 40 tuber-bearing Solanum species. Accessions identified in a broad screen as having high antioxidant activity were fine screened via high performance liquid chromatography (HPLC) to determine specific phenolic compounds present. The objective of the study was to identify tuber-bearing Solanum species that are higher in antioxidants than in currently available cultivated varieties and breeding lines, and to investigate the variability for this trait among accessions within species and genotypes within accessions. The identified accessions will be used as a source of germplasm to develop potato cultivars containing increased levels of antioxidant compounds. In a broad screen for total antioxidant activity, the 40 tuber-bearing species showed a wider range of variability than cultivated varieties and breeding lines. Solanum pinnatisectum and S. jamesii accessions consistently ranked among the highest in antioxidant activity and phenolic content. Based on the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay, antioxidant activity in the wild species ranged from 48 to 824 μg trolox eq/gfw. HPLC analysis revealed that the phenolic content of these species was primarily composed of chlorogenic and caffeic acids. Other phenolics identified were p-coumaric acid, rutin hydrate, vanillic acid, epicatechin, t-cinnamic acid, gallic acid, and salicylic acid. The highest phenolic content found among the accessions was five-fold higher than the highest of the cultivated genotypes.
Resumen
La actividad antioxidante total fue evaluada en 40 especies tuberíferas de Solanum. Las accesiones que mostraron actividad antioxidante en un tamizado amplio fueron sometidas a un tamizado más fino por medio de cromatografía líquida (HPCL) para determinar los compuestos fenólicos específicos presentes. El objetivo de este estudio fue identificar las especies tuberíferas de Solanum que tienen contenido más alto de antioxidantes que las variedades y líneas de mejoramiento e investigar la variabilidad de esta característica entre las accesiones dentro de especies y genotipos. Las accesiones identificadas serán usadas como fuente de germoplasma para desarrollar cultivares de papa que contengan niveles altos de compuestos antioxidantes. En un tamizado amplio para actividad total antioxidante, las 40 especies tuberíferas mostraron un rango más amplio de variabilidad que las variedades cultivadas y que las líneas mejoradas. Las accesiones de Solanum pinnatisectum y S. jamesii dieron consistentemente grados más altos en actividad antioxidante y contenido fenólico. En base a la prueba 2, 2-difenil-1-picrilhidrazil (DPPH), la actividad antioxidante en las especies silvestres fue de 48 a 824 μg trolox eq/gfw. El análisis HPLC reveló que el contenido de estas especies estaba principalmente compuesto de los ácidos clorogénico y cafeico. Otros fenoles identificados fueron ácido p-cumárico, rutina hidrato, ácido vanílico, epicatequín, ácido t-cinámico, ácido gálico y ácido salicílico. El contenido fenólico en las accesiones fue cinco veces más alto que en los genotipos cultivados.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much research has been conducted on the benefits of various phytochemicals in fruits and vegetables and their significant impact on human health. The importance of phytochemicals, including antioxidants, in tying up free radicals and thus fighting deadly diseases including cancer, stroke, and heart-related health problems is now widely recognized. A number of classes of chemicals including polyphenols (flavonoids, flavanols, flavones, and isoflavones), carotenoids (carotenes, epoxycarotenes, xanthophylls, etc.) and vitamins and minerals have been linked to the reduction and prevention of various diseases.
Many consumers recognize the potato (Solanum tuberosum L.) as an important source of carbohydrates, but few recognize it as an important source of vitamin C and potassium. Though there is increasing interest, relatively little is known about the important phytochemicals contained in this most-consumed vegetable. Based on the 1997–1998 National Food Consumption Survey, white potatoes ranked first in consumption among vegetables in the USA, and data from PMA (Produce Marketing Association), UFFVA (United Fresh Fruit and Vegetable Association), and ERS USDA ranked potato first among vegetables by sales, promotion, or consumption (Kolasa 1993). Therefore, considering consumption estimates, even modest antioxidant levels in potato probably play a major role in maintaining a healthy population. Unlike crops such as blueberries, potatoes have not been considered among foods important for their high antioxidant content. This is unfortunate considering the per capita consumption of potatoes in the USA is about 130 lbs (National Potato Council 2006–2007), while that of blueberries stands at 13.9 oz (North American Blueberry Council 2005). In addition, blueberries are much more expensive than potatoes.
