Abstract
Based on mathematical modelling, this review article describes the mechanism of expansion in the circumference of vascular cambium due to radial growth leading to increase in the tree-trunk diameter, and emphasizes upon the huge difference in the rate of symplastic growth of cambial initials in two different directions, viz. radial and circumferential. On the basis of anatomical evidence regarding the role of symplastic and intrusive growths, the long-standing hypothesis that the intrusive growth contributes to the increase of cambium circumference has been falsified. It has been shown with the help of mathematical calculations as well as anatomical observations that only symplastic growth of the initial cells (in circumferential direction) is responsible for the expansion of cambial circumference.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As early as in 1923, Bailey stated that the initial cells of the vascular cambium elongate and divide, causing an increase in the circumference of the cambial cylinder. This type of cell growth resulting in elongation of the cambial initials was later termed as intrusive growth (Sinnott & Bloch, 1939; Majumdar, 1941). That this intrusive growth normally follows anticlinal divisions of fusiform cambial initials and leads to an increase in the cambial circumference of woody plants is a common belief that has been repeatedly mentioned in the literature (e.g. Philipson et al., 1971; Mauseth, 1988; Harris, 1989; Romberger et al., 1993; Larson, 1994) and continues to persist in the recent works (Dickison, 2000; Evert, 2006; Carlquist, 2010; Hejnowicz, 2012; Schmitt et al., 2016). The role of intrusive growth in increasing the cambial circumference is normally described with reference to non-storied cambium, which consists of relatively long fusiform initials (Evert, 2006; Hejnowicz, 2012). It is believed that the increase in the cambial circumference is achieved through apical intrusive growth of the anticlinally divided initial between the radial (anticlinal) walls of the adjacent initial cells. Such a growth is supposed to occur at the corners or edges of the initials (Hejnowicz & Zagórska-Marek, 1974). The intrusive growth of the initial cells supposedly follows a separation of radial walls of the adjacent cells (Hejnowicz, 1980), which involves lysis of middle lamella (Wenham & Cusick, 1975; Hejnowicz, 1980).
However, interpretation of anatomical changes in the cambial tissue (based on precise analysis of a series of thin (3 μm) transverse sections and the reconstruction of tangential shape of the neighbouring cambial cells) has revealed in the recent past that the elongating cells grow intrusively between the tangential (periclinal) walls rather than the radial walls of adjacent cells lying in front of the growing cell tips (Włoch & Połap, 1994; Włoch et al., 2001, 2002; Kojs et al., 2002, 2004a, 2004b; Jura et al., 2006; Karczewska et al., 2009; Włoch et al., 2009, 2013; Wilczek et al., 2018). This change in interpretation of the location of intrusive growth of cambial initials (from along the radial walls to the tangential walls) is not a minor issue, as it involves far-reaching consequences in relation to cambial functionality. For example, if the intrusive growth of cambial initials does not occur between the radial walls, it will have nothing to do with the increase in the circumference of the cambial cylinder. Some recent studies have confirmed that (1) the location of intrusive growth is different from one previously assumed, and hence (2) it does not contribute to the expansion of the cambial circumference (Karczewska et al., 2009; Włoch et al., 2009; Wilczek et al., 2018). This change in the interpretation of the location of intrusive growth also necessitates a reconsideration of the role of intrusive growth in relation to various cell events occurring on the initial surface of the cambium.
Since the latest studies have questioned several old assumptions regarding the cambial structure and function (Włoch et al., 2001; Kojs et al., 2002, 2004a, 2004b; Jura et al., 2006; Włoch et al., 2009, 2013), critical evaluation of reports on the exact location of intrusive growth becomes indispensable for a proper understanding of cell events on the initial surface of the cambium. Karczewska et al. (2009) and Wilczek et al. (2018) have described how cells grow in layers of the cambial cylinder and revealed that the growth of radial walls of the cambial cells is much faster than the growth of their tangential walls. Differences in growth rates between the radial and tangential walls of the cambial initials provide a deeper insight of the dynamics of symplastic growth of the cambial cells. Previous mathematical models described the cambial events as the events on a plane surface, and did not take into account the diverse rates of symplastic and intrusive growths of cambial initials in a three-dimensional space (Bannan, 1957; Hejnowicz, 1961, 1967). However, the actual role and significance of the cellular events of cambium can be explained only when the cylindrical surface of this meristem is taken into account for its mathematical modelling (Forest et al., 2004).
The present review aims at familiarizing the readers with the latest reports concerning the mode of cambial growth, with particular emphasis on the location of intrusive growth of fusiform cambial initials and, consequently, on the issue of contribution of this growth towards increase in the cambial circumference. This review also presents a mathematical analysis of intensity of the symplastic growth of cambial initials, keeping in view that this meristematic tissue, located between the secondary phloem and secondary xylem, has a cylindrical shape. This analysis allows to elucidate accurately the underlying principles of the radial growth in woody plants.
