Summary
A morphology-based phylogeny and taxonomic revision of Prepusa Mart. and Senaea Taub. are presented. Based on studies of herbarium collections, five species of Prepusa and two species of Senaea are recognised. All are endemic to montane habitats in the Brazilian states of Bahia, Espírito Santo, Minas Gerais and Rio de Janeiro. Morphological descriptions, identification keys, illustrations and distribution maps for each species are provided. Prepusa and Senaea are morphologically, geographically, and phylogenetically isolated within Helieae, and their close relationship is supported by 6-merous flowers. Phylogenetic analyses of 33 morphological characters using both parsimony and Bayesian methods provide a consistent picture of the relationships of Prepusa and Senaea. The two genera are monophyletic and sister to one another. There is some support for the relationships within Prepusa.
Resumo
São apresentadas a filogenia morfológica e as revisões taxonômicas de Prepusa Mart. e Senaea Taub. Baseado no estudo de coleções de herbários, cinco espécies de Prepusa e duas de Senaea são reconhecidas. Todas são endêmicas a habitats montanos dos estados brasileiros da Bahia, Espírito Santo, Minas Gerais e Rio de Janeiro. São apresentados descrições morfológicas, chaves de identificação, ilustrações e mapas da distribuição de cada espécie. Prepusa e Senaea são morfológica, geográfica e filogeneticamente isolados entre as Helieae e a presença de flores 6-meras sustenta sua proximidade filogenética. Análises filogenéticas de 33 caracteres morfológicos usando parcimônia e métodos Bayesianos apresentam um quadro consistente das relações de Prepusa e Senaea. Os gêneros são monofiléticos e irmãos entre si. Há sustentação para as relações entre as espécies de Prepusa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prepusa Mart. and Senaea Taub. are members of Gentianaceae. They are rare herbs, shrubs or small trees restricted to montane habitats in the states of Bahia, Espírito Santo, Minas Gerais and Rio de Janeiro. Prepusa was described by Martius (1827). Although sometimes considered to be derived from the Latin word “praeputium” (for example, Barroso 1986), the protologue indicates that the name comes from the Greek “πρɛπουσα” meaning conspicuous, a reference to the remarkable overall appearance of the plants. The showiness of these plants is particularly striking when they are in flower; they are characterised by large, inflated calyces that are at least half the length of the corolla. Prepusa montana was the first species described (Martius 1827), and four species were added later by other researchers: P. connata Gardner (1839), P. hookeriana Gardner (1842), P. alata Porto & Brade (1935), and P. viridiflora Brade (1949). Senaea was established by Taubert (1893) and the protologue indicates the genus was named for a Brazilian botanist referred to only as “Sena”. This genus has traditionally contained two species; S. coerulea , described by Taubert (1893) and S. janeirensis, described later by Brade (1932). Although Prepusa and Senaea share a few morphological features, these two genera are clearly distinguished by calyx size, Senaea having much smaller calyces. Apart from the original descriptions, these genera have only been treated in a few 19th century monographs (e.g., Grisebach 1839, 1845; Progel 1865; Gilg 1895) and in two recent local floras (Harley & Simmons 1986; Cordeiro 1987).
A close relationship between Prepusa and Senaea has been suggested based on several shared morphological features; specifically, both have 6-merous flowers, membranaceous calyces, and a distinctive pollen type (Nilsson 2002; Struwe et al. 2002). They have also been tentatively placed in the tribe Helieae based on morphological characteristics, including a broadly bilamellate stigma and pollen shed as tetrads (Nilsson 2002; Struwe et al. 2002). However, their position has been considered uncertain because the characteristic floral features of these two genera are very different from those of most Helieae. The floral characteristics that link Prepusa and Senaea do not occur in Helieae; furthermore, the characteristic calycine dorsal glandular areas and thickened calyx keels found in many Helieae are absent from both Prepusa and Senaea. Recent phylogenetic analyses of molecular and morphological data have suggested a direct link between Prepusa and Senaea, and have also supported their relationship with Helieae (Struwe et al. submitted). However, these analyses have included either P. montana alone or this species along with both Senaea species. None of the herbaceous species of Prepusa were considered in these analyses and so the relationships of these to the woody taxa and to the remainder of the tribe are uncertain.
To improve our understanding of these two rare and endangered genera we performed a detailed morphological study of all seven species, as well as other members of Helieae. Based on these data, we performed phylogenetic analyses to test the monophyly of the genera and the relationships within and between them. We also produced a taxonomic revision of both genera providing field keys, illustrations, morphological descriptions, and geographic distribution maps. It is our hope that this new information will help efforts to conserve these rare plants and will serve as a basis for future studies on the tribe.
Material and Methods
Detailed morphological studies were based on herbarium material from AAU, ALCB, BHCB, BM*, BR*, C, CEN, CEPEC, CHRB*, CTES, ESA*, G*, HRB, HUEFS, IAN*, INPA*, K*, LIL, M, MBM*, MBML, MO, NY*, P, R*, RB*, S, SP*, SPF*, UB, UEC*, UPCB* and US* (* denotes herbaria visited by the authors). Only mature or fully developed structures were examined. Flowers and fruits were rehydrated in boiling water. All material was examined using a SZ Olympus dissecting microscope. Measurements were made using either a microscope interocular scale or calipers. Morphological descriptions follow Radford et al. (1974) and Stearn (1992) for two-dimensional shapes, and Weberling (1989) for inflorescences. Illustrations are based on both dried and rehydrated material. Preliminary conservation assessments follow IUCN (2001) guidelines.
For the phylogenetic analysis, we assembled a data set that included all species of Prepusa and Senaea. To ensure a proper rooting within the ingroup, 11 species from the tribe Helieae (i.e., Aripuana cullmaniorum, Calolisianthus pedunculatus, Celiantha bella, Chelonanthus viridiflorus, Chorisepalum carnosum, Helia oblongifolia, Irlbachia nemorosa, Macrocarpaea rubra, Symbolanthus calygonus, Tachia grandiflora, and Tetrapollinia caerulescens) and Curtia verticillaris, from the most basally placed tribe Saccifolieae, were included as outgroups. Based on our detailed morphological investigations, we identified 33 characters, shown in Appendix A with character delimitation, character states and commentaries. With the exception of pollen aggregation (character 32), all characters were scored based on direct examination of herbarium sheets. Pollen data were taken from Nilsson (2002). Characters and character states were designed for resolving relationships among Prepusa and Senaea species.
Phylogenetic analyses were performed using both parsimony and Bayesian approaches. For the parsimony analysis, a branch and bound search was performed using PAUP* version 4.0b10 (Swofford 2003). All characters were treated as unordered and equally weighted; multistate characters were treated as uncertain. Tree lengths, consensus trees, consistency index (CI) and retention index (RI) were calculated using PAUP. Bootstrap values were calculated with 1000 replicates. Decay indices were calculated using PAUP* and MacClade version 4.08 (Maddison & Maddison 2005).
The Bayesian analysis used the standard discrete model as implemented in MrBayes 3.1 (Huelsenbeck & Ronquist 2001; Ronquist & Huelsenbeck 2003). Searches used default settings for heating scheme (i.e., three “heated” chains and one “cold” chain), as well as for the priors on rate matrix (0 – 100), branch lengths (0 – 10), and gamma shape parameter (0 – 10). Simultaneous, independent pairs of searches were initiated from random start trees and run for five million generations, sampling from the posterior distribution of trees every 100 generations (for a total of 50,000 samples). Burnin was determined by plotting likelihood scores versus generation number and inspection of PSRF values for all model parameters. Posterior probabilities were calculated using the post-burnin sample of trees.
Results & Discussion
Phylogenetic Analyses
It was not possible to score all characters for all taxa; in one case the character was inapplicable. Specifically, character 13 (i.e., bracteoles subtending terminal flower) could not be scored for Tachia grandiflora because in this species the flowers are axillary. In addition, 2.6% of the cells were coded as missing because it was not possible to determine the character states based on the available material.
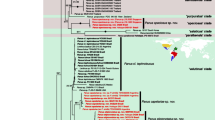
Parsimony analysis resulted in 37 most parsimonious (MP) trees, 91 steps long (CI = 0.42, RI = 0.64). In all trees, the same set of relationships is recovered for Prepusa and Senaea; furthermore, the remaining representatives of Helieae form a clade in all trees. Differences between MP trees are restricted to relationships within this core Helieae clade (Fig. 1). In parsimony analyses, Prepusa and Senaea are sister to one another, although this relationship receives only limited bootstrap support. The two species of Senaea are also sister; this relationship is more strongly supported. Prepusa is recovered as monophyletic in all MP trees, but bootstrap support is less than 50%. Within this clade, P. montana branches first, followed by P. viridiflora; P. alata is sister to the pairing of P. connata and P. hookeriana. These relationships receive moderate to strong bootstrap support. Bremer support values are consistent with those from bootstrapping. Specifically, relationships that are poorly supported by bootstrapping also receive low decay indices.
Majority-rule consensus of 37 most parsimonious trees. Support for nodes is given by the decay index, parsimony bootstrap, and Bayesian posterior probabilities (in that order). A dash indicates that the corresponding node support value was either 0 for the decay index or was less than 50% for the parsimony bootstraping or posterior probability.