Cultivated potatoes do in fact contain significant levels of important antioxidants, including phenolic acids, flavonoids, and carotenoids, among others (Al-Saikhan et al. 1995; Al-Saikhan 2000; Arai et al. 2000; Gazzani et al. 1998; Lachman et al. 2000; Yamamoto et al. 1997; Dao and Freidman 1992; Freidman 1997; Reddivari et al. 2007). Furthermore, investigations on the effects of cooking on potatoes indicate that many antioxidant compounds are retained even after boiling, microwaving, and frying (Tudela et al. 2002). Although a number of studies have investigated the level of compounds with antioxidant activity in cultivated potato, little is known about the levels of these important compounds in wild tuber-bearing species. Since antioxidants serve as plant defense compounds, it is likely that, due to natural selection, some wild species contain higher levels of these compounds than do cultivated varieties. If levels of these compounds in wild species are significantly higher than those of cultivated potato, it could be beneficial to introgress wild genes responsible for elevated levels of these compounds into the gene pool of cultivated potato. Wild germplasm could serve as a source of important health-benefiting compounds in this fourth most important food crop, after wheat, rice, and maize. It is important to note, however, that some antioxidants, such as glycoalkaloids, can be toxic if consumed in large amounts. Thus, when selecting parents and offspring, it will be important to minimize levels of toxic compounds.
In a study conducted by Lewis et al. (1998a), phenolic content in the flesh of eight wild tuber-bearing species was found to range from 84 to 274 μg/gfw, while that in the flesh of cultivated potato was 157 μg/gfw. A more diverse phenolic profile was observed in the flesh of wild species, which contained protocatechuic acid, chlorogenic acid, and p-coumaric acid. Flavanoids, however, were significantly higher in S. tuberosum than in the wild species. Since intensive selection in wild tuber-bearing species has not been practiced by breeders, it is expected that the genetic base for many traits, including antioxidant activity, is broader than that of cultivated varieties. Past studies have utilized a “mini” core collection of wild species to screen for traits for which there were no a priori clues to performance. The collection has been successfully used to screen for traits such as nitrogen uptake and biomass production, male fertility under heat stress, and efficient accumulation of tuber calcium (Bamberg et al. 1993; Bamberg 1995; Errebhi et al. 1998). Tubers generated from these accessions are in the same family, but not clones of one another, so an accession is composed of a heterogeneous group of related genotypes.
The objective of this investigation was to identify tuber-bearing Solanum species that are higher in antioxidants than currently available cultivated varieties and breeding lines. To this end, the antioxidant activity among a broad collection of wild tuber-bearing potato species was determined, as well as the variability in antioxidant activity of accessions within species and genotypes within accessions.
Materials and Methods
Plant Materials
There are virtually unlimited numbers of genotypes within the many accessions within potato species. Accessions were analyzed through stepwise fine screening which incorporated tubers grown under different conditions. The primary intent was to preliminarily characterize the limits of antioxidant variation at the species, accession, and genotype levels. Three groups of Solanum species were used in this study.
-
Group 1.
One accession each of 20 species was selected for analysis of antioxidant activity, along with two accessions of an additional three species (Table 1). An accession consists of the progeny grown from botanical seed produced by a single plant, thus a single accession is a heterogeneous mixture of full sib genotypes. Tissue for analysis was collected from bulked tubers of up to 18 greenhouse-reared plants of each accession. Materials for this group were chosen from available greenhouse-grown tubers to represent a broad taxonomic representation of potato species diversity.
-
Group 2.
