Abstract
The history of cellular events in the storeyed cambium of Lonchocarpus sericeus (Poir.) DC was analysed on the basis of changes in the cell arrangement in successive layers and strata of axial parenchyma in the xylem. The mechanism of formation of the regular interlocked grain was investigated. Inclination of fusiform cells changes intensively whereas height and position of storeys in the successive layers of axial parenchyma are constant. As a result, new contacts between cells are formed by means of the intrusive growth of ends of cells belonging to one storey between the tangential walls of cells of the neighbouring storey and unequal periclinal divisions, which give a new shape to the initials. The concept of intrusive growth between the radial walls of the fusiform initials in the formation of xylem with interlocked grain should be revised on this basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on storeyed cambium date back to the beginning of the twentieth century. Initially, research on the structure of this tissue was focused not on the mechanisms of its formation or rearrangement, but on mechanisms of its maintenance during the increase of the cambial surface (Bailey 1920). It was concluded that maintenance of the storeyed structure of cambium was closely related to the longitudinal anticlinal divisions of the initial cell which produced two daughter initial cells of equal length (reviewed in Iqbal and Ghouse 1990), and to the lack of intrusive growth after the division (reviewed in Larson 1994). These concepts lasted until the 1970s, when studies on the storeyed structure of cambium were resumed in connection with the research on the domain pattern and wave-oscillatory phenomena in the cambium (Hejnowicz and Zagórska–Marek 1974; Zagórska–Marek 1975; Włoch and Zagórska-Marek 1982; Włoch and Bilczewska 1987).
The history of cambium is recorded in the cell arrangement of xylem and phloem cells; it may be reconstructed from a series of tangential sections (Hejnowicz 1961, 1964, 1968; Hejnowicz and Krawczyszyn 1969; Pyszyński 1972; Włoch 1976, 1985, 1987; Włoch et al. 2001, 2002). The continuous analysis of cellular events in the successive layers of xylem in broad-leaved trees is not possible due to the intrusive growth of fibres and vessel members, which heavily disturbs the original arrangement of the cambial cells. However, it is possible to analyse the history of cambium on the basis of changes of the cell arrangement in the successive layers and strata of axial parenchyma. Parenchymatous cells do not grow intrusively during differentiation and hence their arrangement reflects the arrangement of cells in the cambium at the moment of formation of the layer in question. The layer of terminal parenchyma is formed in the xylem of the most broad-leaved trees, especially in those living in temperate zones, at the boundaries of annual increments at the end of the season of cambial activity. The comparison of cellular events in the subsequent layers of terminal parenchyma allows the reconstruction of changes occurring during a certain period of life of a tree. Such an analysis is possible only when the derivatives of the same cambial cell in successive layers of axial parenchyma can be located. The changes of cellular arrangement in the annual increment are usually too large for a continuous reading of cell events in subsequent layers of parenchyma.
The arrangement of fusiform cells in tangential sections allows the distinction of two types of cambium: storeyed and nonstoreyed (Bailey 1920). The storeyed cambium is evolutionarily younger than the nonstoreyed one. Its fusiform cells are usually shorter than cells in nonstoreyed cambium, and arranged in a storeyed pattern. It is generally assumed that the difference in cell arrangement in the two types of cambium is a result of different ways of increasing the cambial surface (Beijer 1927; Butterfield 1972; Cumbie 1984). The increase of cambial surface in nonstoreyed cambium occurs due to anticlinal oblique divisions and the intrusive growth of ends of the shortened cells (Philipson et al. 1971; Romberger et al. 1993; Larson 1994). As for the storeyed cambium, its cells divide longitudinally and their intrusive growth is connected only with a change of their inclination (Hejnowicz and Zagórska-Marek 1974; Krawczyszyn 1977; Włoch and Połap 1994). Intrusion into another storey occurs occasionaly (Włoch and Zagórska–Marek 1982; Zagórska–Marek 1984).
Some woody plants growing in humid tropical zones and characterised by storeyed cambium periodically form layers or strata of axial parenchyma, as described for Entandrophragma cylindricum (Meliaceae) (Hejnowicz and Zagórska-Marek 1974; Zagórska-Marek 1975), Sterculia oblonga (Sterculiaceae) (Wagenführ and Scheiber 1974) and for Lonchocarpus sericeus (Fabaceae) (Kojs 2000). The last-mentioned species is investigated in this study.