Inspection of likelihood plots and PSRF values for all model parameters indicated that independent searches had reached stationarity and converged within 500 generations; the initial 500 generations were discarded as burnin. The 50% majority-rule tree for post-burnin topologies was highly similar to that recovered using parsimony. Specifically, Prepusa and Senaea are sister to one another, and the same relationships within Prepusa are recovered. Bayesian support values are generally consistent with those from the parsimony bootstrap analysis (see Fig. 1).
Additional analyses that included only a single representative of Helieae as an outgroup (Curtia verticillaris was not used) provide increased support for the monophyly of each of the genera, as well as for relationships within Prepusa. For example, when using Aripuana cullmaniorum and Symbolanthus calygonus as outgroups, bootstrap support for all nodes is above 80%. A possible explanation is that in the broader context of the tribe, character conflict leads to reduced support values. For example, P. montana shares a woody habit with both Senaea species, as well as with Aripuana cullmaniorum, Chorisepalum carnosum, and Tachia grandiflora; prominent decurrent ridges on stem are present not only in Senaea but also in several other taxa sampled for this analysis. Indeed, it seems likely that homoplasy is a general problem for morphology-based phylogenetic analysis of Gentianaceae since the diversity of the group makes it difficult to identify features that are unique to specific taxa (e.g. Mészáros et al. 2002).
Our phylogenetic analyses are clearly consistent with the monophyly of Prepusa and Senaea, and with a direct link between them (Fig. 1). Inspection of the data matrix indicates that the sister group relationship between these taxa is based on the shared presence of membranaceous calyces (character 17), 6-merous corollas (ch. 23), and cylindric styles (ch. 33). The first two of these characters have commonly been used to distinguish these genera from other Gentianaceae. In contrast, Helieae generally have fleshy, sometimes leathery, calyces with dorsally thickened lobes that often have dorsal glandular areas; they also differ in that they have 5-merous corollas and flattened styles. Inspection of the matrix also indicates that monophyly of Prepusa is supported by only one character; specifically, winged calyces (ch. 21). Within Prepusa, the clade containing P. viridiflora, P. alata. P. connata and P. hookeriana is supported by several characters. These include leaves arranged in a basal rosette (ch. 3), connate leaf bases (ch. 8), non-prominent leaf midribs on abaxial surface (ch. 10), and connate bract bases (ch. 12). Within this subclade, the group formed by P. alata, P. connata, and P. hookeriana is supported by the red- to magenta-coloured leaf margins and apices (ch. 6), the presence of papillae on the inner side of calyces (ch. 19), and the reddish calyces (ch. 15). Finally, the link between P. connata and P. hookeriana is supported by urceolate calyces (ch. 16). The monophyly of Senaea receives moderate support. This link is supported by two characters; papillate corollas (ch. 26) and stamens exserted beyond the corolla (ch. 30).
It is important to note that while these characters appear to be useful for resolving phylogenetic relationships within Prepusa and Senaea, at least some of them are homoplastic when considered across Helieae as a whole. Given the small scope of this analysis and the observation that Prepusa and Senaea share character states with other Helieae, it should be clear that detailed discussion of morphological evolution within the tribe is simply not possible. However, it should be said that given the morphological diversity of Helieae it seems likely that the shared characters represent independent evolutions.
Biogeography and Conservation
Prepusa and Senaea occur in the campo de altitude and campo rupestre areas of eastern Brazil. These vegetation types are characterised by a mosaic distribution, often forming vegetation islands associated with rocky outcrops and sandy soils (Giulietti & Pirani 1988; Safford 1999). The individual islands are isolated from one another by areas of lower altitude that are both climatically and edaphically very different. Prepusa alata, P. connata, P. hookeriana, P. viridiflora and S. janeirensis occur in the campos de altitude; this vegetation type is associated with montane areas close to the Atlantic coast and the surrounding vegetation is Atlantic forest. In contrast, P. montana and S. coerulea occur in campo rupestre. This vegetation type occurs in more inland areas and the individual patches are isolated from one another by the distinctive cerrado vegetation. Similar patterns of disjunct distribution among campos rupestres and campos de altitude have been reported for other plant groups in eastern Brazil. For example, Bradea Standl. (Rubiaceae), Hindsia Benth. (Rubiaceae), Hockinia Gardner (Gentianaceae), Schlumbergera Lem. (Cactaceae), and Wunderlichia Riedel ex Benth & Hook. f. (Asteraceae) have species that occur in both the campos rupestres and campos de altitude. Giulietti & Pirani (1988), Safford (1999), and Safford & Martinelli (2000) provide excellent discussions of plant distribution patterns in the campos rupestres and campos de altitude.
The patchwork distribution of campos de altitude and campos rupestres has been suggested as an explanation for their high levels of endemism and species richness (Giulietti & Pirani 1988; Safford 1999). If this is the case, it seems reasonable to suggest that geographic isolation may have played a role in the diversification of Prepusa and Senaea. Paleobotanical evidence suggests that open floristic assemblages were more widely distributed in the Tertiary (Miocene) and have become more restricted and fragmented in response to climatic changes, chiefly during the glaciation cycles of the Quaternary (Safford 1999). This scenario implies that current species distribution may have arisen as the result of vicariance followed by allopatric speciation. That is, the distributions of formerly widespread taxa were fragmented, forming “vegetation refugia” (Veloso et al. 1991). Ancestral populations of Prepusa and Senaea in these refugia subsequently diverged in isolation, resulting in the contemporary species distributions. Additional analyses of biogeographic patterns using phylogeny, biogeographic methods, and GIS are planned for this group; we hope these will help us understand the historical context of diversification in these groups.
All Prepusa and Senaea species are local endemics and currently most are restricted to protected areas (Maps 1 and 2). The most widespread species, P. montana, occurs in Parque Nacional (P.N.) da Chapada Diamantina, Parque Estadual (P.E.) do Morro do Chapéu, and the Parque Municipal de Mucugê. This species is also known to occur outside these parks. Based on its relatively widespread distribution, P. montana is considered to be “vulnerable” based on the IUCN guidelines (IUCN 2001). Prepusa viridiflora and P. hookeriana each occur in two locations, and these species are considered “endangered.” Specifically, P. viridiflora is found in P.E. da Pedra Azul and P.E. do Forno Grande, while P. hookeriana occurs in P.N. do Itatiaia and P.N. da Serra dos Órgãos. The remaining species are all placed in the “critically endangered” category. Three of these are known only from a single locality: P.E. do Desengano (S. janeirensis and P. alata) and P.N. da Serra dos Órgãos (P. connata). Although placed in the “critically endangered” category S. coerulea may well be extinct as it has not been found since 1982, despite researchers commonly visiting the localities where it was previously reported. Unfortunately, the localities for S. coerulea are unprotected.
Although these species’ occurrence within parks provides some level of protection, they are highly dependent on the maintenance of these protected areas since their actual distribution is much more restricted, and they usually occur on just a single mountain peak. In all cases, loss of habitat, mainly due to fire, local habitat disturbance by cattle and humans, as well as increasing pressure from nearby human settlements, is of great concern. Not only do these activities greatly reduce the chance of finding additional populations outside of protected areas, but these also threaten the parks themselves. Careful management of these areas and the populations themselves is needed in order to assure longer-term survival.
Taxonomic Treatment
Key to Genera
-
1. Calyx large (19 – 46 × 14 – 33 mm) and conspicuous, half as long or longer than the corolla; corolla lobes much shorter than corolla tube. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Prepusa
-
1. Calyx small (6.9 – 12 × 5 – 8.5 mm) and not conspicuous, much shorter than the corolla; corolla lobes almost as long as corolla tube. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Senaea
Prepusa Mart. (1827: 120). Type species: Prepusa montana Mart.
Herbs to shrubs or small trees, glabrous, unbranched or branched. Stems cylindrical from base to apex, with numerous, discontinuous, thin, vertical ridges (not extending from interstipular line to node directly below). Leaves clustered at base of stem, forming a basal rosette or clustered at branch apices, green to greenish-yellow, sessile, fleshy; margin entire, hyaline, reddish or green; venation acrodromous, primary and secondary veins conspicuous, tertiary veins inconspicuous. Inflorescence terminal, dichasium; bracts and bracteoles leaf-like, with entire, hyaline margins. Flowers actinomorphic, perfect, 6-merous, pedicellate. Calyx reddish, green or yellowish, campanulate or urceolate, inflated, marcescent; lobes equal; colleters present on inside of calyx. Corolla white, cream, yellow, yellowish-green or brown, funnel-shaped or campanulate with a constriction at the level of stamen insertion and widening above, membranaceous or marcescent, not papillate; lobes aestivation contort. Stamens 6, unequal or equal in length, included, inserted in the lower third of the corolla tube; filaments filiform, very rarely twisted when dry; anthers ovoid to ellipsoid, sagittate at base, dorsifixed, with sterile appendix at apex, introrse. Ovary ellipsoid; style slender, not twisted when dry; stigma bilamellate; placentation parietal; ovules numerous. Fruit a capsule, unilocular, 2-valvate, many-seeded, with persistent calyx, corolla, and style.
Prepusa comprises five species restricted to the campos rupestres and campos de altitude of the eastern Brazilian states of Bahia, Espírito Santo and Rio de Janeiro (Map 1).
