Abstract
The genus Panus and many of its species have a wide geographic distribution, and in-depth up-to-date taxonomic review is needed that includes critical review of type materials within a phylogenetic frame. In order to recover the phylogenetic relationships within Panus species and their morphological boundaries and to critically analyze the diversity recorded for Brazil, we carried out fieldwork in poorly explored areas in the country and morphological and literature revisions of fungarium specimens, including several type materials. We present a comprehensive phylogeny of Panus and discuss several taxonomic and nomenclatural implications in order to achieve stability for species of the genus. Four new species are proposed, P. capelariae, P. pachysporus, P. speciosus, and P. stiptonotatus. Panus campinensis and P. thailandicus (an endophytic species) are proposed as new combinations in the genus, based on a morphological revision and phylogenetic evidence of their types, respectively. Additionally, Endopandanicola is synonymized within Panus, and P. parvus is synonymized within P. strigellus. The occurrence of P. conchatus, P. convivalis, P. fulvus, P. similis, and P. tephroleucus in Brazil is rejected due to morphological and phylogenetic evidences. For P. conchatus and P. similis, we present bases for the recognition of its sensu stricto status. We also discuss nomenclatural issues surrounding the Lentinus velutinus complex that include the basionym elucidation, its sensu stricto delimitation, and an epitypification based on a new sequenced specimen from the type locality. Our comprehensive assessment of Panus in Brazil has led to the confirmation of ten species supported by morphological and/or molecular data, which are critically discussed, and an identification key is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panus Fr. was proposed by Fries (1838) and encompasses wood-inhabiting species characterized by centrally to eccentrically stipitate basidiomata, infundibuliform to cyathiform pilei, and a hymenophore with decurrent lamellae. Species are distinguished by a dimitic hyphal system featuring unbranched skeletal hyphae, cystidia ranging from thin to thick-walled, and ellipsoid, smooth, thin-walled basidiospores (Corner 1981). Panus exhibits remarkable diversity and wide geographic distribution, with many species described during the nineteenth century (Fries 1821; 1830; Léveillé 1844; Berkeley and Curtis 1869; Berkeley and Broome 1873; Corner 1981). However, the genus remains underrepresented in molecular phylogenetic studies, and its phylogenetic relationships remain poorly understood. Only a limited number of species have molecular data available, with the majority of them having been recently described, such as P. subfasciatus Thongbai, Karun., C. Richt. & K.D. Hyde, P. roseus (Karun., K.D. Hyde & Zhu L. Yang) N. Vinjusha & T.K.A. Kumar, and P. paraibensis V. Galvão, Koroiva & Wartchow (Tibpromma et al. 2017; Vinjusha and Kumar 2022; Galvão et al. 2023, respectively). Furthermore, little or nothing is known about the species diversification and biogeographical patterns, character evolution, or their ecology.
Panus was previously classified as an infrageneric taxon of Lentinus Fr. (Pegler 1971, 1972, 1983), viz., Lentinus subg. Panus (Fr.) Pegler, and consequently, many Panus species remain under Lentinus. Therefore, those taxa should be revised, including the type material reexamination, in order to assess their taxonomic limits and generic position. There are ca. 150 names of Panus listed in Index Fungorum (http://indexfungorum.org, accessed 01/05/2023), of which most of their type specimens and information (morphological, molecular, and locality) are not easily accessible. This is mainly because around 95% of these names were proposed between the eighteenth and twentieth centuries. Consequently, they exhibit challenges such as poor preservation and small size, rendering them less amenable to loan requests (Dayarathne et al. 2016).
Few studies have explored the diversity of Panus in the Neotropics, with the majority of them describing new species solely based on morphological data. In Brazil, 12 Panus species have been recorded: Panus ciliatus (Lév.) T.W. May & A.E. Wood (e.g., Rick 1907), P. conchatus (Bull.) Fr. (Spegazzini 1889), P. convivalis Corner (Corner 1981), P. fulvus (Berk.) Pegler & R.W. Rayner (https://specieslink.net/, accessed 01/05/2023), P. hymenorhizus Speg. (Spegazzini 1889), P. neostrigosus Drechsler-Santos & Wartchow (e.g., Spegazzini 1889; Rick 1907; Pegler 1983; Drechsler-Santos et al. 2012; Vargas-Isla et al. 2015; Sanuma et al. 2016), P. paraibensis (Galvão et al. 2023), P. parvus Drechsler-Santos & Wartchow (Drechsler-Santos et al. 2012), P. similis (Berk. & Broome) T.W. May & A.E. Wood (e.g., Teixeira 1946; Meijer 2006; Cavalcante et al. 2021), P. strigellus (Berk.) Chardón & Toro (e.g., Rick 1930; Pegler 1983; Sanuma et al. 2016; Cavalcante et al. 2021), P. tephroleucus (Mont.) T.W. May & A.E. Wood (e.g., Pegler 1983, 1997), and P. velutinus (Fr.) Fr. (e.g., Fries 1830; Berkeley 1843; Spegazzini 1889; Teixeira 1946; Batista et al. 1966; Pegler 1983; Sanuma et al. 2016; Cavalcante et al. 2021).