Symplastic and Intrusive Growths in the Cylindrical Cambium
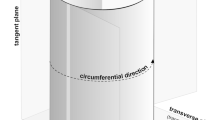
Let us first understand that the fusiform cambial initials may grow in three directions, viz. axial, radial and circumferential directions (Fig. 1). The circumferential direction has often been described as the tangential direction (Iqbal & Ghouse, 1990; Evert, 2006; Jura et al., 2006; Kwiatkowska & Nakielski, 2011; Wilczek et al., 2011b), which describes better the dimensions of individual cambial cells as observed in tangential or transverse sections (e.g. Włoch et al., 2009; Beck, 2010; Wilczek et al., 2011a; Włoch et al., 2013; see also Fig. 1). On the contrary, when the growth of the whole cambial tissue in a trunk or branch is analysed, the cylindrical shape of the tissue should be taken into consideration (e.g. Karczewska et al., 2009; Fig. 1), which necessitates the use of the term ‘circumferential direction’. Cambial cells grow intrusively as well as symplastically (Kojs et al., 2004b; Evert, 2006; Jura et al., 2006; Miodek et al., 2013). The difference between these two types of cell growth is that new cell contacts are created due to the intrusive growth, while the existing ones remain intact during the symplastic growth. In the case of intrusive growth, a part of the individual cell grows between the walls of adjacent cells (Beck, 2010), which results in the establishment of new cell contacts (Kojs et al., 2004b). This type of cell growth occurs in the circumferential and axial directions in a normally developed cylinder of vascular cambium (Wilczek et al., 2018; Fig. 2). Symplastic cell growth, on the other hand, refers to the growth wherein the walls of contiguous cells in a tissue grow simultaneously (Beck, 2010), thus, keeping the contacts between cells intact and unchanged. This kind of growth, in reference to a single cell, occurs mainly in radial and, to a much lesser extent, in circumferential direction (Wilczek et al., 2018).
Directions of intrusive and symplastic growths of cambial cells based on Wilczek et al. (2018). Intrusive growth concerns growth of a single cambial cell, which enlarges largely in axial and/or circumferential direction (white arrows). Symplastic growth in the cambial tissue results in the cell expansion mainly in radial and, to a lesser extent, in circumferential direction (black arrows). Cambial cells are shown above fragments of stems. Xy xylem, VC vascular cambium, Ph phloem
Circular Symmetric Growth of Initial Cells in the Cambial Cylinder
Before discussing the mode of cell growth in the vascular cambium, let us examine the mutual relationships of the component cells in this cylindrical meristem. Mathematical formula for the surface (S) of a cylinder is S = 2πrh (where r is radius; h is height of the cylinder; and π ≈ 3.14). Since the symplastic growth of the circular cambial tissue occurs in radial direction, and not in axial direction (Wilczek et al., 2018), the formula C = 2πr may be used to calculate the increase in the cambial circumference (C). The same formula may be applicable to the perimeter of the Earth (circumference of a sphere). In order to imagine and comprehend the growth dynamics of a single cambial initial cell more comfortably, let us use the analogy to a single cube of soil with edges 1 m in length, located on the perimeter of the Earth.
We know that the equatorial radius of the Earth is r = 6,378,140 m (6,378.14 km) and the equatorial circumference is C = 2πr = 40,054,719.20 m (40,054.72 km). First let us work out how much increase will accrue to the Earth’s radius (Δr), if 1 m is added (ΔC = 1 m) to the perimeter of the Earth (assuming the Earth as a geometrically spherical figure). Apparently, addition of 1 m to the perimeter of 40,054,719.20 m (over 40 million meters) is just negligible because Δr / r = ΔC / C, and hence the radius will increase only by 16 cm (Δr = ΔC × r / C = 1 m × 6,378,140 m / 40,054,719.20 m = 0.16 m = 16 cm). Addition of 1 m to the perimeter of the Earth will not make any perceptible difference if observed from a long distance, e.g. from a space station. However, it may be a noticeable change to a person standing on the Earth surface. Interestingly, according to the mathematical calculation, adding 1 m to the perimeter of an object of any size would increase its radial dimension (Δr) only by 16 cm. On the other hand, if we add a 1 m high layer of soil to the Earth’s surface, thus increasing the Earth’s radius by 1 m, the Earth’s circumference measured along the equator would increase by ΔC = 2π × Δr = 2 × 3.14 × 1 = 6.28 m, thus making the total circumference equal to 40,054,719.20 m + 6.28 m = 40,054,725.48 m. In other words, the Earth’s circumference was composed of 40,054,719.20 sections of 1 m each. Now, in order to determine the increase in the width of each of these 1 m sections, the added 6.28 m would have to be divided by 40,054,719.20 m (given that the C / ΔC = r / Δr). This will reveal that each 1 m section of the altered Earth’s circumference has increased only by ΔC1 = 0.16 μm, which is only a 6,250,000th part of a meter. In conclusion, every single 1 m cube constituting the 40,054,719.20 m Earth’s perimeter (around the equator) if enlarged by 1 m in a radial direction, would grow only by ΔC1 = 0.16 μm in circumferential direction. The growth in circumferential direction is therefore negligible in comparison to one in the radial direction, as the former is Δr / ΔC1 = 1,000,000 μm/0.16 μm = 6,250,000 times smaller than the latter. In simple words, the increase in circumferential direction of 1 m cube would be more than 6 million times smaller than the increase in radial direction. This is an important realization for understanding the huge difference between growth rates of tangential versus radial walls of a single cambial (initial) cell.