Key to the species of Prepusa
-
1. Stems woody, shrub or small tree; leaf apex obtuse and emarginate; corolla lobe margin entire, rarely slightly sinuate (Bahia). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4. P. montana
-
1. Stems not woody (herbaceous) or woody only at base (subshrub); leaf apex acuminate or acute; corolla lobe margin crenulate, very rarely entire (Rio de Janeiro and Espírito Santo):
-
2. Calyx winged:
-
3. Calyx reddish, winged from base to the apex of the calyx tube; calyx lobes transversely elliptic, widely elliptic or depressed ovate, with caudate to mucronate apex; corolla campanulate, longer than calyx (Rio de Janeiro). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1. P. alata
-
3. Calyx greenish-brown, winged from base, but not reaching the apex of the calyx tube; calyx lobes triangular, with acute to acuminate apex; corolla funnel-shaped, shorter than to as long as calyx (Espírito Santo). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
5. P. viridiflora
-
-
2. Calyx not winged:
-
4. Bracts 34 – 61 × 18 – 32 mm, connate to \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – \({\raise0.7ex\hbox{$4$} \!\mathord{\left/ {\vphantom {4 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of length, forming a bilabiate sheath; calyx lobe apex caudate; filaments twisted when dry
2. P. connata
-
4. Bracts 19 – 27 × 5 – 9 mm, connate to only \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) – \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of length; calyx lobe apiculate to mucronate; filaments not twisted when dry. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3. P. hookeriana
-
-
1. Prepusa alata Porto & Brade (1935: 222). Type: Brazil, Rio de Janeiro, Santa Magdalena (sic), Pedra das Flores, 1400 m, Nov. 1933, J. Santos Lima 185 (holotype RB!; isotype RB!).
Herbs, woody at base, 20 – 80 cm tall, unbranched. Stems 2.5 – 4.0 mm in diam. below inflorescence, internodes 5 – 18 mm long at base of plant, 35 – 60 (– 116) mm long below inflorescence. Leaves elliptic to narrowly elliptic, oblanceolate to obovate, 22 – 86 × 11 – 26 mm, base connate less than \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, margin reddish and straight, apex acute to acuminate; 1 – 3 pairs of secondary veins. Inflorescence 90 – 205 mm long, 1 – 4-flowered; bracts elliptic to obovate, 29 – 33 × 13 – 20 mm, base connate to c. \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, apex acute; bracteoles 1 pair per flower, inserted either at the base or at c. \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of pedicel length (from base), narrowly elliptic, oblanceolate or narrowly oblong, 10 – 40 × 1.6 – 13.5 mm, base connate to \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) – \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of length, apex acute or acuminate; pedicel 50 – 156 mm long, 0.7 – 3.0 mm in diam. Calyx reddish, campanulate, 20 – 30 × 14 – 17 mm at anthesis, papillate on the inner side, minutely papillate on the outer side only close to the apex, dorsally winged from base to apex, wings 1.5 – 4.5 mm wide; lobes elliptic to widely ovate, 2.7 – 4.5 × 5.1 – 6.0 mm, apex caudate or mucronate. Corolla white, cream, to pale yellow, campanulate, 36 – 43 mm long, 1.5 – 1.6 times longer than calyx; tube 25 – 31 mm long, 2.2 – 3.2 mm wide at base, 7.2 – 8.0 mm wide below filament insertion, 10 – 13 mm above filament insertion, 10 – 20 mm wide at mouth; lobes ovate, elliptic or obovate, 10 – 15 × 6.5 – 8.1 mm, margin crenulate (very rarely entire), apex apiculate. Filaments unequal in length, not twisted when dry, 21 – 30 mm long; anthers 4.1 – 5.0 mm long, attached to filament c. 2.1 mm from anther base. Ovary 11 – 14 mm long; style 13 – 21 mm long; stigma lobes broadly elliptic, 2.0 – 2.5 mm long. Fruit not seen. Fig. 2.
Distribution. Brazil, Rio de Janeiro. Occurring only on Pedra do Desengano (Parque Estadual do Desengano). Map 1.
Specimens Examined. Rio de Janeiro: Santa Maria Madalena, Parque Estadual do Desengano, Pedra do Desengano, 17 Sept. 1986, Farney & Caruso 1195 (CEPEC, MG, RB!) & 24 March 2002, Gomes et al. 153 (CHRB!, K, SPF!, SP!) & 5 Oct. 1988, Martinelli et al. 13140 (RB!) & March 1934, Santos Lima & Brade 14101 (RB!).
Habitat. Campo de altitude; 1400 – 1800 m.
Conservation Status. CR B1ab(i,ii)+2ab(i,ii). This species is categorised as Critically Endangered due to its small extent of occurrence, confined to only one mountain peak.
Phenology. Flowering specimens have been found in March, Sept. and Oct.
Etymology. The species epithet ‘alata’ was given because of the winged calyx.
2. Prepusa connata Gardner (1839: 225 – 226); Grisebach (1845: 81); Progel (1865: 243); Gilg (1895: 96). Type: Brazil, Rio de Janeiro, near the summits of Organ Mts, 1500 m, May 1837, Gardner 541 (holotype BM!; isotypes CGE!, G! (2 sheets), K!, NY! (2 sheets), P!, S!, US!).
Prepusa campanulata Grisebach (1845: 81) nom. nud.
Herbs, not woody at base, 28 – 69 cm tall, unbranched. Stems 2.5 – 6.8 mm in diam. below inflorescence, internodes 6 – 55 (– 105 – 110) mm long at base of plant, (8 –) 35 – 75 (– 105) mm long below inflorescence. Leaves elliptic to narrowly elliptic, oblanceolate to obovate or oblong, 34 – 120 × 16 – 33 mm, basal leaves base connate less than \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, apical leaves base connate \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – \({\raise0.7ex\hbox{$3$} \!\mathord{\left/ {\vphantom {3 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of length, margin green and straight, apex acute to acuminate and mucronate to apiculate; 2 (– 5) pairs of secondary veins. Inflorescence 65 – 230 mm long, 3 – 7-flowered; bracts elliptic to obovate, 34 – 61 × 18 – 32 mm, base connate to \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – \({\raise0.7ex\hbox{$4$} \!\mathord{\left/ {\vphantom {4 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of length, forming a bilabiate sheath, apex acute, acuminate or obtuse; bracteoles 1 pair per flower, inserted at the base of pedicel, lanceolate or oblanceolate, 9 – 24 × 1 – 5 mm, base connate to c. \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, apex acuminate; pedicel 51 – 150 mm long, 0.9 – 2.4 mm in diam. Calyx reddish, greenish-red, whitish-pink, or purple-red, urceolate, 20 – 29 × 15 – 24 mm at anthesis, papillate on the inner side, not papillate on the outer side, not dorsally winged; lobes widely depressed ovate, 0.8 – 1.8 × 4.8 – 7.2 mm, apex caudate. Corolla whitish-rose, white, cream or pale yellow tinged red, campanulate, 34 – 38 mm long, 1.3 – 1.9 times longer than calyx; tube 24 – 29 mm long, 2.8 – 3 mm wide at base, 5.8 – 7.0 mm wide below filament insertion, 10 – 12 mm wide above filament insertion, 10 – 12 mm wide at mouth; lobes elliptic, 9 – 12 × 5.1 – 6.9 mm, margin crenulate, apex caudate or mucronate. Filaments unequal in length, twisted when dry, 19 – 23 mm long; anthers c. 4.6 mm long, attached to filament c. 2 mm from anther base. Ovary 13 – 16 mm long; style c. 17 mm long; stigma lobes obovate, c. 2.6 mm long. Fruit not seen. Fig. 3.
Distribution. Brazil, Rio de Janeiro. Occurring only on Serra dos Órgãos (Parque Nacional da Serra dos Órgãos). Map 1.
Specimens Examined. Rio de Janeiro: Serra dos Órgãos, Frade, 7 Aug. 1869, Glaziou 3813 (BR!, C!, P!, R!); Nova Friburgo, 13 June 1999, Franzen 42 (MBM!) & 13 May 1888, Glaziou 17238 (C!, IAN!, MO!, NY!) & 18 May 1891, Glaziou 18372 (BM!, BR!, C!, G!, K!, NY!(2 sheets), P!); Petrópolis, Morro do Cuca, 27 Jan. 1983, Simonis & Martinelli 20 (RB!) & Vale das Videiras, 21 April 1974, Martinelli 240 (BR, CEN!, CEPEC!, F, GUA!, K, LIL, MBM!, MO, MG, RB!) & 1 July 1975, Martinelli 602 (RB!) & 12 June 1977, Martinelli 2561 (RB!) & 10 Oct. 1979, Martinelli & Santos 6125 (RB!) & 25 Aug. 1983, Martinelli et al. 9335 (RB!) & 27 May 1995, Ribeiro et al. 2280 (GUA!) & between Araras and Vale das Videiras, Pico Pindoba, s.d., Martinelli 9889 (RB!) & 18 June 1985, Martinelli et al. 11148 (RB!); Teresópolis, Parque Nacional da Serra dos Órgãos, Pico da Pedra da Cruz, 6 June 2004, Pederneiras 16 (R!); Without locality, s.d., Gardner s.n. (R!).
Habitat. Campo de altitude; 1500 – 1800 m.
Conservation Status. CR B1ab(i,ii)+2ab(i,ii). This species is only found on the Organ Mountains, so its area of occupancy is very limited, leading to a classification as Critically Endangered.