This group was composed of materials grown in fields in Weslaco, TX and Miami, FL (Table 1). This group represents the members of the “mini” core collection (noted in Introduction above) which successfully produced tubers in field conditions. The 24 species examined in the second group included ten species from Group 1 along with an additional 14 species. Sixteen species, along with many of the accessions from each species, were common between the two locations. For 17 species, three accessions were analyzed. Exceptions included two species in which four accessions were analyzed, two species in which two accessions were analyzed, and three species in which one accession was analyzed. The bulked tubers from each accession were collected from these field-grown plants.
-
Group 3.
This group consisted of tubers from greenhouse-grown plants of selected species (Table 1). The composition of this group aimed to allow examination of multiple populations of the Mexican diploid species S. pinnatisectum (which had previously been identified as having very high antioxidant levels) and several of its close relatives, namely, S. brachistotrichum, S. bulbocastanum, S. cardiophyllum, S. jamesii, S. nayaritense. The numbers of populations examined was determined by availability. Many populations of a non-Mexican species, S. infundibuliforme, and limited numbers of five other species were included for comparison. This set of materials aimed particularly at examining variation among accessions and among genotypes within accessions. Thus, Group 3 was subdivided for analysis based on the type of sample analyzed. Entries in Group 3a included multiple accessions from 12 different species and were used to examine variation among accessions within species. Group 3b included multiple genotypes within a single accession of six different species and was used to examine variation among individual genotypes within accessions within species.
Extraction of Phenolics
A single extraction was performed for both the evaluation of antioxidant activity and the determination of individual antioxidant components. For each replication, a small group of tubers was chopped and a 5 g sample was weighed and stored at −20°C until extractions were performed. Separate tubers were used for each replication.
For the evaluation of potato antioxidant activity and the HPLC analysis of phenolics, antioxidants were extracted from the 5 g tuber samples by mixing 15 mL of methanol and homogenizing with a tissuemizer (Ultra-turrax, Tekmar Corporation, Cincinnati, OH). Homogenized samples were centrifuged at 15,000 rpm for 15 min in a refrigerated centrifuge (Beckman model J2–21) using a J-17 rotor, and the supernatant was stored at −20°C until analysis.
DPPH Assay for Total Antioxidant Activity
The determination of antioxidant activity was based on the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay described by Brand-Williams et al. (1995). DPPH, a stable radical, absorbs at 515 nm and, upon reduction by an antioxidant species, a decrease in absorbance is observed. The DPPH assay was chosen because the change in color (from purple to yellow) provides an easy and rapid assay to evaluate the antiradical activities of tuber extracts. This assay measures the ability to quench radicals through single electron transfer and/or hydrogen atom transfer activities, and may be broadly classified as antioxidant activity. It does not, however, measure other types of antioxidant activity such as the quenching of singlet oxygen (Prior et al. 2005). This study dealt with a large number of samples, and the DPPH assay was used as a broad screen to identify those samples that were high in antioxidant activity, as defined by the limits of this assay.
DPPH stock solution was prepared by dissolving 24 mg of DPPH in 100 mL of methanol. The stock was diluted ~10:55 until the spectrophotometer at 515 nm read 1.1. Two-thousand-eight-hundred-fifty microliters of the dilute DPPH was allowed to react with 150 μL of the tuber methanol extract for 15 min, and was read on the spectrophotometer at 515 nm. All genotypes were analyzed in triplicate.
A standard regression line was prepared using trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid—a commonly used synthetic antioxidant), and absorbance readings were converted to μg equivalents of this compound/gfw. Three samples were prepared separately for each concentration and assayed in the same manner as described above for the tuber samples.