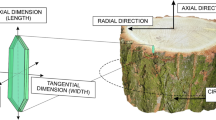
Let us make analogous calculations for the vascular cambium. As a regular tissue, the cambium of trees forms a cylinder composed of the initial cells, which do not grow symplastically in height, but grow in thickness through successive divisions and subsequent expansion (Steeves & Sussex, 1989; Evert, 2006; Beck, 2010; Crang et al., 2018). Given this, the changes (ΔC) in the circumference (C = 2πr) of this cylinder can be calculated the same way. Cambial initials, having an average width of 20 μm and thickness of 10 μm (5 μm right after periclinal division) in the majority of dicotyledonous trees (Esau, 1965; Iqbal, 1994; Larson, 1994; Iqbal, 1995; Dickison, 2000; Kojs et al., 2004b, Evert, 2006) as seen in transverse sections (Fig. 3), are arranged on the perimeter of the cambial cylinder in mature tree-trunks analogous to 1 m cubes of soil around the equator of the Earth. Every initial cell divides periclinally, i.e. tangential to the surface of the trunk, and gives rise to secondary xylem and secondary phloem cells (Beck, 2010; Crang et al., 2018; Miodek et al., 2020). A cambial circumference of 1 m (1,000,000 μm), would include as many as 50,000 cells of an average width of 20 μm (i.e. N = 1,000,000 μm / 20 μm = 50,000).
Transverse section showing vascular cambium of Robinia pseudoacacia L. located between derivative tissues (secondary phloem and secondary xylem). Probable locations of initial cells are marked by asterisks. Note that cells in the initial layer of the cambium maintain mutual contacts. ri – radial dimension of initial cell; Ci – part of circumference of cambial cylinder per one initial (i.e. tangential dimension of initial cell); xy xylem, ph phloem, r ray
Let us assume that a tree growing in the temperate zone forms annual rings of secondary xylem (wood) measuring about 3 mm (3,000 μm) in thickness. Microscopic studies of transverse sections have revealed that the differentiating wood cells of axial system (excluding vessel elements) have, on an average, a width and a thickness of 20 μm each (Wilczek et al., 2018). It can therefore be approximated that within the annual ring, about 150 wood cells (3,000 μm / 20 μm = 150) can be deposited in a single radial row. This annual increment of secondary tissue, comprising 150 layers of wood cells, increases the cambial circumference by approx. 18,840 μm (ΔC = 2π × Δr = 6.28 × 3,000 μm = 18,840 μm). Therefore, in this freshly enlarged circumference of the cambial cylinder, 942 new initial cells (18,840 μm / 20 μm = 942) would be deposited. If the 1 m cambial circumference initially comprised 50,000 cells, it implies that over a period of one growing season, every 53rd (50,000 / 942 = 53.08) initial cell needs to divide anticlinally into two sister cells (which later grow to the average width of the mother cell in accordance with the laws of circular symmetry). It also means that in the cambial cylinder in question, almost 8,000 (50,000 × 150 / 942 = 7,961.78) new periclinal divisions would be added per each anticlinal division, and that on an average only 6,28 initials divide anticlinally in each new layer of cells (942 / 150 = 6,28). But is this true in reality? Not exactly; the radial growth of the cambium generates mechanical stresses which are relaxed by the dynamic cambial cell readjustments accompanied by numerous anticlinal divisions and intrusive growth of the cambial initials (Włoch et al., 2013). But these excessive anticlinal divisions connected with the cambial cell readjustments (not with the growth of cambial circumference) are the source of confusing information and misinterpretation about the role of both, excessive anticlinal cell divisions and intrusive growth, in connection with increase of the cambial circumference. This aspect will be discussed ahead.
As mentioned earlier, an average cambial initial cell is approx. 10 μm thick and resembles a flattened rectangle (when observed in transverse section). As a consequence of each periclinal division of the initial cell, two cells of a thickness of nearly 5 μm are formed. They subsequently grow to the normal size of an initial cell before the occurrence of the next periclinal division (Fig. 4). This makes it easy to calculate the extent of the increase caused to a cambial circumference of 1 m due to addition of one cell layer on the inner side of the cambium. Thus, when a new layer of cells (daughter cells) is added to the cambial radius on its inner side (xylem side) as a result of a periclinal division of the initials, and the newly produced cells grow symplastically in radial direction by 10 μm, the resulting increase in the cambial circumference (ΔCL = C × Δr / r = 1,000,000 μm × 10 μm / 159,235.67 μm = 62.80 μm) is equivalent to the width of approximately three initial cells of 20 μm each (as 62.80 μm / 20 μm = 3.14). It means that even if only three initial cells out of a total of 50,000 undergo anticlinal division, followed by the symplastic growth of the two sister cells to attain the width of the mother cell (20 μm), it would be enough to harmonize this radial increase of 10 μm (CL / ΔCL = r / Δr = 1,000,000 μm / 62.80 μm = 159,235.67 μm / 10 μm). In other words, occurrence of a single anticlinal division per 15,923.57 cells (50,000 / 3.14 = 15,923.57) would be sufficient. However, if only those anticlinal divisions (followed by symplastic growth in circumferential direction) connected with the process of increase of cambial circumference had occurred, it would have been almost impossible to observe anticlinal divisions in a small sector of the cambium covered by a microscopic field. However, several anticlinal divisions per microscopic field are commonly observed in the cambium of most of the plant species during the active period of cambial growth (Wilczek, 2012; Miczajka et al., 2014).