Storeyed arrangement of fusiform cells may be accompanied by storeyed arrangement of rays. This type of cambium is described as double-storeyed (Romberger et al. 1993). The height of rays is usually smaller than the length of surrounding fusiform cells (Włoch and Szendera 1989).
Storeyed cambium is marked by the capability of intensive, cyclic changes of inclination of cells, which results in the interlocked grain in xylem (Krawczyszyn and Romberger 1979, 1980; Hejnowicz and Romberger 1979; Włoch 1987).
Anticlinal oblique divisions occur rarely in storeyed cambium. The rearrangement of cells is a result of the oriented intrusive growth at the apical and lateral radial edges of fusiform cells (Hejnowicz and Zagórska–Marek 1974; Włoch and Zagórska–Marek 1982; Zagórska–Marek 1984). The intrusive growth of cells on a given tangential surface of cambium occurs irregularly. On the basis of studies on the small-leaved linden ( Tilia cordata) and Entandrophragma cylindricum, it was concluded that growth activity of a certain cell end changes periodically. Groups of cell ends marked by growth activity can be distinguished on the cambial surface. These groups alternate with groups of inactive ends, and both groups form a pattern of growth activity (Włoch and Bilczewska 1987; Włoch 1988). Growth activity of fusiform initials in the cambium is described by (1) bifurcation of the cell ends and (2) range of displacement of cell end along the boundary of the storey in relation to the ends of cells of the neighbouring storey. Bifurcation of the cell ends represents on intermediate stage between two successive positions of the cell end at the boundary of the storey (Hejnowicz and Zagórska–Marek 1974; Romberger et al. 1993).
In the development of cambium it is possible to distinguish cell events (like anticlinal divisions, intrusive growth) occurring in one of two possible configurations: right Z and left S. Regions with cell events occurring in a certain configuration have been called domains. High frequency of cell events in the domains migrating along the stem causes a periodical change of inclination of cambial cells. Wavy grain of phloem and xylem is formed on the tangential surface (Hejnowicz 1964, 1975).
In many tropical trees, among them in Lonchocarpus sericeus, high frequency of cellular events allows for the formation of xylem with interlocked grain. The inclination of cells changes periodically in large areas of the subsequent layers of xylem, which reflect the fast migration of large domains in the cambium (Hejnowicz and Romberger 1973, 1979).
Recent observations concerning the mechanisms of formation of spiral arrangement of cells in Pinus sylvestris (Włoch et al. 2002) and the mechanisms of intensive changes of cell inclination in spruce tumour (Włoch et al. 2001) urge for testing the hypothesis concerning the rearrangement of cells in the cambium based on the intrusive radial growth.
The aim of this study is to find an answer to the question whether the change in orientation of fusiform initials of L. sericeus is connected with the intrusive growth of initial cell between radial walls or tangential walls.
Materials and methods
The material used in our studies was a piece of wood of Lonchocarpus sericeus (Poir.) DC of uncertain age, 12 cm long, 2 cm wide and 9 cm thick, with regular interlocked grain, obtained from the collection of wood samples in the Botanical Garden—Centre for Biological Diversity Conservation of the Polish Academy of Sciences (Warsaw).
A small sample of 7 mm ×5 mm ×4 mm, characterised by the fast reorientation of cells, was excised from this piece of wood. The sample was boiled in distilled water for 5 days, 5 h per day and left for at least 2 weeks in a mixture of ethanol and glycerol (3:1). In this mixture, samples may be stored for a long time. Serial tangential sections ca. 30 µm thick were prepared using a sliding microtome. Consecutive sections were attached to microscope slides with Haupt’s adhesive. The slides were boiled for 15 min in 98% ethanol and then put into cold ethanol several times in order to expel air from cell lumina. After three changes of xylene, specimens were embedded in Canada balsam without staining. In order to study the character of the processes occurring in the cambium of L. sericeus, a series of photographs of four subsequent layers of axial parenchyma was taken. For a better visualisation of cell events, drawings were made of the photographs. The derivatives of initial cambial cells in subsequent layers were identified and marked by numbers for better distinction. Semi-thin transverse sections were prepared as described earlier (Włoch et al. 2002).