Phenology. Flowering specimens have been collected in Jan., April to Aug. and Oct.
Etymology. Prepusa connata is named after its connate bracts.
Note. Prepusa campanulata is not a validly published name. In 1845, when Grisebach cited this name as a synonym of P. connata, he created a nomem nudun.
3. Prepusa hookeriana Gardner (1842: 3909); Grisebach (1845: 81); Progel (1865: 244); Gilg (1895: 96). Type: Brazil, Rio de Janeiro, Summit of Organs Mts. Locis apertis humidis, 2050 m, 18 April 1841, Gardner 5823 (holotype BM!; isotypes CGE!, G! (3 sheets), K!, NY! (2 sheets), P!, RB!).
Herbs, not woody at base, 31 – 53 cm tall, unbranched. Stems 1.5 – 4.9 mm in diam. below inflorescence, internodes 2 – 5 mm long at base of plant, (55 –) 105 – 183 mm long below inflorescence. Leaves elliptic to narrowly elliptic, oblanceolate or linear, 16 – 82 (– 150) × 4 – 13 mm, base attenuate, cuneate or connate less than \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, margin reddish and straight, apex acute to acuminate, some apiculate; 1 – 2 pairs of secondary veins. Inflorescence 65 – 145 mm long, 1 – 5-flowered; bracts obovate to oblanceolate, 19 – 27 × 5 – 9 mm, base connate \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) – \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of length, apex acute to acuminate and mucronate to apiculate; bracteoles 1 pair per flower, inserted at the base or rarely at c. \({\raise0.7ex\hbox{$3$} \!\mathord{\left/ {\vphantom {3 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of the pedicel length (from base), oblanceolate or rarely lanceolate, 8 – 24 × 2.0 – 7.7 mm, base attenuate, apex acute and apiculate; pedicel 31 – 110 mm long, 0.4 – 2.0 mm in diam. Calyx reddish, pink or rose, urceolate, 19 – 33 × 14 – 25 mm at anthesis, papillate on the inner side, minutely papillate on the outer side close to the apex, not dorsally winged; lobes transversely elliptic to narrowly transversely elliptic, 0.7 – 3.0 × 3.7 – 5.8 mm, apex apiculate to mucronate. Corolla whitish-rose, white, cream, or pale yellow tinged red, campanulate, 26 – 32 mm long, c. 1.4 times longer than calyx; tube 18 – 23 mm long, 1.4 – 1.9 mm wide at base, 5.0 – 6.2 mm wide below filament insertion, 8 – 11 mm wide above filament insertion, 10 – 11 mm wide at mouth; lobes elliptic, obovate, widely obovate or widely ovate, 6 – 12 × 3.3 – 8.1 mm, margin crenulate, apex apiculate. Filaments unequal in length, not twisted when dry, 9 – 13 mm long; anthers 3.6 – 4.3 mm long, attached to filament c. 2 mm from anther base. Ovary c. 10 mm long; style c. 7 mm long; stigma lobes elliptic, c. 2.4 mm long. Fruit not seen. Fig. 4.
Distribution. Brazil, Rio de Janeiro. Occurring only on Serra dos Órgãos (Parque Nacional da Serra dos Órgãos) and on the Serra da Mantiqueira (Parque Nacional do Itatiaia) Map 1.
Specimens Examined. Rio de Janeiro: Itatiaia, Parque Nacional do Itatiaia, near Abrigo Rebouças, 13 April 1979, Shepherd & Kirszanzaft 9958 (UEC!); Serra dos Órgãos, 27 Feb. 1933, Brade 12464 (R!) & July 1975, Camerilo B640 (K!) & 1964, Duarte s.n. (RB!) & 18 April 1841, Gardner s.n. (BM!) & 3 April 1870, Glaziou 4099 (C!) & 15 March 1888, Glaziou 16236 (R!) & 1887, Moura s.n. (R!) & s.d., Saldanha 7357 (RB!); Nova Friburgo, Caledônia, 15 May, Cappeli s.n. (RB!) & Aug. 1951, Cappeli s.n. (RB!); Petrópolis, Parque Nacional da Serra dos Órgãos, Morro Açu, 4 April 1972, Bacia 534 (R!) & 30 Sept. 1929, Brade 9502 (R!) & 29 March 1937, Dionysio s.n. (RB!) & 6 March 1910, Lützelburg s.n. (M!(2 sheets), NY!) & 21 Feb. 1944, Vianna 116 (RB!) & trail to Morro Açu, 31 Aug. 1985, Farney et al. 795 (K, NY!, RB!); Teresópolis, Feb. 1941, Ribeiro s.n. (R!) & Jan. 1952, Vidal 182 (R!) & Feb. 1952, Vidal 330 (R!) & Parque Nacional da Serra dos Órgãos, 29 April 1962, Santos et al. 1221 (HB!, R!) & Campo das Antas, 2 May 1930, Brade s.n. (RB!) & 22 May 1948, Carris s.n. (GUA!, RB!) & 13 July 1956, Castellanos 21660 (LIL!) & 27 March 1883, Gabinete de Botânica da Escola Politécnica 7351 (R!) & 16 April 1972, Kirkbride Jr. et al. 1723 (C!, NY!, UB!, US!, R!) & 2 Feb. 1983, Martinelli & Simonis 9055 (RB!, US!) & Feb. 1942, Santos & Frota Pessoa s.n. (R!) & 1 Feb. 1983, Simonis & Martinelli 26 (NY!, RB!) & Pedra da Baleia, Feb. 1953, Vidal 6431 (R!) & Feb. 1953, Vidal 6447 (R!) & 10 May 1981, Vilaça & Ribeiro 127 (GUA!) & between Pedra da Baleia e Pedra do Dinossauro, 3 March 1981, Carauta et al. 3670 (GUA!) & Pedra do Sino, 8 Oct. 1929, Brade 9620 (R!) & 2 May 1931, Brade 10753 (BHCB!, R!) & 12 Jan. 1960, Flaster 64 (R!) & 17 March 2000, Gajardo & Sazima 3 (UEC!) & 16 June 1946, Silva s.n. (RB!) & 9 Dec. 1960, Strang 272 (GUA!) & Feb. 1952, Vidal 597 (R!) & July 1953, Vidal 6480 (R!) & trail to Pedra do Sino, 22°27′6″S 43°0′4″W, 12 March 2001, Costa et al. 508 (SP!, SPF!); Without locality, s.d., Gardner s.n. (K!) & s.d., Gardner s.n. (S!) & s.d. Glaziou 3814 (BR!), & March 1884, Glaziou 15242 (C!, G!, K!, P!, RB!) & May 1887, Glaziou 16363 (C!, K!, P!, RB!).
Habitat. Campos de altitude; found only once growing on swampy ground (Shepherd et al. 9958); 850 – 2700 m.
Conservation Status. EN B1ab(i,iii,iv)+2ab(i,iii,iv). There are only two known localities for this species, each from a different mountain chain. The localities are about 100 km apart and the species is here classified as Endangered.
Phenology. Flowering specimens have been found from Jan. to May and July to Oct.
Etymology. Prepusa hookeriana was named in honour of W. J. Hooker (1785 – 1865), a famous British botanist.
Vernacular Name. Cravina-do-campo (Portuguese).
4. Prepusa montana Mart. (1827: 121); Grisebach (1839: 206); Grisebach (1845: 81); Progel (1865: 243); Gilg (1895: 96). Type: Brazil, Bahia, Serra do Sincorá, 660 m, 1817 – 1820, Martius 2108 (holotype M).
Shrubs to small trees, 1 – 3 m tall, branched. Stems 4.5 – 7.3 mm in diam. below inflorescence, internodes 32 – 150 mm long below inflorescence. Leaves elliptic, oblanceolate or obovate, 33 – 140 × 13 – 64 mm, base attenuate, not connate, margin green and revolute, apex obtuse and emarginate; 1 – 2 pairs of secondary veins. Inflorescence 102 – 330 mm long, 2 – 18-flowered; bracts elliptic or obovate, 21 – 58 × 10 – 36 mm, base attenuate, apex obtuse; bracteoles 1 pair per flower, inserted at \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$2$}}\) of pedicel length (from base), oblanceolate, 8 – 17 × 1.7 – 6.0 mm, base attenuate, apex obtuse; pedicel 22 – 64 mm long, 1.3 – 2.0 mm in diam. Calyx greenish-yellow, yellowish-green, cream, to light green, campanulate, 22 – 33 × 24 – 33 mm at anthesis, not papillate, dorsally winged from base to apex, wings 1.5 – 4.5 mm wide; lobes transversely elliptic or depressed ovate, 6 – 9 × 8.9 – 13 mm, apex caudate. Corolla greenish-yellow, pale yellow, or cream, campanulate, 30 – 43 mm long, 1.3 – 1.6 times longer than calyx; tube 20 – 31 mm long, 2 – 6 mm wide at base, 6.5 – 11.0 mm wide below filament insertion, 13 – 22 mm above filament insertion, 13 – 24 mm wide at mouth; lobes widely elliptic or oblong, 8 – 19 × 6.4 – 11.0 mm, margin entire or very rarely slightly sinuate, apex caudate, mucronate or cuspidate. Filaments slightly unequal or equal in length, not twisted when dry, 13 – 29 mm long; anthers 5.5 – 7.2 mm long, attached to filament 1.9 – 3 mm from anther base. Ovary 13 – 20 mm long; style 8 – 20 mm long; stigma lobes widely elliptic, obovate or depressed ovate, 1.2 – 3.0 mm long. Fruit not seen. Fig. 5.