HPLC Analysis of Phenolic Compounds
HPLC analysis was performed on a subset of samples to identify and quantify which phenolic compounds were present in the tuber extracts. Accessions were chosen based on results of the DPPH analysis from Groups 2 and 3a and were analyzed in triplicate via HPLC. A total of 47 accessions were analyzed. These included those which were the highest in antioxidant activity (25 accessions), the accessions which were the lowest in antioxidant activity (ten accessions), and accessions representing several species that were not included in the other two groups. When available, samples extracted from tubers in two locations in the field (field replications) were analyzed. Since the accessions which were high in antioxidant activity were primarily S. pinnatisectum and S. jamesii, it was possible that accessions high in one particular compound (though not total activity) would be overlooked if only the highest were examined via HPLC. To reduce the possibility of overlooking an accession that could contribute to the level of one particular compound when used as parents, a survey of species was conducted. The reduction in numbers was necessary because of both monetary and time constraints involved in the HPLC analysis.
Concentrating the Samples
A 7 mL sample of the 5 g methanol extract was dried to completion in a heated speed vac, and resuspended in 1.5 mL of methanol for analysis of phenolic compounds on the HPLC. Prior to injection, the concentrated samples were filtered through a 0.45 μm syringe filter.
The Compounds Analyzed
Based on the phenolic compounds previously reported in the literature for potato, the following 19 compounds were selected for this analysis: Rutin hydrate, chlorogenic acid, gallic acid, protocatechuic acid, catechin, p-hydroxybenzoic acid, caffeic acid, vanillic acid, (−) epicatechin, p-coumaric acid, syringic acid, sinapic acid, 4,5,7-trihydroxyflavanone, ferulic acid, myricetin, saliclylic acid, quercetin dihydrate, t-cinnamic acid, and kaempherol (Al-Saikhan et al. 1995; Lewis et al. 1998a, b; Rodriguez de Sotillo et al. 1994). Standards for each compound were obtained from Acros Organics (Pittsburgh, PA).
The HPLC System
The samples were run on a system equipped with a binary pump (Waters 515), an autoinjector (Waters 717 plus), a photodiode array (PDA) detector (Waters 996), and a column heater (SpectraPhysics SP8792), and results were analyzed using Waters Millennium 3.2 software. Compounds were separated on a 4.6 × 150 mm, 5 μm, Atlantis C-18 reverse-phase column (Waters, Milford, MA), which was maintained at 40°C. The Atlantis column was chosen based on its ability to separate polar compounds using conventional reverse-phase chromatography. For analysis of phenolic compounds, the following gradient system was used: Solvent A (acetonitrile), solvent B (water/HCL, adjusted pH 2.3); gradient (min/%A) 0/85, 5/85, 30/0, 35/0. The column was brought back to initial conditions and allowed to equilibrate for 11 min before injection. All solvents were filtered and degassed before use. Nine point standard calibration curves were prepared for all standards, except tryptophan, and each was analyzed at its lambda max.
Statistical Analysis
The effect of species, accession and genotype on antioxidant activity was determined by analysis of variance (ANOVA) using the SAS general linear model (GLM) procedure (SAS 2000). Pairwise multiple comparisons were determined by LSD.
Results and Discussion
DPPH Assay for Total Antioxidant Activity
Standard Curve
Absorbance was determined for trolox between 0 and 900 μM trolox, and a regression line was estimated. The resulting equation was as follows: \({{y = 888.12x + 3.4883\;{\text{where}}\;y = {\text{ $ \mu $ g}}\;{\text{trolox}}\;{\text{equivalents}}} \mathord{\left/ {\vphantom {{y = 888.12x + 3.4883\;{\text{where}}\;y = {\text{ $ \mu $ g}}\;{\text{trolox}}\;{\text{equivalents}}} {{\text{gfw}}\;{\text{and}}\;x = {\text{absorbance}}\;{\text{at}}\;515\;{\text{nm}}}}} \right. \kern-\nulldelimiterspace} {{\text{gfw}}\;{\text{and}}\;x = {\text{absorbance}}\;{\text{at}}\;515\;{\text{nm}}}}\). The calibration data fit the linear equation very closely with an R 2 value of 0.998. The values reported in the subsequent discussion are based on the aforementioned equation and are reported in μg trolox eq/gfw.