Diagram showing a periclinal division of an initial cell of vascular cambium, and the subsequent radial growth of initial cell as well as the daughter cell. An average cambial initial cell is approx. 10 μm thick, 20 μm wide, and resembles a flattened rectangle when observed in transverse section. As a result of the periclinal division of the initial cell, two derivative cells (new initial and daughter cell) of a thickness of 5 μm are formed. They grow radially to attain the original size (10 μm) of the initial cell, i.e. the size before the division
Knowing that during the course of radial growth of a new single layer of cambial cells, a one-meter circumference of the cambial initial layer (composed of 50,000 cells, each with nearly 20 μm tangential width) will increase only by ΔCL = 62.80 μm, we can determine the extent of tangential expansion (in circumferential direction) of each component cell by dividing the increase in one-meter circumference (ΔCL) by the total number of initial cells (N) on the cambial circumference (i.e. 62.80 μm / 50,000 cells = 0.001256 μm). The width of a single initial cell would grow only by 0.001256 μm, which is far less than the thickness of the radial wall of the cambial cell, which is approx. 1 μm. However, if we consider that an initial cell, after each periclinal division, grows in radial direction only by Δr = 5 μm, it comes out that the increase in its width would be limited to ΔCi = Ci × Δr / r = 20 μm × 5 μm / 159,235.67 μm = 0.000628 μm, which is half of the previous value (0.001256 / 2 = 0.000628). Thereafter, comparing the growth of an initial cell in radial direction (Δr = 5 μm) with the relative growth in circumference (ΔCi = 0.000628 μm) reveals that the growth of this cell in the radial direction is approx. 8,000 times greater than in the circumferential direction (Δr / ΔCi = 5 μm / 0.000628 μm = 7,961.78). This means that after the deposition of one layer of derivatives, change of tangential dimension of each cell is negligible in comparison to the corresponding increase in its radial dimension. This information is summarized in Fig. 5.
Increase of tangential dimension of an initial cell (0.000628 μm) visualized in comparison to the thickness of radial cell wall (1 μm). Such increase of tangential dimension of an initial cell relates to initial, which grows 5 μm in radial direction in cambial tissue of 1 m circumference. CI – cambial initial
Let us see how often during the growing season in the temperate zone an average cambial initial undergoes periclinal division in a tree-trunk of 1 m circumference. As already stated, an increment of 150 wood cells accrues in every radial row in a 3 mm thick annual ring. Usually, after the division of an initial cell on the wood side, a four-cell complex – called Sanio’s four – is formed because each derivative cell divides twice (Mahmood, 1968). This means that during the growing season in the temperate zone each initial cell divides about 38 times (150 / 4 = 37.50). Since cells divide periclinally for about 4 months (120 days), each initial cell divides, on an average, every 3rd day (120 / 37.50 = 3.2 ≈ 3). In other words, in a given radial row on the wood side of the cambium, 1.25 cells (150 / 120 = 1.25), on an average, are deposited per day. Since each cell on the wood side is supposed to grow to a thickness of 20 μm, their mother cambial initial is supposed to be pushed away from the pith by about 12.50 μm (1.25 × 10 μm = 12.50 μm) in radial direction, thus causing a centrifugal dislocation of the cambial initial layer, described as an ‘outward growth’ of the cambium (Karczewska et al., 2009). Moreover, as a consequence of their own growth and the growth of their immediate derivatives (formed as a result of periclinal division), new initial cells move in radial direction by 12.50 μm (1.25 × 10 μm = 12.50 μm) per day. According to these calculations, the girth of the cambial cylinder will increase by ΔC = 2πΔr = 6.28 × 25 μm = 157 μm per day. Therefore, for a radius increase (Δr) of 25 μm, the tangential dimension of a single initial cell in a tree-trunk with cambial circumference of 1 m would increase by merely 0.00314 μm (157 μm / 50,000 = 0.00314 μm). Thus, the extent of radial growth of a cell is 7,961.78 times (25 μm / 0.00314 μm = 7,961.78) larger in comparison to its tangential growth. Therefore, this number (7,961.78) is the same as the one obtained with Δr = 5 μm and ΔCi = 0.000628 μm, which further confirmed that the growth of an initial cell is 7,961.78 times greater (Δr / ΔCi = 7,961.78) in the radial direction than in the circumferential direction.
In short, a single cell of the vascular cambium with a circumference of 1 m grows approx. 8,000 times faster in the radial than in the circumferential direction. This fact was hardly ever taken into account (Karczewska et al., 2009; Wilczek et al., 2018) in earlier studies focusing on the radial growth of cambium, despite its great importance in understanding the role of cellular events in this meristematic tissue. Many researchers have mistakenly thought of a larger expansion of cambial initial cells in circumferential direction – perhaps due to the peculiar shape of these cells, which in anatomical cross-sections appear as radially flattened rectangle, with their longer side being parallel to the circumferential direction. Such an assumption leads to an erroneous concept of the cambial growth. Moreover, cell growth and cellular events are usually studied in small anatomical sections, and the fact that the cambium has a geometrical shape, which could be approximated to a cylinder, is often ignored. In order to have a clear understanding of the cambial cell dynamics and the significance of different cellular events on the initial surface, it is also essential to discuss the negligible role of intrusive growth and elimination of initial cells with reference to the expansion of cambial circumference.
Evidence from Anatomical Analyses
Development of a hypothesis of circular symmetrical growth of cylindrical tissue for describing the growth of cambial initial cells requires incorporation of a circle-circumference equation (Karczewska et al., 2009; Wilczek et al., 2018). The model of symplastic growth of the cambial tissue has shown that the cambial cell in a single file grows almost entirely anisotropically (unidirectionally) in radial direction, and thus (considering the relatively small increase in width, i.e., the tangential dimension of a single cambial cell) there seems to be no need for so frequent anticlinal divisions as are actually observed in the microscopic studies of the cambium (Karczewska et al., 2009). It is noteworthy that according to the mathematical model of symplastic growth of vascular cambium, only a few anticlinal divisions, together with the subsequent symplastic growth (in circumferential direction) of the newly produced sister cells plus all other initials constituting the cambial circumference, are sufficient enough to cope with the increase of the cambial circumference.