Among those species, only P. neostrigosus, P. paraibensis, and P. strigellus have reliable and verified molecular data, alongside detailed morphological descriptions (Vargas-Isla et al. 2015; Galvão et al. 2023). For a few other Panus species reported in Brazil, molecular data exist from other countries (e.g., P. conchatus, P. similis, and P. velutinus). However, these data still require verification to serve as references for their respective taxa, ensuring taxonomic and phylogenetic stability for future research on the genus. Panus strigellus and P. tephroleucus were originally described from Cuba (Berkeley and Curtis 1869) and Suriname (Pegler 1983), respectively, and their presence in Brazil may indeed reflect their true distributional range (Maia et al. 2015; Vargas-Isla et al. 2015). However, for other species recorded in Brazil, such as P. ciliatus (type from Indonesia), P. conchatus (type from Sweden), and P. fulvus and P. similis (both types from Sri Lanka), their wider distribution raises questions and could potentially involve misidentifications (Putzke 1994; Maia et al. 2015; Putzke and Putzke 2002).
Multiple studies have shown that Agaricomycetes species with occurrences on multiple continents, usually referred to as “cosmopolitan species,” often consist of several species with restricted distribution ranges that represent a species complex (Palacio et al. 2017; Peintner et al. 2019; Motato-Vásquez et al. 2020; Olou et al. 2020; Liu et al. 2021). Among the species described based on Brazilian specimens, P. velutinus has been widely recorded worldwide and its delineation remains ambiguous, leading to suggestions that it constitutes a species complex (Pegler 1983; Douanla-Meli and Langer 2010). In light of these uncertainties, a taxonomic revision of Panus in Brazil is warranted, and species with broad and disjointed distributions should be subjected to further study to ascertain their taxonomic status and potentially unveil undescribed species in the Neotropics.
To ensure a comprehensive phylogenetic analysis and to provide an in-depth assessment of Panus diversity in Brazil, we conducted field expeditions in poorly explored regions of the Brazilian Atlantic Forest. We performed detailed morphological examinations of both newly collected specimens and specimens from fungarium collections. Furthermore, we conducted molecular phylogenetic analyses utilizing DNA sequences from both ITS and nrLSU regions. In addition to proposing new taxa, we reevaluated the boundaries of several Panus species and conducted a thorough review of all recorded occurrences of Panus names in Brazil.
Material and methods
Specimens and morphological descriptions
Specimens were collected during field surveys in the Atlantic Rainforest of Southern Brazil [classification according to Oliveira-Filho (2015)]. They were subsequently deposited at the FLOR herbarium. Additionally, we examined specimens from other herbaria, namely, BAFC, IAC, FLOR, LISU, LPS, K, SP, TENN, UPS, and URM. Herbarium acronyms follow Thiers (continuously updated). Color determinations follow Küppers (1994). For the observation and description of microscopic characteristics, we manually prepared sections and mounted them in a solution of 5% potassium hydroxide with aqueous phloxine 1%. We also employed Melzer’s reagent (IKI) to assess the amiloidicity reaction. The hyphal system was described based on Teixeira (1995). The terminology for the basidia, pleurocystidia, and cheilocystidia shapes follows Vellinga and Noordeloos (2001). The description of pileipellis follows Pegler (1983). Microscopic structures were measured (N = 20) using a micrometer ruler eyepiece attached to an optical microscope (Olympus CX22LED OM). In basidiospore measurements, the “[a/b/c]” at the beginning indicates “a” number of basidiospores measured from “b” number of basidiomata taken from “c” number of collections. Basidiospores were measured in lateral view, with a minimum of 20 basidiospores for each basidioma. The terminology used to describe the basidiospore shapes follows Largent et al. (1977). The Q value represents the length-to-width quotient interval for all measured basidiospores; Qm represents the average of all calculated Q values for all measured basidiospores, while Lm and Wm represent the average lengths and widths of all measured basidiospores, respectively.