Moreover, pieces of wood were fractured tangentially through the layer of axial parenchyma, stuck to the aluminium stub for a scanning electron microscope and coated with gold in a sputter coater (Pelco SC-6, Ted Pella, USA). The tangential surface of xylem parenchyma was observed in a TESLA BS 301 scanning electron microscope, equipped with digital image acquisition system.
Results
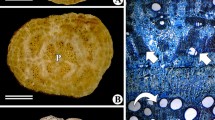
Lonchocarpus sericeus (Poir.) DC is a tree with a very regular, storeyed structure of cambium and interlocked grain in the xylem. In the studied sample, the changes of the grain inclination were observed eight times (four complete cycles of change of cell orientation). Xylem is diffuse-porous with paratracheal confluent parenchyma (Carlquist 1988). Light bands of axial parenchyma 3–15 cells wide are observed alternately with dark bands of fibres in the transverse section (Fig. 1). Vessels are solitary or in radial multiples of 2–3 (Fig. 2).
Transverse section of xylem of Lonchocarpus sericeus. Diffuse-porous xylem. Vessels are single or sometimes in radial multiples of 2–3. Paratracheal confluent parenchyma is well distinguished as light bands. Dark bands represent fibres
Transverse section of xylem of L. sericeus. Radial group of two vessel lumina. The boundary between storeys is without rays. When the section runs through the storey below or above the boundary, rays are clearly visible ( lateral part of the photograph)
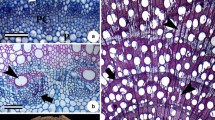
On the basis of the arrangement of parenchyma cells in the tangential section through the xylem it is possible to reconstruct the primary arrangement of the fusiform initials in the cambium at the time when a given layer of xylem was being deposited (Fig. 3). Fusiform initials divide transversely during differentiation of axial parenchyma, forming bi-cellular strands, reflecting the shape of the fusiform initials. Boundaries of storeys are recognised in the axial parenchyma on the basis of the remarkable zigzag outline of the overlapping ends of fusiform cells from two neighbouring storeys, widening of the cell ends and the pattern of rays. In the bands of axial parenchyma, the double storeyed arrangement is visible. Rays are usually triseriate; however, mono- and biseriate rays, as early stages of ray formation, also occur (unpublished data). Rays enclosed within storeys of fusiform cells do not reach the boundaries of the storeys. Fusiform cells usually have widened, club-like ends. The fusiform cells with bifurcated ends were not observed in axial parenchyma.
Tangential section through the xylem axial parenchyma of L. sericeus. The double storeyed arrangement comprises fusiform cells and rays enclosed within the storey. Each fusiform cell is divided transversely ( arrows)
Transverse section through the axial parenchyma of L. sericeus beyond the boundary between storeys. Radial cells are elongated ( asterisk). Fusiform cells are rounded and shifted in neighbouring files ( arrows). Intercellular spaces ( arrowheads) formed during differentiation of parenchymatous cells are visible
Transverse section through the boundary between storeys. Rays are lacking. Ends of fusiform cells belonging to neighbouring storeys are different in size ( white arrows)
In the transverse section through the axial parenchyma, half way along a storey, rounded fusiform cells occur between rays (Fig. 4), whereas at the boundary of the storeys rays are lacking and fusiform cells with different diameters are polygonal (Fig. 5).
Cells in a given radial file of axial parenchyma are derivatives of the same initial cell. On the basis of photographs of two successive tangential sections through one stratum of axial parenchyma the changes of local contacts between fusiform cells at the boundary of the storeys were observed. These changes arose during deposition of one layer of cells (Fig. 6A–D). Contacts between cells (cell numbers 3 and 11 and cell numbers 10 and 18), visible in one layer of parenchyma (Fig. 6A, B) are lost in the nearest layer; at the same time, new contacts between two other pairs of cells (2 and 12, and 9 and 19) are formed (Fig. 6C, D).