-
Group 1.
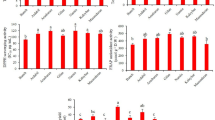
A wide range of variation in antioxidant activity was found among the 23 species analyzed in Group 1. Antioxidant activity among the 23 species of Group 1 ranged from 48 to 818 μg trolox eq/gfw, with an average value of 320 μg trolox eq/gfw (Table 1). There was a 17-fold difference between the species with the lowest antioxidant activity (S. brachistotrichum) and that with the highest (S. pinnatisectum). Analysis of variance revealed significant differences among (p < 0.01) most of the accessions (data not shown) and species (p < 0.01; Table 1). Due to the number of species analyzed, mean separation analysis revealed little about which accession would be useful in a breeding program to enhance antioxidant levels above what is already available in cultivated potato. In order to visually observe the distribution of antioxidant activity, individual accessions were graphed. When graphed, three accessions appeared to be above the rest of the cluster (Fig. 1). Interestingly, these three accessions were also above the cultivated potato genotypes which were analyzed in another study (Hale 2003). Significantly different from most other individual accessions was the S. commersonii accession PI 320266 (778 μg trolox eq/gfw), followed by accessions of S. pinnatisectum (PI 184764, 744 μg trolox/gfw), and S. jamesii (PI 564050, 622 μg trolox/gfw).
-
Group 2.
Less variation in antioxidant activity was found among the 24 species analyzed in Group 2. In the tubers harvested in Weslaco, TX, antioxidant activity ranged from 268 to 817 μg trolox/gfw, with an average value of 525 μg trolox/gfw (Table 1). There was a three-fold difference between the species with the lowest antioxidant activity (S. stenotomum) and that with the highest (S. pinnatisectum). In the Florida-harvested tubers, antioxidant activity ranged from 100 (S. bulbocastanum) to 862 μg trolox/gfw (S. pinnatisectum), with an average value of 304 μg trolox/gfw. The antioxidant activity of many species was higher than that of the cultivated genotypes of S. tuberosum which were analyzed previously (Hale 2003). Analysis of variance revealed significant differences among species (p < 0.01; Table 1) and accessions within species (p < 0.01; data not shown).
-
Group 3.
Antioxidant activity differed among the species in Group 3 (p < 0.01; Table 1) as well as among the accessions within species (p < 0.01; Table 2). The species with the highest average antioxidant levels were S. pinnatisectum (864 μg/gfw), S. jamesii (664 μg/gfw), S. sambucinum (653 μg/gfw), and S. tarijense (599 μg/gfw), while the species that was lowest was S. nyaritense (61). There was a 14-fold difference between the high and low species (Table 1). Ranges in antioxidant activity for each species are shown in Table 2. Significant differences were found among accessions within each species for all species except S. nyaritense and S. pinnatisectum (Table 2).
The 119 entries in Group 3b were each composed of a single genotype from one of six accessions. Each of the six accessions represented a different species. These entries were used to assess the amount of variation that existed within single accessions. Analysis of variance indicated significant differences among genotypes within accessions (p < 0.01; Table 3). Antioxidant activity was highest in S. jamesii (743 μg/gfw) followed by S. chacoense (394 μg/gfw) and S. microdontum (349 μg/gfw). There were no significant differences in AOA among the means of the remaining three species (Table 3). When the data were plotted by genotypes and species, most individual genotypes of S. jamesii were higher than the other species (Fig. 2). With the exception of individual genotypes within the S. jamesii accession, a majority of the populations of individual genotypes were between 100 and 500 μg trolox/gfw.
HPLC Analysis of Phenolic Compounds
As discovered in previous studies on cultivated varieties (Yamamoto et al. 1997 as cited in Lachman et al. 2000; Rodriguez de Sotillo et al. 1998, 1994; Lewis et al. 1998b), the primary phenolics identified in the wild tubers were chlorogenic and caffeic acids, which were both present in all but 11 of the accessions analyzed. Salicylic acid, p-coumaric acid, vanillic acid, epicatechin, t-cinnamic acid, rutin hydrate, and gallic acid were identified in some of the accessions as well.