Also, variation in the frequency of anticlinal divisions does not affect the rate of increase of the cambial circumference and the average width of the initial cells. In other words, anticlinal divisions per se do not contribute to the increase of the cambial circumference, because the total tangential dimension of two sister cells is the same as the tangential dimension of the mother initial cell before the occurrence of anticlinal division. Therefore, the enlargement of cambial cylinder may be considered to be due only to a minute gradual expansion of tangential dimensions of initial cells (both divided and undivided cells) through symplastic growth. In the cambium of perennial stems, the average cell width undergoes minor changes only (Kojs et al., 2004a). Symplastic enlargement of the cambial cylinder can be traced easily by comparing the tangential dimensions of cambial cells depositing in juvenile and mature woods.
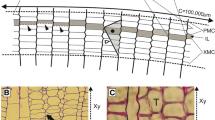
That a substantial contribution to the enlargement of cambial circumference is made by the symplastic growth of the cambial initials was substantiated, inter alia, by the study of Laburnum anagyroides (Włoch et al., 2009). Recent studies have demonstrated that the intrusive growth of elongating initial cells takes place between the tangential walls of adjacent initial cells and their immediate derivatives (Włoch et al., 2001; Kojs et al., 2002, 2004a, 2004b; Jura et al., 2006; Włoch et al., 2009, 2013). Figure 6 shows that despite the intrusive growth of a cambial initial CI, the neighbouring initial cells, between which the intrusive growth is taking place, do not move apart and their total tangential dimension does not increase. Tangential walls of the initial cell a (adjacent to the intrusively growing cambial initial), and its immediate derivative b are partially separated. Jura et al. (2006) demonstrated that the separated fragments of the tangential walls transform into oblique walls, which later appear as the radial walls in the derivative cells progressively formed due to successive unequal periclinal divisions.
Intrusive growth of cambial initial cell seen in transverse section of Pinus sylvestris L. The intrusively growing initial cell CI, as well as its derivatives (resulting from periclinal divisions) are marked in white. Intrusive growth of the initial cell occurred between tangential walls of neighboring initial cell a and its immediate derivative b deposited on phloem side. Files of cells on the left and right sides of the initial CI did not move apart within the initial layer despite its intensive intrusive growth. Note that parts of tangential walls of cells a and b were transformed into oblique walls. More details in the text. Cells belonging to eliminated radial file are indicated by black stars. CI – cambial initial cell. a – initial cell in radial file on the left side of initial CI. b – derivative cell of initial cell a deposited on phloem side. Xy xylem, Ph phloem
No effect of intrusive growth of the initials could be observed on the increase of cambial circumference (i.e. the tangential dimension of the analysed fragment of tissue) of the non-storied {e.g., Pinus sylvestris (Jura et al., 2006; Włoch et al., 2013; see Fig. 7)} or storied {e.g., Tilia cordata (Włoch & Połap, 1994), Wisteria floribunda (Jura et al., 2006) and Laburnum anagyroides (Włoch et al., 2009)} vascular cambium. These findings, therefore, negate the existence of two different patterns of circumferential growth of the cambium described in most of the textbooks and research papers: (1) the non-storied cambium grows through oblique anticlinal divisions, followed by an apical intrusive growth of the sister cells produced, and (2) the storied cambium grows through radial anticlinal divisions followed by a tangential enlargement of the sister cells produced (e.g. Larson, 1994; Evert, 2006; Beck, 2010).
Intrusive growth of cambial initial cells seen in tangential section of Pinus sylvestris L. a initial surface before intrusive growth of cambial initial marked as CI. b initial surface after intrusive growth of initial cell CI. This scheme represents a hypothetical arrangement of cells corresponding to the situation seen in Fig. 6 (position of transverse plane shown in Fig. 6 was indicated by thick, horizontal line). c superimposed tangential sections a and b. Intrusively growing part of initial cell CI has been hatched. Note that initial cells on the left and right sides of initial CI had not moved apart, despite its intrusive growth. Arrows indicate directions of intrusive growth of initials
The latest research on intrusive growth of cambial cells (Kojs et al., 2004a, 2004b; Jura et al., 2006; Włoch et al., 2009, 2013) confirm a fundamental role of intrusive growth in the rearrangement of cells on the so-called initial surface (Włoch & Połap, 1994; Włoch et al., 2013). It may be noted that intrusive growth of the initial cell CI between tangential walls of the initial cell a and its immediate derivative b begins the process of displacement of the initial cell a from the initial surface (Fig. 6). Therefore, the initial cell a would be replaced by another initial cell (CI). This phenomenon could be interpreted as a competition for space on the initial surface. However, it seems to be a passive process, in which the radial tensile stress operating in the vascular cambium plays the main role (Hejnowicz, 1980). Under this stress, the tangential walls of initial cells and their immediate derivatives would be separated, and the neighbouring initial cells could grow into the created microspaces (Kojs, 2012; Wilczek et al., 2018). When a growing initial cell succeeds in replacing the other initial cell, the replaced cambial cell loses the status of being an initial. This process taking place on the initial surface involves a gradual transformation of tangential walls into radial ones, giving rise to the characteristic slants that are visible in transverse sections (Jura et al., 2006). It may be noted that in the vast majority of initials, after the occurrence of anticlinal division, one of the formed sister cells loses its status of being the initial cell due to the intrusive growth of neighbouring cells as well as its own successive unequal periclinal divisions (Włoch & Połap, 1994; Włoch et al., 2001; Kojs et al., 2002, 2004a, 2004b; Jura et al., 2006; Włoch et al., 2009, 2013).