Comparison of two successive layers of one stratum of xylem axial parenchyma. A and C show tangential sections through two successive layers of axial parenchyma. Fusiform cells with ends that have changed their contacts at the boundary between storeys ( shaded). B and D are enlarged views of the boundary between storeys. Local change of contacts occurred between cells no. 3 and no. 11, and between cells no. 10 and no. 18, belonging to different storeys. In B+D superimposed drawing show the range of intrusive growth of fusiform initials. Arrows indicate orientation of intrusive growth
The observed changes of contacts between fusiform cells at the boundary of the storeys occur by the oriented intrusive growth of the cell end between tangential walls of cells in the neighbouring file. These changes of orientation of fusiform cells are possible due to their shifted arrangement in neighbouring radial files (Figs. 4, 5), which facilitates intrusive growth between tangential walls of cells of neighbouring radial files. Tile-like arrangement of fusiform cell ends at the boundary of the storeys also implies the intrusive growth between tangential walls (Fig. 7).
The result of this type of intrusive growth is the formation of club-like widenings of the cell ends. These can be easily distinguished in tangential sections (Figs. 3, 6) and transverse sections through the boundary between the storeys (Fig. 5).
Over a longer period of time, measured by the formation of successive strata of axial parenchyma, the oriented intrusive growth causes a change of inclination of the cell axes; at the same time the position of the middle parts of cells remains constant. Superimposition of the tangential sections from two successive strata of axial parenchyma, 0.7 mm from each other, allows for the observation of numerous changes of contacts between cell ends (Fig. 8). Due to high frequency of these events, all lower ends of the upper storey and upper ends of the lower storey are displaced in the opposite direction in relation to the other storey and new contacts between cells are established.
Comparison of cell arrangement in two layers ( A and B) from two successive strata of xylem parenchyma 0.7 mm away from each other. Rays are shaded. Cells from layer A are outlined with a dotted line. Rays and fusiform cells are numbered. A+B reveals the change of contacts between all cells belonging to neighbouring storeys
The described examples illustrate the way a change of contact occurs between fusiform cells at the boundary of storeys and reveal a high capability of changes in shape of fusiform cell ends (Fig. 9). Usually, growth activity at the ends of the same initial cell differs at the same moment: activity at one end is accompanied by a lack of activity at the other end (Fig. 10A+B II), it declines after a period of time and is followed by an increased activity at the other end (Fig. 10B+C II). However, simultaneous activity of both ends of the fusiform initial, lasting over the deposition of two strata of axial parenchyma, was also observed (Fig. 10B+C III, C+D III cell no. 1). The pattern of growth activity shows that over a long period of time all cells become active (Figs. 9A+D, 10A+D).
Maps of cell pattern from four consecutive strata of axial parenchyma ( A–D) serving for investigation of cambial activity. Rays are shaded. Fusiform initials and rays are numbered. Selected regions representing different growth activity in successive strata, are circled and numbered with Roman numerals I, II , III. In A+D, pictures of two most remote strata are superimposed. Despite intensive reorientation of cells, the position of boundaries between storeys is constant
Selected fragments of the maps with different growth activity of cambium, which are presented in Fig. 9, are drawn in columns I, II , III. Rays are shaded. Fusiform cells and rays are numbered. Growth activity of cell ends is marked with dots. GA growth activity according to an arbitrary scale: + low activity, ++ medium activity, +++ high activity. A+B, B+C , C+D and A+D shows the superimposition of cell arrangement in four successive strata of axial parenchyma: A, B , C , D ( dashed and continuous line). Dashed line marks the plate of longitudinal anticlinal division
The comparison of position of cells in two successive strata of parenchyma reveals that the growth activity on the initial surface of the cambium is not homogenous; it may occur in a single cell (Figs. 6, 10B+C III) or in groups of several cells (Fig. 10A+B I, B+C II), surrounded by regions of inactive cells (like in Fig. 6 or Fig. 10B+C I, C+D II, A+B III).
Discussion
Discovery of wave-oscillatory phenomena in cambium aroused interest in the investigation of mechanisms of cell reorientation in storeyed cambium (Hejnowicz and Zagórska–Marek 1974; Zagórska–Marek 1975; Hejnowicz 1975; Krawczyszyn and Romberger 1979). It seems very likely that the same processes, different in details, are the bases of cambial rearrangement in Tilia cordata, Entandrophragma cylindricum and Lonchocarpus sericeus. Up to the present, studies have shown occurence of intrusive growth of lateral radial edges of fusiform initials in the storeyed cambium, leading to the transitional bifurcation of a cell end and—as a consequence—to reorientation of the fusiform initials.