When analyzed by species, chlorogenic acid levels ranged from 0 to 1,330 μg/gfw, while caffeic acid levels ranged from 35 to 242 μg/gfw (Table 4). Total phenolic levels, calculated by adding the individual compounds quantified, ranged from 35 to 1,584 μg/gfw (Table 4). These levels have a much greater range than those found in named varieties and advanced breeding lines of tetraploid S. tuberosum analyzed under the same conditions. The values in cultivated S. tuberosum, ranged from 26 to 341 μg chlorogenic acid/gfw, 33 to 41 μg caffeic acid/gfw, and 60 to 396 μg total phenolics/gfw (Hale 2003). A typical chromatogram is shown in Fig. 3.
Vanillic acid had a small range in concentration of 0 to 12 μg/gfw. Species containing vanillic acid included S. pinnatisectum, S. cardiophyllum, S. commersonii, S. sambucinum and S. tarijense. Species containing p-coumaric acid included S. gourlayi, S. oplocense, S. demissum, S. stoloniferum, S. pinnatisectum, S. cardiophyllum, and S. polytrichon. Species containing epicatechin included S. cardiophyllum and S. brachistortrichum. Trans-cinnamic acid was identified in S. pinnatisectum, S. oplocense, and S. cardiophyllum. Rutin hydrate was identified in S. stoloniferum and S. gourlayi (Table 4). While gallic acid was identified in only one accession of S. brachistortrichum, it is possible that it was contained in other accessions as well. Due to its immediate elution from the column, it co-eluted with the void volume; therefore, the spectra were frequently contaminated by other compounds. Compounds were only quantified if they matched both the retention time and spectra of the standard compounds. Although there were peaks observed other than for chlorogenic acid, caffeic acid, vanillic acid, p-coumaric acid, epicatechin, t-cinnamic acid, rutin hydrate, salicylic acid and gallic acid, and they sometimes matched retention times with the standards analyzed, none of the spectra in the library matched these peaks. Had the analysis been based solely on retention time, as it is in many studies, perhaps gallic acid and many other compounds would have been identified and quantified.
Analysis of variance for chlorogenic acid revealed significant differences between accessions (p < 0.01) and species (p < 0.01), while no differences were found between replications (Table 5). Analysis of variance for caffeic acid showed that there were no significant differences between accessions or species. Analysis of variance for total phenolics showed a significant effect for both accessions and species (p < 0.01; Table 5).
All of the accessions that were high in antioxidant activity in the broad screen, were also high in chlorogenic acid. A correlation analysis was performed between the results from the DPPH assay and chlorogenic acid content as quantified via HPLC analysis. Pearson’s correlation coefficient was calculated as 0.56, and linear regression revealed that 31% (R 2 = 0.31) of the variability in the DPPH analysis could be explained by chlorogenic acid content (Table 6). A correlation analysis was performed between the results from the DPPH assay and caffeic acid, and less of a linear relationship was seen than with chlorogenic acid. Pearson’s correlation coefficient was calculated as 0.29, and linear regression revealed that 8% (R 2 = 0.08) of the variability in the DPPH assay could be explained by caffeic acid content. Pearson’s correlation coefficient between antioxidant activity in the broad screen and total phenolics was 0.64, and linear regression revealed 41% (R 2 = 0.41) of the variation in the DPPH assay could be explained by total phenolics as calculated.