As mentioned earlier, until recently it was a common concept that multiplicative (anticlinal) divisions produce initial cells, which are responsible for the increase in circumference of the vascular cambium (Carlquist, 2010; Beck, 2010). The multiplicative activity of initial cells in stems during a period ranging from few to many years of growth has been shown typically through diagrams of transverse sections of xylem (Bannan, 1950, 1960; Evert, 1961). Interestingly, the number of anticlinal divisions observed in a non-storied cambium (characterised by oblique/pseudo-transverse anticlinal divisions) is usually in excess (Bannan, 1960), i.e., the number of initial cells formed as a result of anticlinal divisions is far greater than the requirement to cope with the circumferential growth of the cambial cylinder. We should remember that the newly formed initials may be displaced from the initial surface by the adjoining initials growing intrusively (Kojs et al., 2004a, 2004b; Jura et al., 2006). When the rate of radial growth of the cambium is low, and the number of multiplicative divisions is far greater, even up to 100% of the added initial cells lose their initial cell status (Evert, 1961, 2006). At this point, a question arises about the role of intrusive growth in the vascular cambium, especially in the non-storied cambium, which is characterized by a large number of oblique anticlinal divisions. Typically, the observed high intensity of intrusive growth in the non-storied cambium entails a change in the arrangement (inclination/orientation) of cambial cells (Hejnowicz & Brański, 1966; Włoch, 1976; Hejnowicz, 2012), which may be related to relaxation of shear stresses (Włoch et al., 2013). As suggested by Włoch et al. (2013), intrusive growth of cells in the cambium helps in readjustment of initial cells in accordance with the direction of shearing stresses. In storied cambium, where anticlinal divisions occur much less frequently than in non-storied cambium, such stresses are relaxed quickly by means of intrusive growth of the initial cells at the borders of storeys (Kojs, 2012; Włoch et al., 2013). Thus, the frequency of radial longitudinal divisions, together with the symplastic growth of sister cells in circumferential direction is adequate for the required increase in the cambial circumference (Cumbie, 1984; W. Włoch, unpubl.). The extent of intrusive growth of initial cells in the storied cambium is not related to the frequency of anticlinal divisions. Rearrangement of cells – due to intrusive growth – leads to the formation of various types of wood grain, e.g. spiral grain in the case of non-storied cambia, and interlocked grain in storied cambia (Włoch et al., 2002; Evert, 2006). In trees of tropical rainforests, quick rearrangement of double-storied cambium (where both fusiform initials as well as rays are storied) allows to form interlocked grain, which enhances the mechanical strength of tree-trunks; such trees may grow fast in height (with a simultaneous, relatively slow growth in thickness), and win the strong competition for light (Kojs et al., 2002, 2003, 2004b; Wilczek et al., 2014). Recent studies have shown that intrusive growth also participates in the development of storied structure of cambium – intrusive growth of chosen initials in the initially non-storied cambium leads to the formation of continuous borders of stories of fusiform cells (Kojs et al., 2004a; Wilczek, 2012; Miczajka et al., 2014).
Conclusion
Taking into account the mathematical model of symplastic growth of the cambial cylinder, and the results of the latest anatomical studies, it may be concluded that intrusive growth of cambial initials does not play any role in the growth of cambial circumference. Mathematical analysis shows that symplastic growth of a single initial cell is overwhelmingly larger in the radial than in the circumferential direction, and the expansion of the circumference of cambial cylinder could be explained simply by the synchronous symplastic growth of initials in circumferential direction. The notion that intrusive growth of initial cells between radial walls is the mechanism of growth of cambial circumference is incorrect, although it still continues to exist in the literature. Recent studies have not only falsified the hypothesis of intrusive growth mechanism for increase in the cambial circumference, but also provided strong arguments for localization of intrusive growth of the initial cells between tangential walls of the neighbouring initials and their immediate derivatives.
Abbreviations
- S:
-
Surface of a cylinder (S = 2πrh)
- h:
-
Height of cambial cylinder
- C:
-
Circumference of cambial cylinder (C = 2πr)
- Ci :
-
Tangential dimension of initial cell (part of cambial circumference occupied by one initial).
- ΔC:
-
Change in circumference
- ΔCL :
-
Increase in circumference of layer of cambial initials after addition of one cell layer on xylem side (ΔCL = 2πΔrL)
- ΔC1 :
-
Increase in the Earth’s perimeter after addition of 1 m to its radius
- ΔCi :
-
Increase in cambial circumference per one fusiform initial (ΔCi = ΔCL / N)
- N:
-
Number of cambial initial cells on the cambial circumference
- r:
-
Radius
- ri :
-
Radial dimension of one initial cell
- Δr:
-
Change in radius
- ΔrL :
-
Change in radius of cambial cylinder caused by the deposition of one cell layer on xylem side
References
Bailey, I. W. 1923. The cambium and its derivative tissues IV. The increase in girth of the cambium. American Journal of Botany 10: 499–509.