DNA extraction, amplification, and sequencing methods
Genomic DNA was extracted from dried materials stored in silica gel using a CTAB protocol (Góes-Neto et al. 2005). The primer pairs ITS1F-ITS4 (White et al. 1990; Gardes and Bruns 1993) and LR0R-LR5 (Vilgalys and Hester 1990) were used to amplify the nuc rDNA internal transcribed spacer region ITS1-5.8S-ITS2 (ITS) and the nuc rDNA 28S (nrLSU), respectively. Amplification parameters for each region were as follows: ITS − 94 °C for 5 min, followed by 35 cycles 94 °C for 3 min, 50 °C for 30 s, and 72 °C for 1 min, and a final extension at 72 °C for 10 min; and nrLSU − 94 °C for 5 min, followed by 35 cycles 94 °C for 1 min, 50 °C for 1 min 20 s, and 72 °C for 1 min 30 s, and a final extension at 72 °C for 10 min. The PCR products were purified with Polyethylene Glycol according to Sambrook et al. (1989). The sequencing of both markers was conducted with the same primers used in the amplification, and it was done by capillary electrophoresis in an ABI3730 device, using BigDye 3.1 polymer (Myleus Biotechnology, Belo Horizonte, Brazil).
Phylogenetic analyses
The generated chromatograms were manually verified with Geneious 9.0.5 (Kearse et al. 2012), and the final sequences were deposited at GenBank (https://www.ncbi.nlm.nih.gov/). GenBank accession numbers for newly provided sequences and additional downloaded sequences for the analyses are listed in Table S1. Sequences of Cerrena unicolor (Bull.) Murrill were used as outgroups based on Justo et al. (2017). The sequences were aligned in MAFFT 7 with strategy Q-INS-i for ITS and G-INS-i for nrLSU (Katoh 2013) and then manually edited using MEGA 7 (Kumar et al. 2016).
We carried out phylogenetic analyses based on two datasets: ITS + nrLSU combined and only ITS. From the combined dataset, a total of 208 ingroup specimens were included. All phylogenetic analyses were performed online using the CIPRES Science Gateway (Miller et al. 2010). Phylogenetic tree of only the ITS dataset was reconstructed based on maximum likelihood analysis. All of the following methods were applied to the combined dataset. We defined partitions a priori (ITS + nrLSU) to estimate the best-fit partition scheme and substitution models in PartitionFinder 2 (Guindon et al. 2010; Lanfear et al. 2017) under the linked model of branch lengths, greedy search algorithm (Lanfear et al. 2012), and Akaike information criterion for model selection.
The maximum likelihood (ML) analyses were carried out in RAxML 8.2.9 (Stamatakis 2014). For each partition, GTR + G model was applied. The analysis initially included 1000 rapid bootstrap inferences, followed by a thorough ML search. To assess node reliability, we conducted rapid bootstrapping replicates, with the program employing an extended majority rule (MRE)-based bootstopping criterion (Pattengale et al. 2009).
The Bayesian inference (BI) was performed in MrBayes 3.2.6 (Ronquist et al. 2012). For each partition, we employed partition-specific models determined as the best-fit models. In these partitioned mixed-model analyses, the substitution matrix, base frequencies, and gamma shape parameter were unlinked between data partitions, and the rate prior was set to variable [prset applyto = (all), ratepr = variable], allowing for variations in rates among partitions. We set to execute two independent runs, each initiated from random trees, employing four simultaneous independent chains. The analysis spanned 5,000,000 generations, with tree sampling occurring at every 1000th generation. Convergence diagnostics were computed every 10,000th generation. We assessed the minimal effective sample sizes (ESS > 200) and checked for convergence between runs using Tracer 1.7 (Rambaut et al. 2018). To ensure robust results, we discarded the initial 25% of sampled trees as burn-in. The remaining trees were used to construct a 50% majority-rule consensus tree and to estimate Bayesian posterior probabilities (BPPs) for the branches.
Clades with BPP values above 0.95 and bootstrap (BS) values above 70% were considered moderately supported, and those with BPP = 0.99–1 and BS = 99–100% were considered highly or fully supported (Felsenstein 1985; Hillis and Bull 1993; Soltis and Soltis 2003; Huelsenbeck and Rannala 2004). Only both moderately and highly/fully supported clades were deemed for taxonomic decisions. The recovered topology in the phylogenetic trees were organized in clades both moderately and highly supported main clades, being most inclusive as possible in order to reflect the phylogenetic relationships of the infrageneric lineages. In cases where a clade represents a single species, it was retained unless closely related clades lacked substantial support. The alignments and trees were deposited at Harvard Dataverse (Sousa-Guimarães et al. 2022).