The DPPH assay was used in this study as a broad screen to determine which samples would be run on the HPLC. When the accessions were ranked by antioxidant activity and then by total phenolics, the results did not always match exactly. The discrepancy between these two analyses could be explained by at least two factors. The first is that the DPPH assay accounts for the total antioxidant activity of the methanol extract, and there are antioxidants other than phenolics present in this extract. For example, vitamin C is known to be an abundant antioxidant in potato, but was not analyzed in this study. Thus, the estimated antioxidant activity probably involved more than just the phenolics analyzed via HPLC. Secondly, in the HPLC analysis, there were large peaks detected but not identified. As is clear from the chromatogram shown in Fig. 3, some major peaks were not quantified due to the lack of a spectral match in the library of the 19 phenolics used. These unidentified peaks, possibly phenolic glycosides, could have antioxidant activity which contributed to the total antioxidant activity determined by the DPPH assay.
Since standard compounds were chosen based on what is known for cultivated potato, no novel compounds in the wild species were identified. Since there were peaks in the HPLC analysis that remained unidentified, it is possible that wild species could be a source of novel compounds not found in cultivated varieties. Although this is a possibility, any novel compounds were very minor constituents of the samples, since all major peaks were identified.
Phenolics were significantly higher in many accessions than in cultivated varieties. In particular, S. pinnatisectum and S. jamesii accessions consistently ranked among the highest in antioxidant activity and phenolic content; however, these species are not easily crossed with the cultivated S. tuberosum. Some populations of S. chacoense, S. megistracrolobum, S. microdontum, and S. spegazzinii were also high in antioxidant activity, and also more easily crossed to tuberosum, making them better candidates for incorporation into a breeding program aimed at increasing antioxidant activity. Promising accessions, along with their endosperm balance numbers, ploidy levels, and suitability for crossing with S. tuberosum are shown in Table 7. In cultivated varieties and advanced breeding lines analyzed at the same time as the wild accessions, antioxidant activity ranged from 104 to 648 μg trolox eq/gfw, while the averages of the wild species ranged in activity from 48 to 824 μg trolox eq/gfw (Hale 2003).
Since individual genotypes were analyzed for only six accessions (Group 3b), recommendations for future breeding efforts are based on accessions which were composed of a bulk of multiple genotypes. Many accessions were higher in antioxidant activity than the highest of the cultivated genotypes. Over half of the accessions analyzed for phenolic content by HPLC were higher in individual compounds than were their cultivated counterparts. In addition, more compounds were identified in the wild species than were identified in the cultivated genotypes (Hale 2003). Promising accessions for use in breeding for enhanced phytochemical levels are listed in Table 7. These accessions were chosen based on their antioxidant activity as well as levels of individual phenolic components.
In conclusion, several tuber-bearing Solanum species were identified with as much as five-fold higher antioxidant activity than that found in currently available cultivated varieties (Hale 2003). Phenolics such as chlorogenic acid, caffeic acid and, to a much lesser degree, others play an important role in contributing to the antioxidant activity found in these wild species. Solanum pinnatisectum and S. jamesii accessions consistently ranked highest in antioxidant activity and phenolic content. Breeding strategies must be developed which provide for the efficient incorporation of beneficial phytochemicals found in wild species into cultivated genotypes.
References
Al-Saikhan, M.S. 2000. Antioxidants, Proteins, and Carotenoids in Potato (Solanum tuberosum, L.). Ph.D. dissertation. College Station, TX: Texas A&M University.
Al-Saikhan, M.S., L.R. Howard, and J.C. Miller Jr. 1995. Antioxidant activity and total phenolics in different genotypes of potato (Solanun tuberosum, L.). Journal of Food Science 602: 341–343.
Arai, Y., S. Wantanabe, M. Kimira, K. Shimoi, R. Mochizuki, and N. Kinae. 2000. Dietary intakes of flavonols, flavones, and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. Journal of Nutrition 130: 2243–2250.
Bamberg, J.B. 1995. Screening potato (Solanum) species for male fertility under heat stress. American Potato Journal 721: 23–33.
Bamberg, J.B., J.P. Palta, L.A. Peterson, M. Martin, and A.R. Krueger. 1993. Screening tuber-bearing Solanum (potato) germplasm for efficient accumulation of tuber calcium. American Potato Journal 70: 219–226.
Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft + Technologie 28: 25–30.
Dao, L., and M. Freidman. 1992. Chlorogenic acid content of fresh and processed potatoes determined by ultraviolet spectrophotometry. Journal of Agricultural and Food Chemistry 40: 2152–2156.
Errebhi, M., C.J. Rosen, F.I. Lauer, M.W. Martin, J.B. Bamberg, and D.E. Birong. 1998. Screening of exotic potato germplasm for nitrogen uptake and biomass production. American Journal of Potato Research 75: 93–100.
Freidman, M. 1997. Chemistry, biochemistry, and dietary role of potato polyphenols. A review. Journal of Agricultural and Food Chemistry 45: 1523–1540.
Gazzani, G., A. Papetti, M. Daglia, F. Berte, and C. Gregotti. 1998. Protective activity of water soluble components of some common diet vegetables on rat liver microsome and the effect of thermal treatment. Journal of Agricultural and Food Chemistry 46: 4123–4127.
Hale, A.L. 2003. Screening Potato Genotypes for Antioxidant Activity, Identification of the Responsible Compounds, and Differentiating Russet Norkotah Strains Using AFLP and Microsatellite Marker Analysis. Ph.D. dissertation. College Station, TX: Texas A&M University.
Kolasa, K.M. 1993. The potato and human nutrition. American Potato Journal 70: 375–384.
Lachman, J., K. Hamouz, M. Orsak, and V. Pivee. 2000. Potato tubers as a significant source of antioxidants in human nutrition. Rostlinná Výroba 46: 231–236.
Lewis, C.E., J.R.L. Walker, J.E. Lancaster, and K.H. Sutton. 1998a. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. II: Wild, tuberous Solanum species. Journal of the Science of Food and Agriculture 77: 58–63.
Lewis, C.E., J.R.L. Walker, J.E. Lancaster, and K.H. Sutton. 1998b. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I. Coloured cultivars of Solanum tuberosum L. Journal of the Science of Food and Agriculture 77: 45–57.
National Potato Council. 2006–2007. Potato Statistical Yearbook. Washington, DC: National Potato Council.
North American Blueberry Council, 2005. Accessed at http://www.blueberry.org.
Prior, R.L., X. Wu, and K. Schaich. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry 53: 4290–4302.
Reddivari, L., A.L. Hale, and J.C. Miller Jr. 2007. Determination of phenolic content, composition and their contribution to antioxidant activity in specialty potato selections. American Journal of Potato Research 84: 275–282.
Rodriguez de Sotillo, D., M. Hadley, and E.T. Holm. 1994. Phenolics in aqueous potato peel extract: Extraction, identification, and degradation. Journal of Food Science 59: 649–651.
Rodriguez de Sotillo, D., M. Hadley, and C. Wolf-Hall. 1998. Potato peel extract a non mutagenic antioxidant with potential antimicrobial activity. Journal of Food Science 63: 907–910.
SAS. 2000. SAS Software Version 9.0. Cary, NC: SAS Institute.
Tudela, J.A., E. Cantos, J.C. Espin, F.A. Tomas-Barberan, and M.I. Gil. 2002. Induction of antioxidant flavanol biosynthesis in fresh-cut potatoes. Effect of domestic cooking. Journal of Agricultural and Food Chemistry 50: 5925–5931.
Yamamoto, I., K. Takano, H. Sato, I. Kamoi, and T. Miamoto. 1997. Natural toxic substances polyphenols, limolene, and allyl isothiocyanate in several edible crops. Tokyo Nogyo Daigaku Nogaku Shuho 41: 239–245.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hale, A.L., Reddivari, L., Nzaramba, M.N. et al. Interspecific Variability for Antioxidant Activity and Phenolic Content Among Solanum Species. Am. J. Pot Res 85, 332–341 (2008). https://doi.org/10.1007/s12230-008-9035-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-008-9035-1