Bannan, M. W. 1950. The frequency of anticlinal divisions in fusiform cambial cells of Chamaecyparis. American Journal of Botany 37: 511–519.
Bannan, M. W. 1957. The relative frequency of the different types of anticlinal divisions in conifer cambium. Canadian Journal of Botany 35: 875–884.
Bannan, M. W. 1960. Cambial behavior with reference to cell length and ring width in Thuja occidentalis L. Canadian Journal of Botany 38: 177–183.
Beck, C. B. 2010. An introduction to plant structure and development. Cambridge University Press, Cambridge.
Carlquist, S. 2010. Comparative wood anatomy. Systematic, ecological, and evolutionary aspects of dicotyledon wood. Springer-Verlag, Germany.
Crang, R., S. Lyons-Sobaski & R. Wise. 2018. Plant Anatomy: a concept-based approach to the structure of seed plants. Springer Nature Switzerland AG. Cham.
Cumbie, B. G. 1984. Origin and development of the vascular cambium in Aeschynomene virginica. Bulletin of the Torrey Botanical Club 111: 42–50.
Dickison, W. C. 2000. Integrative plant anatomy. Academic Press, San Diego.
Esau, K. 1965. Plant anatomy. Wiley, New York.
Evert, R. F. 1961. Some aspects of cambial development in Pyrus communis. American Journal of Botany 48: 479–488.
Evert, R. F. 2006. Esau’s plant anatomy: meristems, cells and tissues of the plant body: their structure, function, and development. Wiley, New Jersey.
Forest, L., J. San Martin, F. Padilla, F. Chassat, F. Giroud & J. Demongeot. 2004. Morphogenetic processes: application to cambial growth dynamics. Acta Biotheoretica 52: 415–438.
Harris, J. M. 1989. Spiral grain and wave phenomena in wood formation. Springer-Verlag, Berlin.
Hejnowicz, Z. 1961. Anticlinal divisions, intrusive growth, and loss of fusiform initials in nonstoried cambium. Acta Societatis Botanicorum Poloniae 30: 729–758.
Hejnowicz, Z. 1967. Interrelationship between mean length, rate of intrusive elongation, frequency of anticlinal divisions and survival of fusiform initials in cambium. Acta Societatis Botanicorum Poloniae 36: 367–378.
Hejnowicz, Z. 1980. Tensional stress in the cambium and its developmental significance. American Journal of Botany 67: 1–5.
Hejnowicz, Z. 2012. Anatomia i histogeneza roślin naczyniowych. Organy wegetatywne. PWN, Warszawa.
Hejnowicz, Z & S. Brański. 1966. Quantitative analysis of cambium growth in Thuja. Acta Societatis Botanicorum Poloniae 35: 395–400.
Hejnowicz, Z & B. Zagórska-Marek. 1974. Mechanism of changes in grain inclination in wood produced by storeyed cambium. Acta Societatis Botanicorum Poloniae 43: 381–398.
Iqbal, M. 1994. Structural and operational specializations of the vascular cambium of seed plants. Pp. 211–271. In: M. Iqbal (ed.), Growth patterns in vascular plants. Dioscorides Press, Portland.
Iqbal, M. 1995. Structure and behaviour of vascular cambium and the mechanism and control of cambial growth. Pp. 1–67. In: M. Iqbal (ed.), The cambial derivatives. Gebrüder Borntraeger, Stuttgart.
Iqbal, M & A. K. M. Ghouse. 1990. Cambial concept and organisation. Pp. 1–36. In: M. Iqbal (ed.), The vascular cambium. Research Studies Press/Wiley, Taunton.
Jura, J., P. Kojs, M. Iqbal, J. Szymanowska-Pułka & W. Włoch. 2006. Apical intrusive growth of cambial fusiform initials along the tangential walls of adjacent fusiform initials: evidence for a new concept. Australian Journal of Botany 54: 493–504.
Karczewska, D., J. Karczewski, W. Włoch, J. Jura-Morawiec, P. Kojs, M. Iqbal & J. Krawczyszyn. 2009. Mathematical modeling of intrusive growth of fusiform initials in relation to radial growth and expanding cambial circumference in Pinus sylvestris L. Acta Biotheoretica 57: 331–348.
Kojs, P. 2012. A qualitative model of symplastic and intrusive growth of the vascular cambium of broadleaved trees – a biomechanical perspective. (pp. 344–345). In: 7th Plant Biomechanics International Conference. Clermont-Ferrand.
Kojs, P, W. Włoch, A. Rusin, W. Szendera, Duda, J. J Jura & T. Rusin. 2002. Od niefunkcjonalnej do funkcjonalnej piętrowości kambium. Modele piętrowości. Biuletyn Ogrodów Botanicznych 11: 93–104.
Kojs, P, W. Włoch, A. Rusin & W. Szendera. 2003. Storeyed structure of cambium as an adaptive strategy to environmental conditions in trees forming the canopy and emergent layer of the tropical rain forests. Biuletyn Ogrodów Botanicznych 12: 23–29.
Kojs, P, A. Rusin, M. Iqbal, W. Włoch & J. Jura. 2004a. Readjustments of cambial initials in Wisteria floribunda (Willd.) DC for development of storeyed structure. New Phytologist 163: 287–297.
Kojs, P, W. Włoch & A. Rusin. 2004b. Rearrangement of cells in storeyed cambium of Lonchocarpus sericeus (Poir.) DC connected with formation of interlocked grain in the xylem. Trees 18: 136–144.