It is worth noting that intrusive growth of radial edges may take place only in the space between radial walls of neighbouring initial cells. This statement is in agreement with the current paradigm of the initial layer of cambium (Eames and MacDaniels 1947; Foster 1949; Committee on Nomenclature 1964; Esau 1965; Wilson et al. 1966; Brown 1971; Wodzicki and Brown 1973; Fahn 1974; Schmid 1976; Larson 1994).
The mentioned bifurcation of fusiform cell ends was recognized in Entandrophragma cylindricum and Tilia cordata as a transitional stage in the formation of new contacts between cambial cells (Hejnowicz and Zagórska-Marek 1974; Zagórska–Marek 1975; Włoch and Zagórska-Marek 1982). However, later works show that the displacement of cell end along the boundary of the storeys is not necessarily preceded by a stage of bifurcated ends. The example of L. sericeus shows that bifurcated ends may be entirely lacking during the rearrangement of cells in the cambium. Therefore, the question arises whether the observed intrusive growth occurs at the radial edge and, therefore, takes place between radial walls.
The results presented in this paper bring into question the issue of whether the rearrangement is accomplished by intrusive growth between radial walls. Club-like widenings of cell ends cannot be interpreted as a consequence of intrusive growth of lateral radial edges (Fig. 11A) between radial walls, because this type of growth should always lead to bifurcation of cell ends. In the observed cases, club-like widenings indicate that intrusive growth must have taken place on para-apical longitudinal (tangential) edges (Fig. 11B). However, both club-like widenings and bifurcations can be formed in consequence of the intrusive growth of longitudinal (lateral and para-apical) edges, with different location and/or length of the growing edge (Fig. 11B–D).
A Drawing of a fusiform initial with named edges. B–D Hypothetical possibilities of the intrusive growth occurring on different edges of fusiform initials. B Growth along the whole para-apical longitudinal (tangential) edge leading to club-like widening of the cell end. C Growth of the lateral longitudinal (tangential) edge leading to bifurcation of the cell end. D Growth of the para-apical longitudinal (tangential) edge leading to the bifurcation of the cell end
A shifted arrangement of cells in neighbouring radial files at the boundary of storeys promotes the intrusive growth of para-apical longitudinal edges between tangential walls of neighbouring cells in the file. Although growth between radial walls may also occur, as in tumourous cambium or in wound callus, and leads to disturbances of cambial structure (Włoch 1981; Savidge and Farrar 1984).
The intensive rearrangement connected with the intrusive growth between radial walls should lead to an increase of cambial surface. However, in our study the increase of cambial surface has only been observed as a consequence of anticlinal divisions followed by symplastic growth (Larson 1994).
The changes of contacts between fusiform cells comprise transitional widening of the cell ends on the tangential surface as well as elimination of part of this end by unequal periclinal division. In L. sericeus, a typical cell end that is changing its orientation is club-like. This widening perhaps originates at the site of minimal stress in the wall, and is therefore in the region of low wall rigidity (Hejnowicz 1980), in this case at the side edge. Club-like widenings, like bifurcated ends, may also be regarded as a transitional stage of cell rearrangement. Unequal periclinal division eliminates this widening. The role of unequal periclinal divisions is important in both Tilia cordata (Włoch and Połap 1994) and Entandrophragma cylindricum (Hejnowicz and Zagórska-Marek 1974) and as well in Lonchocarpus sericeus.
In the process of cyclic intensive change of orientation of cells in storeyed cambium, such cell events as intrusive growth between tangential walls and unequal periclinal divisions of fusiform initial cells play a special part. The initial cell grows intrusively between radially deflected tangential walls of the neighbouring initial cell and its derivative. It leads to a transitional overlapping of ends or edges of neighbouring initial cells. Since periclinal divisions are always parallel to the initial surface, this means that the initials with deflected ends divide into two derivative cells of unequal length, and the shorter cell remains the initial (Włoch and Połap 1994; Kojs 2000; Włoch et al. 2001, 2002).