Distribution. Brazil, Bahia. Occurring in the Chapada Diamantina in the Parque Nacional da Chapada Diamantina, Parque Estadual do Morro do Chapéu, Parque Municipal de Mucugê, and outside the boundaries of these parks. Map 1.
Specimens Examined. Bahia: Andaraí, Serra Andaraí–Capa Bode, road to Mucugê, 30 Oct. 1978, Martinelli et al. 5425 (RB!) & 27 Oct. 1978, Martinelli et al. 5507 (RB!); Igatu, s.d., Gusmão 305 (ALCB!, HRB!, SP!); Morro do Chapéu, 17 May 1975, Barroso et al. s.n. (SPF!) & 26 Sept. 1965, Duarte & Pereira 9208 (GUA!, RB!) & 11 Sept. 1956, Pereira 2020 (GUA!, RB!) & 26 Sept. 1965, Pereira & Duarte 10118 (HB!, M!) & 21 Sept. 1985, Pinto 116/85 (HRB!, UB!) & 27 July 1975, Souza & Brito s.n. (ALCB!) & 11°38′34′S 40°55″45″W, 26 Aug. 1980, Orlandi 268 (HRB!, HUEFS!) & 11°6′0″S 41°2′0″W, 3 Oct. 1991, Freire-Fierro et al. 1749 (SPF!) & c. 7 km S of Morro do Chapéu, 16 Feb. 1971, Irwin et al. 32295A (MO!, NY!, US!) & 16 Feb. 1971, Irwin et al. 32327 (MO!, NY!) & Ferro Doido river, 14 Oct. 1981, Hatschbach 44263 (C!, MO!) & 19.5 km SE of Morro do Chapéu on the BA 052 highway to Mundo Novo, 11°38′0″S 41°2′0″W, 31 May 1980, Harley et al. 22878 (AAU!, CEPEC!, K!, NY!, SPF!, US!) & Ferro Doido waterfall, s.d., Martinelli et al. 5259 (RB!) & 27 Oct. 1978, Martinelli et al. 5275 (GUA!, RB!) & 14 June 1975, Pereira & Gusmão s.n. (ALCB!) & 11°37′24″S 41°0′3″W, 10 July 2000, Oliveira et al. 64 (HUEFS!, SPF!) & 11°37′40″S 41°0′5″W, 28 June 1996, Hind et al. 3165 (ALCB!, CEPEC!, HUEFS!, IBGE, SPF!) & 20 km from Morro do Chapéu, 11°37′42″S 41°0′1″W, 30 Aug. 2003, Cavalcanti et al. 3214 (CEN, SPF!) & 25 Sept. 1985, Wanderley et al. s.n. (SP!) & road Lage do Batata-Morro do Chapéu, km 66, 11°27′S 41°7′W, 28 June 1983, Coradin et al. 6230 (CEN!, RB!) & road Morro do Chapéu-Jacobina, km 7, 11°6′0″S 41°2′0″W, 3 Oct. 1990, Freire-Fierro et al. 1767 (CHRB!, SPF!) & road to Bonito, 3 km SW, 10 July 1999, Peixinho & Vanilda s.n. (HUEFS!, SP!) & road to Utinga, 8 Sept. 1990, Lima 3900 (CEPEC!, K!, SPF!) & c. 5 km from Morro do Chapéu, 11°35′51″S 41°9′48″W, 18 July 2001, Souza et al. 26365 (ESA!, SP!) & 28 Jan. 2005, Paula-Souza et al. 4866 (ESA!) & summit of Morro do Chapéu, 16 July 1979, Hatschbach & Guimarães 42398 (AAU!, C!, CTES!, INPA!, MBM!, MO!, SPF!, UB!, US!) & c. 8 km SW of the town of Morro do Chapéu to W of the road to Utinga, 11°35′0″S 41°12′0″W, 30 May 1980, Harley et al. 22755 (CEPEC!, K!(2 sheets), SPF!) & transmission tower, 26 April 1999, Amorim et al. 3009 (CEPEC!, NY!, SP!) & 30 Aug. 1990, Hage et al. 2330 (CEPEC, HUEFS!, SP!, SPF!) & 30 Oct. 1978, Martinelli 5243 (CEPEC, K, MG, RB!) & c. 6 km S of Morro do Chapéu, 16 June 1981, Mori & Boom 14469 (NY!); Mucugê, 15 June 1984, Hatschbach & Kumrow 47930 (BR!, HUEFS!, MBM!, MO!, US!, UPCB!) & 1 km N of Mucugê, 13°0′0″S 41°23″7″W, 10 Oct. 1987, Guedes et al. 1543 (ALCB!) & 10 Oct. 1987, Queiroz et al. 1846 (HUEFS!, MBM!, NY!, UEC!) & Projeto de Sempre-Viva center, trail to Tiburtino, near Piabinha and Cumbuca rivers, 12°59′36″S 41°20′29″W, 25 March 2000, Giulietti et al. 1926 (HUEFS!) & road Mucugê-Abaíra, c. 3 km to Mucugê, near the bridge, 13°14′0″S 41°20′29″W, 11 Aug. 1992, Ganev 816 (HUEFS!, K!, SP!, SPF!, NY!) & road Mucugê-Cascavel, km 3 – 6, near Paraguaçu river, 20 July 1981, Menezes et al. 1452 (CHRB!, HUEFS!, K!, SPF!) & 20 July 1981, Menezes et al. 5823 (K!, SPF!) & 13°00′18.9″S 41°23″29.9″W, 21 July 2006, Calió et al. 116 (SPF!) & road Mucugê-Guiné, 5 km from Mucugê, 7 Sept. 1981, Furlan et al. 1926 (CHRB!, K!, SPF!) & road to Jussiape, 3 km S of Mucugê, 13°0′S 41°24′W, s.d., Mori et al. 12552 (CEPEC!, RB!, US!) & Trilha to Sibéria, 26 June 1993, Ferreira 555 (CTES!, HRB!, MBM!, RB!) & Unidade de Manejo Sustentável, 12°59′59″S 41°20′46″W, 5 April 1997, Bautista & Silva 259 (HRB!); Without locality, 1914, Lützelburg 532 (M!, NY!).
Habitat. Campo rupestre and cerrado, sometimes close to river margins and swamps; 700 – 1400 m.
Conservation Status. VU B1ab(i,iii,iv)+2ab(i,iii,iv). The range of Prepusa montana is highly fragmented but estimated to be less than 20,000 km2 in total. Populations outside of protected areas are threatened by development and the expansion of settlements and agriculture. This species is classified as Vulnerable.
Phenology. Flowering specimens have been collected between Feb. and Oct., with a fruiting specimen found in March.
Etymology. The epithet montana (mountain) reflects the high altitude areas where this species was first found.
5. Prepusa viridiflora Brade (1949: 18). Type: Brazil, Espírito Santo, Castelo, Forno Grande Pico, 12 Aug. 1948, Brade 19278 (holotype RB!; isotypes: RB! (7 sheets)).
Herbs, woody at base, 30 – 70 cm tall, unbranched. Stems 2 – 5 mm in diam. below inflorescence, internodes 2 – 11 (– 24) mm long at base of plant, (22 – 25 –) 86 – 168 (– 251) mm below inflorescence. Leaves elliptic to narrowly elliptic, oblanceolate, lanceolate, obovate or ovate, 25 – 106 × 7 – 30 mm, base connate less than \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, margin green and straight, apex acute or acuminate, some mucronate; 1 – 4 pairs of secondary veins. Inflorescence 61 – 300 mm long, 2 – 7-flowered; bracts elliptic to narrowly elliptic, obovate, oblanceolate or lanceolate, 15 – 23 × 4 – 12 mm, base connate less than \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, apex acute or acuminate; bracteoles 1 – 2 pairs per flower, inserted at \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – 1 of pedicel length (from base), terminal flower commonly lacks subtending bracteoles, oblanceolate, narrowly elliptic or obovate, 8 –16 × 1.5 – 8.0 mm, base attenuate to connate less than \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of length, apex acute, some apiculate; pedicel (16 – 18 –) 37 – 90 (– 135) mm long at anthesis, 0.8 – 2.5 mm in diam. Calyx light green, brownish-green, yellowish-green, campanulate, 33 – 46 × 25 – 33 mm at anthesis, papillate on the inner side, not papillate on the outer side, dorsally winged, wings 2.3 – 4.1 mm wide, not reaching the calyx tube apex; lobes triangular, 11 – 20 × 7.5 – 11.2 mm, apex acute or acuminate. Corolla greenish-yellow, yellow, green, funnel-shaped, 36 – 39 (– 45) mm long, 0.9 – 1.0 times longer than calyx; tube 24 – 25 mm long, 2.5 – 4.5 mm wide at base, 5.7 – 6.5 mm wide below filament insertion, 12 – 23 mm wide at mouth; lobes widely ovate, 12 – 15 × 9.8 – 11.0 mm, margin crenulate, apex caudate. Filaments slightly unequal or equal in length, not twisted when dry, 22 – 26 mm long; anthers 4.9 – 5.0 mm long, attached to filament 1.7 – 2.1 mm from anther base. Ovary 10 – 15 mm long; style 16 – 20 mm long; stigma lobes very widely ovate or oblong, 3.0 – 3.3 mm long. Fruit ovoid, 16 mm long. Fig. 6.