Kwiatkowska, D. & J. Nakielski. 2011. Mechanics of the meristems. Pp. 133–172. In: P. Wojtaszek (ed.), Mechanical integration of plant cells and plants. Springer, Berlin, Heidelberg.
Larson, P. R. 1994. The vascular cambium: development and structure. Springer–Verlag, Berlin.
Mahmood, A. 1968. Cell grouping and primary wall generations in the cambial zone, xylem, and phloem in Pinus. Australian Journal of Botany 16: 177–195.
Majumdar, G. P. 1941. The sliding, gliding, symplastic or the intrusive growth of the cambium cells and their derivatives in higher vascular plants. Journal of the Indian Botanical Society 20: 161–171.
Mauseth, J. D. 1988. Plant anatomy. The Blackburn Press, Caldwell.
Miczajka, S., A. Gizińska, A. Miodek & A. B. Wilczek. 2014. Adjustment of storied pattern of vascular cambium of Caragana arborescens Lam. branches. Nature Journal 47: 45–60.
Miodek, A., A. Gizińska, A. Wilczek & W. Włoch. 2013. Growth of wood fibers in circular-symmetrical annual xylem increment. In: Interdyscyplinarne i aplikacyjne znaczenie nauk botanicznych (pp. 114–115). Streszczenia wystąpień ustnych 56. Zjazdu Polskiego Towarzystwa Botanicznego. Olsztyn.
Miodek, A., A. Gizińska, M. Klisz, T. Wojda, K. Ukalski & P. Kojs. 2020. Direct exposure to solar radiation causes radial growth eccentricity at the beginning of the growing season in Robinia pseudoacacia. IAWA Journal 41: 61–84.
Philipson, W. R., J. M. Ward & B. G. Buterfield. 1971. The vascular cambium, its development and activity. Chapman and Hall Ltd., London.
Romberger, J. A., Z. Hejnowicz & J. F. Hill. 1993. Plant Structure: function and development. Springer-Verlag, Berlin.
Schmitt, U., G. Koch, D. Eckstein, J-W. Seo, P. Prislan, J. Gričar, K. Čufar, H. Stobbe & R. Jalkanen. 2016. The vascular cambium of trees and its involvement in defining xylem anatomy. Pp. 3–24. In: Y. S. Kim, R. Funada & A. P. Singh, (eds), Secondary xylem biology; origins, functions, and applications. Academic Press, Amsterdam.
Sinnott, E. W. & R. Bloch. 1939. Changes in intercellular relationships during the growth and differentiation of living plant tissues. American Journal of Botany 26: 625–634.
Steeves, T. A. & I. M. Sussex 1989. Patterns in plant development. Cambridge University Press, Cambridge.
Wenham, M. W. & F. Cusick. 1975. The growth of secondary wood fibres. New Phytologist 74: 247–261.
Wilczek, A. 2012. The formation of heterogeneous storeys in the cambium on the example of Laburnum anagyroides Medik. Acta Agrobotanica 65: 47–56.
Wilczek, A, J. Jura-Morawiec, P. Kojs, M. Iqbal & W. Włoch. 2011a. Correlation of intrusive growth of cambial initials to rearrangement of rays in the vascular cambium. IAWA Journal 32: 313–331.
Wilczek, A, W. Włoch, M. Iqbal & P. Kojs. 2011b. Position of rays and lateral deviation of vessel elements in the stem wood of some dicotyledonous species with storeyed, double-storeyed, and nonstoreyed cambia. Botany 89: 849–860.
Wilczek, A, Gizińska, A. A Miodek & W. Włoch. 2014. Nowa hipoteza wzrostu promieniowego i przebudowy kambium waskularnego roślin drzewiastych. Kosmos 63: 591–601.
Wilczek, A, M. Iqbal, W. Włoch & M. Klisz. 2018. Geometric analysis of intrusive growth of wood fibres in Robinia pseudoacacia. IAWA Journal 39: 191–208.
Włoch, W. 1976. Cell events in cambium, connected with the formation and existence of a whirled cell arrangement. Acta Societatis Botanicorum Poloniae 45: 313–326.
Włoch, W & E. Połap. 1994. The intrusive growth of initial cells in re-arrangement of cells in cambium of Tilia cordata Mill. Acta Societatis Botanicorum Poloniae 63: 109–116.
Włoch, W, E. Mazur & P. Kojs. 2001. Intensive change of inclination of cambial initials in Picea abies (L.) Karst. tumours. Trees 15: 498–502.
Włoch, W, E. Mazur & M. Bełtowski. 2002. Formation of spiral grain in the wood of Pinus sylvestris L. Trees 16: 306–312.
Włoch, W, J. Jura-Morawiec, P. Kojs, M. Iqbal & J. Krawczyszyn. 2009. Does intrusive growth of fusiform initials really contribute to circumferential growth of vascular cambium? Botany 87: 154–163.
Włoch, W, A. Wilczek, J. Jura-Morawiec, P. Kojs & M. Iqbal. 2013. Modelling for rearrangement of fusiform initials during radial growth of the vascular cambium in Pinus sylvestris L. Trees 27: 879–893.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miodek, A., Włoch, W., Iqbal, M. et al. Controversy over the Mode of Growth of Cambial Cylinder. Bot. Rev. 87, 243–257 (2021). https://doi.org/10.1007/s12229-020-09237-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-020-09237-9