During the rearrangement of cells in the nonstoreyed cambium, the extent of intrusive growth is considerable (Włoch et al. 2002) (Fig. 12A). Whilst the change of contacts between cells in the storeyed cambium, as it is seen in L. sericeus, takes place with a minimum of intrusive growth restricted to the boundary of a storey (Fig. 12 B). It is worth noting that in the case of nonstoreyed cambium, high frequency of intrusive growth and its large range do not cause a rapid change of cell inclination. In contrast, in storeyed cambium, with a high frequency of intrusive growth and a smaller range, the change of inclination may occur rapidly—even during deposition of a single stratum of axial parenchyma. As a result, it leads to the formation of an interlocked grain.
Scheme illustrating the location of initial cells during the growth of cell ends into the space between periclinal walls. Ends of cells grow from one radial file into another. Intrusively growing cells are shaded. A Pinus sylvestris L. Dashed horizontal lines indicate places equivalent to the drawings of transverse sections (Włoch et al. 2002). B Lonchocarpus sericeus (Poir.) DC. The rays are dotted. The cells of upper and lower storeys are numbered. The Arrows indicate orientation of intrusive growth
Conclusion
In L. sericeus, in contrast to the general assumption, intrusive growth leading to the formation of interlocked grain does not start from radial, but from tangential edges. Both intrusive growth between periclinal walls and unequal periclinal divisions take part in the process of changing inclination.
The results presented in the paper argue against the hypothesis of cell rearrangement in the cambium based on concept of intrusive growth between radial walls. Instead, this study shows the possibility of a new interpretation of the mechanism of cell rearrangement in storeyed cambium.
References
Bailey IW (1920) The formation of the cell plate in the cambium of the higher plants. Proc Natl Acad Sci USA 6:197–200
Beijer JJ (1927) Die Vermehrung der Radialen Reihen im Cambium. Rec Trav Bot Neerl 24:631–786
Brown CL (1971) Secondary growth. In: Zimmermann MH, Brown CL (eds) Trees structure and function. Springer, Berlin Heidelberg New York, pp 67–123
Butterfield BG (1972) Developmental changes in the vascular cambium of Aschynomene hispida Willd. N Z J Bot 10:373–386
Carlquist S (1988) Comparative wood anatomy. In: Timell TE (ed) Springer series in wood science. Springer, Berlin Heidelberg New York
Committee on Nomenclature, IAWA (1964) Multilingual glossary of terms used in wood anatomy. Konkordia, Winterthur, Switzerland
Cumbie BG (1984) Origin and development of the vascular cambium in Aeschynomene virginica. Bull Torrey Bot Club 111:42–50
Eames AJ, MacDaniels LH (1947) An introduction to plant anatomy. McGraw–Hill, New York
Esau K (1965) Plant anatomy. Wiley, New York
Fahn A (1974) Plant anatomy, 2nd edn. Pergamon, New York
Foster AS (1949) Practical plant anatomy, 2nd edn. Van Nostrand, New York
Hejnowicz Z (1961) Anticlinal divisions, intrusive growth and loss of fusiform initials in nonstoried cambium. Acta Soc Bot Pol 30:729–758
Hejnowicz Z (1964) Orientation of the partition in pseudotransverse division in cambium of some conifers. Can J Bot 42:1685–1691
Hejnowicz Z (1968) The structural mechanism involved in the changes of grain in timber. Acta Soc Bot Pol 37:347–365
Hejnowicz Z (1975) A model of morphogenetic map and clock. J Theor Biol 54:345–362
Hejnowicz Z (1980) Tensional stress in the cambium and its developmental significance. Am J Bot 67:1–5
Hejnowicz Z, Krawczyszyn J (1969) Oriented morphogenetic phenomena in cambium of broadleaved trees. Acta Soc Bot Pol 38:547–560
Hejnowicz Z, Romberger JA (1973) Migrating cambial domains and the origin of wavy grain in xylem of broadleaved trees. Am J Bot 60:209–222
Hejnowicz Z, Romberger JA (1979) The common basis of wood grain figures is the systematically changing orientation of cambial fusiform cells. Wood Sci Technol 13:89–96