Distribution. Brazil, Espírito Santo. Occurring on Atlantic mountains (Parque Estadual da Pedra Azul and Parque Estadual do Forno Grande). Map 1.
Specimens Examined. Espírito Santo: road Manhuaçu-Vitória, km 89, 7 Sept. 1977, Shepherd et al. 5834 (UEC!); Castelo, Parque Estadual do Forno Grande, 12 May 1949, Brade 19782 (RB!) & 13 Oct. 2000, Fraga & Kollmann 722 (MBML!, RB!) & 13 Oct. 2000, Kollmann & Fraga 3188 (MBML!, SPF!); Domingos Martins, Pedra Azul, 15 June 1985, Hatschbach & Silva 49407 (C!, MBM!, MO!, US!) & 19 Oct. 1994, Vieira & Gurken 650 (HB!) & road Vitória-Belo Horizonte, km 89, 16 June 1984, Shepherd & Pereira s.n. (ALCB! UEC!); Without locality, 1969, Marx s.n. (RB!) & 3 Sept. 1967, Duarte 10469 (HB!, RB!).
Habitat. Campo de altitude; 1000 – 1200 m.
Conservation Status. EN B1ab(i,iii,iv)+2ab(i,iii,iv). Prepusa viridiflora is restricted to two localities, c. 70 km apart, and classified as Endangered.
Phenology. Flowering specimens have been found in May, June, and Aug. to Oct.
Etymology. Prepusa viridiflora was named for its green calyx and corolla.
Senaea Taub. (1893: 515). Type species: Senaea coerulea Taub.
Shrubs to small trees, glabrous, branched. Stems cylindrical at base, cylindrical to quadrangular below inflorescence, with numerous, discontinuous, thin vertical ridges plus two pairs of prominent, continuous vertical ridges that extend from each interstipular line to node directly below. Leaves not clustered at base or at branch apices, green, sessile, fleshy; margin entire, hyaline, green; venation acrodromous, primary and secondary veins conspicuous, tertiary veins inconspicuous. Inflorescence terminal, thyrsoid; bracts and bracteoles leaflike with entire, hyaline margins. Flowers actinomorphic, perfect, 6-merous, pedicellate. Calyx green to purplish-green, campanulate, inflated, membranaceous or marcescent; lobes equal or with deeper divisions between every second or third lobe; colleters present on inside of calyx. Corolla blue to purple, campanulate, with a constriction at the level of stamen insertion and widening above, membranaceous, papillate on inside and outside; lobes aestivation contort. Stamens 6, equal in length, exserted, inserted in the corolla tube, usually in the lower third; filaments filiform, twisted when dry; anthers yellow, narrowly ovoid, sagittate at base, dorsifixed, with sterile appendix on apex, introrse. Ovary ovoid; style slender, flattened and twisted when dry; stigma bilamellate; placentation parietal; ovules numerous. Fruit a capsule, unilocular, 2-valvate, many-seeded, with persistent calyx, corolla, and style.
Senaea comprises two species, which are reported from high altitude areas of the Brazilian states of Minas Gerais and Rio de Janeiro. Map 2.
Key to the species of Senaea
-
1. Leaves oblanceolate, obtuse and mucronate at apex; pedicels papillate; calyx papillate on outside, calyx lobe apices longer than 1.6 mm; corolla 29 – 30 mm long, corolla lobes lanceolate; filaments 16.6 – 17.1 mm long; ovary c. 9 – 10 mm long; style c. 16 mm long. (Minas Gerais). . . . . . . . . . . . . . . . . . . . . . . .
6. S. coerulea
-
1. Leaves elliptic, acute at apex; pedicels not papillate; calyx not papillate on outside, calyx lobe apices shorter than 0.8 mm; corolla 20 – 23 mm long, corolla lobes ovate; filaments 9.2 – 12.0 mm long; ovary c. 6 mm long; style 9.5 – 12.5 mm long. (Rio de Janeiro). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7. S. janeirensis
6. Senaea coerulea Taub. (1893: 516); Gilg (1895: 96). Type: Brazil, Minas Gerais, Biribiry (sic), 29 March 1892, Glaziou 19739, (holotype C; isotypes K!, P!).
Shrubs to small trees, up to 2 m tall, branched. Stems 3.1 – 3.3 mm in diam. below inflorescence, internodes 19 – 60 mm long below inflorescence. Leaves oblanceolate, 61 – 105 × 13 – 31 mm, base long attenuate, margin revolute, apex obtuse and mucronate or mucronulate; 1 – 2 pairs of secondary veins. Inflorescence 90 – 180 mm long, 15 – 31-flowered; bracts oblanceolate, linear or rarely narrowly elliptic, 17 – 77 × 1.2 – 20.0 mm, base attenuate, apex obtuse and mucronate, acute or acuminate; bracteoles 1 pair per flower, inserted at \({\raise0.7ex\hbox{$3$} \!\mathord{\left/ {\vphantom {3 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – \({\raise0.7ex\hbox{$9$} \!\mathord{\left/ {\vphantom {9 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of pedicel length (from base), terminal flower commonly lacks subtending bracteoles, linear, 7.4 – 16.0 × 0.3 – 1.3 mm, base attenuate to truncate, apex acute to acuminate; pedicel 1.9 – 12.0 mm long, 0.7 – 0.9 mm in diam., papillate. Calyx not winged, 6.9 – 12 × 5.0 – 5.8 mm at anthesis, not papillate on the inner side, papillate on the outer side; lobes transversely elliptic or narrowly transversely elliptic, 2.0 – 2.5 × 1.7 – 4.3 mm. Corolla 29 – 30 mm long, 2.5 – 3.3 times longer than calyx; tube 13.5 – 16.1 mm long, 2.0 – 2.5 mm wide at base, 5.0 – 5.2 mm wide below filament insertion, 5.5 – 13 mm wide above filament insertion, 13.0 – 19.3 mm wide at mouth; lobes lanceolate, 13.8 – 16.0 × 4.1 – 6.2 mm, margin entire, ondulate or crenulate, apex acute. Filaments 16.6 – 17.1 mm long; anthers 4.2 – 4.7 mm long, attached to filament at 1.4 – 1.7 mm from anther base. Ovary 9 – 10 mm long; style c. 16 mm long; stigma lobes oblong, 1.8 – 2 mm long. Fruit ovoid, c. 13 mm long. Fig. 7.
Distribution. Brazil. Minas Gerais. Occuring in Cadeia do Espinhaço in scattered locations. Map 2.
Specimens Examined. Minas Gerais: Carmo do Rio Claro, Fazenda Córrego Bonito, 7 Sept. 1961, Andrade & Emmerich 1110 (HB!); Congonhas do Norte, Serra da Mangabeira, close to Rio Preto, 18°5′0″S 43°49′0″W, 23 April 1982, Amaral et al. CFSC 8476 (SP!, SPF!); Diamantina, 20 Jan. 1947, Romariz 127 (RB!) & 1904, Schwacke s.n. (BHCB!).
Habitat. Campo rupestre and cerrado, sometimes, on sandy soils close to river margins.
Conservation Status. CR A4abc; B2ab(i,ii,iii,iv). Senaea coerulea is here classified as Critically Endangered, although it is not certain if this species still exists in the wild, since it has not been collected for over 20 years. The places where it was collected are now mainly occupied by towns or pasture areas, and its extent of occurrence must therefore be very fragmented if the species is still extant.
Phenology. Flowering specimens have been collected in Jan., April (also with fruits), and Sept.
Etymology. Senaea coerulea was named for its blue corolla.
7. Senaea janeirensis Brade (1932: 118). Type: Brazil, Rio de Janeiro, Serra do Imbé, Alto da República, 1500 m, April 1932, Brade & Santos Lima 11784 (holotype R!).
Shrubs to small trees, 40 – 150 cm tall, branched. Stems 3.5 – 6.0 mm in diam. below inflorescence, internodes 23 – 40 mm long below inflorescence. Leaves narrowly elliptic, 50 – 80 × 15 – 28 mm, base long attenuate, margin revolute, apex acute; 2 pairs of secondary veins. Inflorescence 40 – 150 mm long, 11 – 35-flowered; bracts linear, narrowly elliptic or rarely elliptic, 12 – 53 × 1 – 22 mm, base attenuate, apex acute or acuminate; bracteoles 1 pair per flower, inserted at \({\raise0.7ex\hbox{$3$} \!\mathord{\left/ {\vphantom {3 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) – \({\raise0.7ex\hbox{$9$} \!\mathord{\left/ {\vphantom {9 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of pedicel length (from base), terminal flower commonly lacks subtending bracteoles, linear or rarely narrowly oblong, 7.2 – 15.0 × 0.6 – 1.6 mm, base attenuate to truncate, apex acuminate; pedicel 6 – 20 mm long, 0.8 – 1.4 mm in diam., not papillate. Calyx not winged, 6.9 – 8.0 × 5.2 – 8.5 mm at anthesis, not papillate; lobes transversely elliptic or narrowly transversely elliptic, 0.7 – 2.8 × 2.0 – 4.3 mm, apex caudate to mucronate. Corolla 20 – 23 mm long, c. 2.9 times longer than calyx; tube 11 – 12 mm long, c. 3.5 mm wide at base, 5.2 – 6.5 mm wide below filament insertion, 5.4 – 10.0 mm wide above filament insertion, 8 – 10 mm wide at mouth; lobes ovate, 9 – 11 × 4.1 – 5.0 mm, margin entire, ondulate or crenulate, apex acute to acuminate. Filaments 9.2 – 12.0 mm long; anthers 4.8 – 5.3 mm long, attached to filament at 2.0 – 2.5 mm from anther base. Ovary c. 6 mm long; style 9.5 – 12.5 mm long; stigma lobes elliptic, 1.8 – 2.3 mm long. Fruit not seen. Fig. 8.
Distribution. Brazil, Rio de Janeiro. Occurring on Pedra da República and Pedra do Desengano (Parque Estadual do Desengano). Map 2.
Specimens Examined. Rio de Janeiro: Santa Maria Madalena, Nov. 1933, Santos Lima 217 (RB!, U!) & Parque Estadual do Desengano, Pedra da República, 3 March 1935, Santos Lima & Brade 14215 (RB!) & March 1937, Santos Lima 62 (RB!) & Pedra do Desengano, 17 Dec. 1986, Martinelli et al. 12003 (RB!).
Habitat. Campo de altitude; 1500 – 1600 m.
Conservation Status. CR A4b; B1ab(i,ii)+2ab(i,ii). Senaea janeirensis is classified as Critically Endangered due to its small extent of occurrence, restricted to only one mountain peak. It has not been collected for over 20 years.
Phenology. Flowering specimens have been found in March, and Nov. to Dec.
Etymology. Senaea janeirensis was named after its geographic locality, Rio de Janeiro.
References
Barroso, G. M. (1986). Sistemática de angiospermas do Brasil, V. 3, p. 57. Imprensa Universitária, Universidade Federal de Viçosa, Viçosa, Brasil.
Brade, A. C. (1932). Espécies novas de plantas do estado do Rio de Janeiro. Arq. Mus. Nac. Rio de Janeiro 34: 118.
Brade, A. C. (1949). Contribuição para o conhecimento da flora do estado do Espírito Santo – II. espécies novas das famílias Orchidaceae, Rubiaceae e Gentianaceae. Arch. Jard. Bot. Rio de Janeiro 9: 18.
Cordeiro, I. (1987). Flora da Serra do Cipó: Gentianaceae. Bol. Bot. Univ. São Paulo 9: 227 – 242.
Gardner, G. (1839). Icon. Pl. 31: 225. Longman, Rees, Orme, Brown, Green & Longman, London.
Gardner, G. (1842). Bot. Mag. 15: 3909. Stephen Couchman, London.
Gilg, E. F. (1895). Gentianaceae. In: H. G. A. Engler & K. A. E. Prantl (eds.), Nat. Pflanzenfam. 4 (2): 50 – 108. Verlag von Wilhelm Engelmann, Leipzig.
Giulietti, A. M. & Pirani, J. R. (1988). Patterns of geographic distribution of some plant species from the Espinhaço range, Minas Gerais and Bahia, Brazil. In: P. E. Vanzolini & H. R. Heyer (eds.), Proceedings of a workshop on neotropical distribution patterns: 39 – 69. Academia Brasileira de Ciências, Rio de Janeiro.
Grisebach, A. H. R. (1839). Gen. sp. Gent. J. G. Cotta, Stuttgart, Tübingen.
Grisebach, A. H. R. (1845). Gentianaceae. In A. L. P. P. de Candolle (ed.) Prodr.: Gentianaceae. 9. Fortin, Masson et Sociorum, Paris.
Harley, R. M. & Simmons, N. A. (1986). Florula of Mucugê, Chapada Diamantina, Bahia, Brazil. A descriptive check-list of the campo rupestre area. Royal Botanic Gardens, Kew.
Huelsenbeck, J. P. & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754 – 755.
IUCN (2001). IUCN Red List categories and criteria. Version 3.1. IUCN, Gland, Switzerland & Cambridge, U.K.
Maddison, D. R. & Maddison, W. P. (2005). MacClade 4: Analysis of phylogeny and character evolution, vers. 4.08. Sinauer Associates, Sunderland, Massachusetts, U.S.A.
Martius, C. F. P. Von. (1827). Nov. Gen. sp. pl. 2 (2): 121. Wolf, München.
Mészáros, S., De Laet, J., Goethals, V., Smets, E. & Nilsson, S. (2002). Cladistics of Gentianaceae: a morphological approach. In: L. Struwe & V. A. Albert (eds.), Gentianaceae: systematics and natural history, pp. 310 – 376. Cambridge University Press, Cambridge, U.K.
Nilsson, S. (2002). A review of palynology. In: L. Struwe & V. A. Albert (eds.), Gentianaceae: systematics and natural history, pp. 377 – 497. Cambridge University Press, Cambridge, U.K.
Porto, P. C. & Brade, A. C. (1935). Contribuição para a Flora Fluminense. Arq. Inst. Biol. Veg. 13: 222.
Progel, A. (1865). Gentianaceae. In: C. F. P. Von Martius, Fl. Bras. 6 (1): 198 – 247. München.
Radford, A. E., Dickison, W. C., Massey, J. R., & Bell, C. R. (1974). Vascular Plant Systematics. Harper & Row Publishers Inc., New York.
Ronquist, F. & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed model. Bioinformatics 19: 1572 – 1574.
Safford, H. D. (1999). Brazilian Páramos I: an introduction to the physical environment and vegetation of campos de altitude. J. Biogeogr. 26: 693 – 712.
Safford, H. D. & Martinelli, G. (2000). Southeast Brazil. In: S. Porembski, & W. Barthlott (eds.), Inselbergs—Biotic diversity of isolated rock outcrops in tropical and temperate regions, pp. 339 – 389. Springer-Verlag, Berlin, Heidelberg.
Stearn, W. T. (1992). Botanical Latin — History, Grammar, Syntax, Terminology and Vocabulary. 4th edition. Timber Press, Portland, Oregon.
Struwe, L., Albert, V. A., Calió, M. F., Frasier, C., Lepis, K. B., Mathews, K. G. & Grant, J. R. (submitted). Evolutionary patterns in neotropical Helieae (Gentianaceae): evidence from morphology, chloroplast and nuclear DNA sequences. Taxon.
Struwe, L., Kadereit, J. W., Klackenberg, J., Nilsson, S., Thiv, M., von Hagen, K. B. & Albert, V. A. (2002). Systematics, character evolution, and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: L. Struwe, & V. A. Albert (eds.), Gentianaceae: systematics and natural history, pp. 21–309. Cambridge University Press, Cambridge, U.K.
Swofford, D. L. (2003). PAUP*: phylogenetic analysis using parsimony (*and other methods), vers. 4.0. Sinauer Associates Inc., Sunderland, Massachusetts, U.S.A.
Taubert, P. (1893). Plantae glaziovianae novae vel minus cognitae IV. Bot. Jahrb. Syst. 17: 516.
Veloso, H. P., Rangel Filho, A. L. R. & Lima, J. C. A. (1991). Classificação da vegetação brasileira, adaptada a um sistema universal. IBGE, Departamento de Recursos Naturais e Estudos Ambientais, Rio de Janeiro.
Weberling, F. (1989). Morphology of flowers and inflorescences. Cambridge University Press, New York.
Acknowledgements
This research was supported by NSF (grant 317612 to LS), New Work Consortium (planning grant to LS and JRP), FAPESP (Ph.D. fellowship 03/10918 – 3 to MFC), and IAPT Research grant (2006, to MFC). We thank Jason R. Grant for help coding morphological features of Macrocarpaea, Daniela Zappi and Inês Cordeiro for suggesting groups of plants with similar patterns of distribution, Richard Winkworth for helping with analyses, Philip Miarmi for providing the maps, Bobbi Angell for specimen illustrations, and all herbaria and their staff mentioned under Material and Methods for loans. We also thank Thomas Waytt and the two anonymous reviewers for comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Morphological characters with states coded.
1. Habit: herbaceous, not woody (0)/woody only at base (1)/woody from base to apex (2)
2. Prominent decurrent ridges on stems: absent (0)/present (1)
3. Leaf arrangement at base: not in basal rosette (0)/basal rosette (1).
Taxa presenting leaves evenly distributed along stem (and not grouped at base) were coded as “not in basal rosette”.
4. Leaf general texture: membranaceous (0)/fleshy, choriaceous (1)
5. Leaf margin texture: hyaline, membranaceous (0)/not hyaline, chartaceus or choriaceous (1)
6. Leaf apex and margin color: green (0)/red to magenta (1)
7. Leaf apex shape: acute or acuminate (0)/obtuse (1)
8. Leaf connation at base: not connate (0)/connate (1).
Leaf base is attenuate for most species, but some species have leaves connate at base.
9. Leaves venation: acrodromous (0)/brochidrodromous, pinnate (1)
10. Midrib prominence below: absent (0)/present (1)
11. Petiole: absent (0)/present (1)
12. Bracts base: not connate (0)/slightly connate (1)/strongly connate (2).
Bracts were coded as “not connate” when they were attenuate at base, as “slightly connate” when the bracts were connate only \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {10}}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${10}$}}\) of their total length, and as “strongly connate” when the connation was superior than \({\raise0.7ex\hbox{$2$} \!\mathord{\left/ {\vphantom {2 5}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$5$}}\) of the bract total length.