Hejnowicz Z, Zagórska-Marek B (1974) Mechanism of changes in grain inclination in wood produced by storeyed cambium. Acta Soc Bot Pol 43:381–398
Iqbal M, Ghouse AKM (1990) Cambial concept and organisation. In: Iqbal M (ed) The vascular cambium. Research Studies, Taunton, England, 1–36
Kojs P (2000) The mechanisms of cell rearrangement in storied cambium of selected woody species (in Polish). D. Phil. Thesis. Silesian University, Katowice
Krawczyszyn J (1977) The transition form nonstoried to storied cambium in Fraxinus excelsior L. l. The occurrence of radial anticlinal divisions. Can J Bot 55:3034–3041
Krawczyszyn J, Romberger JA (1979) Cyclical cell length changes in wood in relation to storied structure and interlocked grain. Can J Bot 57:787–794
Krawczyszyn J, Romberger JA (1980) Interlocked grain, cambial domains, endogenous rhythms, and time relations, with emphasis on Nyssa sylvatica: Am J Bot 67:228–236
Larson PR (1994) The vascular cambium: development and structure. In: Timell TE (ed) Springer series in wood science. Springer, Berlin Heidelberg New York
Philipson WR, Ward JM, Butterfield BG (1971) The vascular cambium. Its development and activity. Chapman and Hall, London
Pyszyński W (1972) Downward movement of the domain pattern in Aesculus cambium producing wavy-grained xylem. Acta Soc Bot Pol 41:511–517
Romberger JA, Hejnowicz Z, Hill JF (1993) Plant structure: function and development. Springer, Berlin Heidelberg New York
Savidge RA, Farrar JL (1984) Cellular adjustments in the vascular cambium leading to spiral grain formation in conifers. Can J Bot 62:2872–2879
Schmid R (1976) The elusive cambium—another terminological contribution. IAWA Bull 4:51–59
Wagenführ R, Scheiber C (1974) Holzatlas. VEB, Leipzig
Wilson, BF, Wodzicki TJ, Zahner R (1966) Differentiation of cambial derivatives: proposed terminology. For Sci 12:438–440
Włoch W (1976) Cell events in cambium with the formation and existence of whirled cell arrangement. Acta Soc Bot Pol 45:313–326
Włoch W (1981) Nonparallelism of cambium cells in neighbouring rows. Acta Soc Bot Pol 50:625–636
Włoch W (1985) Time-variable frequency of events in domains of Tilia cambium. Acta Soc Bot Pol 54:29–40
Włoch W (1987) Transition areas in the domain patterns of storeyed cambium of Tilia cordata Mill. Acta Soc Bot Pol 56:645–665.
Włoch W (1988) Chiral cell events and domain pattern in the cambium of lime ( Tilia cordata Mill.) (in Polish). D. Phil. Thesis, Silesian University, Katowice
Włoch W, Bilczewska E (1987) Fibrillation of events in the cambial domains of Tilia cordata Mill. Acta Soc Bot Pol 56:19–35
Włoch W, Połap E (1994) The intrusive growth of initial cells in re-arrangement of cells in cambium of Tilia cordata Mill. Acta Soc Bot Pol 63:109–116
Włoch W, Szendera W (1989) The storeyed and non-storeyed arrangement of rays in the storeyed cambium of Tilia cordata Mill. Acta Soc Bot Pol 58:211–228
Włoch W, Zagórska-Marek B (1982) Reconstruction of storeyed cambium in the linden. Acta Soc Bot Pol 51:215–228
Włoch W, Mazur E, Kojs P (2001) Intensive change of inclination of cambial initials in Picea abies (L.) Karst. tumours. Trees 15:498–502
Włoch W, Mazur E, Bełtowski M (2002) Formation of spiral grain in the wood of Pinus sylvestris L. Trees 16:306–312
Wodzicki TJ, Brown L (1973) Cellular differentiation of the cambium in the Pinaceae. Bot Gaz 134:139–146
Zagórska-Marek B (1975) Growth activity of fusiform initials in storeyed cambium. Acta Soc Bot Pol 44:437–552
Zagórska-Marek B (1984) Pseudotransverse divisions and intrusive elongation of fusiform initials in the storied cambium of Tilia. Can J Bot 62:20–27
Acknowledgements
We thank Professors Thomas Speck and Muhammed Iqbal for helpful comments and Dr. Peter Barlow for careful linguistic correction of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kojs, P., Włoch, W. & Rusin, A. Rearrangement of cells in storeyed cambium of Lonchocarpus sericeus (Poir.) DC connected with formation of interlocked grain in the xylem. Trees 18, 136–144 (2004). https://doi.org/10.1007/s00468-003-0292-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-003-0292-9