13. Bracteoles subtending terminal flower: absent (0)/present (1)
14. Calyx merosity: 4 (0)/5 (1)/6 (2)
15. Calyx main colour: green, yellow, cream (0)/red, purple (1)
16. Calyx overall shape: urceolate (0)/campanulate (1).
Calyx was coded as “urceolate” when it was constricted at mouth and as “campanulate” when the mouth was broader or as broad as the calyx tube.
17. Calyx texture: membranaceous (0)/coriaceous (1)
Membranaceous calyces are those with thin texture, while coriaceous calyces are leathery and thick.
18. Calyx papillae on the outside: absent (0)/present (1)
19. Calyx papillae on the inside: absent (0)/present (1)
20. Calyx dorsal thickening: absent (0)/present (1)
The calyx dorsal thickening is a glandular area that is more prominent than the rest of the calyx.
21. Calyx wings: absent (0)/present (1)
22. Corolla symmetry: actinomorphic (0)/zygomorphic (1)
23. Corolla merosity: 5 (0)/6 (1)
24. Corolla basic colour: white, cream, yellow (0)/blue, rose, purple (1)
25. Corolla tube below stamen insertion: not constrained (0)/constrained (1)
26. Corolla papillae on both sides of corolla: absent (0)/present (1)
27. Corolla fleshiness: not fleshy (0)/fleshy (1)
28. Corolla bud apex: acute (0)/round (1)
29. Stamen length: equal (0)/unequal (1)
30. Position of stamens: included in corolla (0)/exserted from corolla (1)
31. Filaments in cross-section: filiform (0)/flattened (1)
32. Pollen aggregation: monads (0)/tetrads (1)/polyads (2)
33. Style cross-section: cylindric (0)/flattened (1)
Appendix B
Appendix C
Index to collectors and material of all analysed specimens. Data are presented in the following sequence: collection, taxon number in the taxonomic treatment, herbarium. Prepusa alata = 1; P. connata = 2; P. hookeriana = 3; P. montana = 4; P. viridiflora = 5; Senaea coerulea = 6; S. janeirensis = 7.
Amaral et al. CFSC 8476 (6) (SP!, SPF!); Amorim et al. 3009 (4) (CEPEC!, NY!, SP!); Andrade & Emmerich 1110 (6) (HB!)
Bacia 534 (3) (R!); Barroso et al. s.n. (4) (SPF!); Bautista & Silva 259 (4) (HRB!); Brade s.n. (3) (RB!), 9502 (3) (R!), 9620 (3) (R!), 10753 (3) (BHCB!, R!), 12464 (3) (R!), 19782 (5) (RB!)
Calió et al. 116 (4) (SPF!); Camerilo B640 (3) (K!); Cappel s.n. (3) (RB!); Cappeli s.n. (3) (RB!); Carauta et al. (3) (GUA!); Carris s.n. (3) (GUA!, RB!); Castellanos 21660 (3) (LIL!); Cavalcanti et al. 3214 (4) (CEN, SPF!); Coradin et al. 6230 (4) (CEN!, RB!); Costa et al. 508 (3) (SP!, SPF!)
Dionysio s.n. (3) (RB!); Duarte s.n. (3) (RB!), 10469 (5) (HB!, RB!); Duarte & Pereira 9208 (4) (GUA!, RB!)
Farney & Caruso 1195 (1) (CEPEC, MG, RB!); Farney et al. 795 (3) (K!, NY!, RB!); Ferreira 555 (4) (CTES!, HRB!, MBM!, RB!); Flaster 64 (3) (R!); Fraga & Kollmann 722 (5) (MBML!, RB!); Franzen 42 (2) (MBM!); Freire-Fierro et al. 1749 (4) (SPF!), 1767 (4) (CHRB!, SPF!); Furlan et al. 1926 (4) (CHRB!, K!, SPF!)
Gabinete de Botânica da Escola Politécnica 7351 (3) (R!); Gajardo & Sazima 3 (3) (UEC!); Ganev 816 (4) (HUEFS!, K!, NY!, SP!, SPF!); Gardner s.n. (2) (R!), s.n. (3) (BM!), s.n. (3) (K!), s.n. (3) (S!); Giulietti et al. 1926 (4) (HUEFS!); Glaziou 3813 (2) (BR!, C!, P!, R!), 3814 (3) (BR!), 4099 (3) (C!), 15242 (3) (C!, G!, K!, P!, RB!), 16236 (3) (R!), 16363 (3) (C!, K!, P!, RB!), 17238 (2) (C!, IAN!, MO!, NY!), 18372 (2) (BM!, BR!, G!, C!, K!, NY!(2), P!); Gomes et al. 153 (1) (CHRB!, K, SPF!, SP!); Guedes et al. 1543 (4) (ALCB!); Gusmão 305 (4) (ALCB!, HRB!, SP!)
Hage et al. 2330 (4) (CEPEC, HUEFS!, SP!, SPF!); Harley et al. 22755 (4) (CEPEC!, K!(2), SPF!), 22878 (4) (AAU!, CEPEC!, K!, NY!, SPF!, US!); Hatschbach 44263 (4) (C!, MO!)
Hatschbach & Guimarães 42398 (4) (AAU!, C!, CTES!, INPA!, MBM!, MO!, SPF!, UB!, US!); Hatschbach & Kumrow 47930 (4) (BR!, HUEFS!, MBM!, MO!, UPCB!, US!); Hatschbach & Silva 49407 (5) (C!, MBM!, MO!, US!); Hind et al. 3165 (4) (ALCB!, CEPEC!, IBGE, SPF!)
Irwin et al. 32295A (4) (MO!, NY!, US!), 32327 (4) (MO!, NY!)
Kirkbride Jr. et al. 1723 (3) (C!, NY!, R!, UB!, US!); Kollmann & Fraga 3188 (5) (MBML!, SPF!)
Lima 3900 (4) (CEPEC!, K!, SPF!); Lützelburg s.n. (3) (M!(2), NY!), 532 (4) (M!, NY!)
Martinelli 240 (2) (BR, CEN!, CEPEC, F, GUA!, K, LIL, MG, MO, MBM!, RB!), 602 (2) (RB!), 2561 (2) (RB!), 5243 (4) (CEPEC, K, MG, RB!), 9889 (2) (RB!); Martinelli & Santos 6125 (2) (RB!); Martinelli & Simonis 9055 (3) (RB!, US!); Martinelli et al. 5259 (4) (RB!), 5275 (4) (GUA!, RB!), 5425 (4) (RB!), 5507 (4) (RB!), 9335 (2) (RB!), 11148 (2) (RB!), 12003 (7) (RB!), 13140 (1) (RB!); Marx s.n. (5) (RB!); Menezes et al. 1452 (4) (CHRB!, HUEFS! K!, SPF!), 5823 (4) (K!, SPF!); Mori & Boom 14469 (4) (NY!); Mori et al. 12552 (4) (CEPEC!, RB! US!); Moura s.n. (3) (R!)
Newton Santos & Frota Pessoa s.n. (3) (R!)
Oliveira et al. 64 (4) (HUEFS!, SPF!); Orlandi 268 (4) (HRB!, HUEFS!)
Paula-Souza et al. 4866 (4) (ESA!); Peixinho & Vanilda s.n. (4) (HUEFS!, SP!); Pederneiras 16 (2) (R!); Pereira 2020 (4) (GUA!, RB!); Pereira & Duarte 10118 (4) (F, HB!, K, M!); Pereira & Gusmão s.n. (4) (ALCB!); Pinto 116/85 (4) (HRB!, UB!)
Queiroz et al. 1846 (4) (HUEFS!, MBM!, NY!, UEC!)
Ribeiro s.n. (3) (R!); Ribeiro et al. 2280 (2) (GUA!); Romariz 127 (6) (RB!)
Saldanha 7357 (3) (RB!); Santos et al. 1221 (3) (HB!, R!); Santos Lima 62 (7) (RB!), 217 (7) (RB!, U!); Santos Lima & Brade 14101 (1) (RB!), 14215 (7) (RB!); Schwacke s.n. (6) (BHCB!); Shepherd & Kirszanzaft 9958 (3) (UEC!); Shepherd & Pereira s.n. (5) (ALCB!, UEC!); Shepherd et al. 5834 (5) (UEC!); Silva s.n. (3) (RB!); Simonis & Martinelli 20 (2) (RB!), 26 (3) (NY!, RB!); Souza & Brito s.n. (4) (ALCB!); Souza et al. 26365 (4) (ESA!, SP!); Strang 272 (3) (GUA!)
Vianna 116 (3) (RB!); Vidal 182 (3) (R!), 330 (3) (R!), 597 (3) (R!), 6431 (3) (R!), 6447 (3) (R!), 6480 (3) (R!); Vieira & Gurken 650 (5) (HB!); Vilaça & Ribeiro 127 (3) (GUA!)
Wanderley et al. s.n. (4) (SP!)
Rights and permissions
About this article
Cite this article
Calió, M.F., Pirani, J.R. & Struwe, L. Morphology-based phylogeny and revision of Prepusa and Senaea (Gentianaceae: Helieae) — rare endemics from eastern Brazil. Kew Bull 63, 169–191 (2008). https://doi.org/10.1007/s12225-008-9030-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-008-9030-1