Results
In this study, we incorporated 57 newly acquired sequences, comprising 34 ITS and 23 nrLSU sequences, as outlined in Table S1. The resultant combined dataset featured a final alignment spanning 1576 base pairs (716 bp for ITS and 860 bp for nrLSU). Among these, 963 were invariable, 198 were variable parsimony-uninformative, and 405 were parsimony-informative.
The optimal evolutionary models were determined through AIC (Akaike information criterion) selection, resulting in the following models: GTR + I + G for ITS and GTR + I + G for nrLSU. In BI analyses, the runs converged to stable likelihood values, specifically − lnL = 10,688.12 and 10,700.54. After discarding the initial 25% of trees as burn-in, 7501 trees were used to compute a 50% majority-rule consensus tree and to estimate the BPP. During ML searches, the combined alignment revealed 775 distinct patterns, with a proportion of gaps and undetermined characters totaling 48.44%. The bootstopping criteria in RAxML indicated that 550 pseudoreplications sufficed to determine internal branch support, with the final ML optimization likelihood recorded as − lnL = 10,571.85.
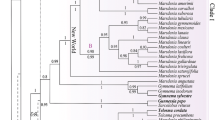
No significant topological conflicts were detected between the ML and BI analyses, thereby allowing us to superimpose both BS and BPPs onto the best-scoring ML tree for the combined ITS + nrLSU dataset (Fig. 1). The topology derived from ITS + nrLSU (Fig. 1) and solely ITS (SUPPL. Fig. 1) trees was congruent and the main clades are as follows. Eleven primary clades have been identified and designated from bottom to top in Fig. 1 and SUPPL. Fig. 1. From Fig. 1, the clades and their respective support values (BS/BPP) are as follows: “ciliatus” (BS = 86/BPP = 1), “neostrigosus” (100/1), “velutinus” (85/1), “paraibensis” (100/1), “asiaticus” (100/1), “speciosus” (100/1), “purpuratus” (100/1), “bambusinus” (52/0.96), “strigellus” (83/1), “roseus” (100/1), and “conchatus” (87/1) clades.
Phylogenetic tree (maximum likelihood) of Panus based on ITS and nrLSU. On branches, support values are given as BS/BPP. The black circles in the branches are BS ≥ 99 and BPP ≥ 0.99. The sequences generated in this study are in bold. Sequences in red are newly proposed taxa. The different colors in the maps distinguish the specimens sampled by country within each clade. Blue indicates countries with up to 15% from the clade sampling; yellow indicates 15–30% and red indicates more than 30% of the specimens in such a clade occur in a unique country
In the following clades, two or more phylogenetic species were recovered: “ciliatus,” “velutinus,” “asiaticus,” “bambusinus,” “strigellus,” “roseus,” and “conchatus.” Most of the eleven main clades, which encompass specimens from multiple countries, exhibit a predominantly pantropical distribution. Notably, this includes the clades “ciliatus” and “speciosus” and the subclade Panus aff. conchatus (Fig. 1 and Table S1). In contrast, the “neostrigosus” clade displays a widespread distribution, while specimens from other clades tend to be more geographically restricted (Fig. 1 and Table S1). Specifically, specimens from the “asiaticus,” “bambusinus,” and “roseus” clades primarily inhabit Asian regions, while the subclade Panus cf. tephroleucus (within the “velutinus” clade) and the “strigellus” clade are concentrated in the Neotropical region. Additionally, the subclade Panus conchatus s.s. is distributed within the temperate region of the Northern Hemisphere (Fig. 1 and Table S1).
Brazilian specimens represent at least nine phylogenetic species distributed as follows: P. ciliatus (SP446150) in the “ciliatus” clade; P. neostrigosus (CC40, Fun81W3, Fun8D3, and INPACM1466) in the “neostrigosus” clade; P. capelariae, P. cf. tephroleucus, P. stiptonotatus, and P. velutinus s.s. in the “velutinus” clade; P. paraibensis in the “paraibensis” clade; P. speciosus in the “speciosus” clade; and P. strigellus in the “strigellus” clade. Panus capelariae, P. stiptonotatus, and P. speciosus are newly proposed species, as detailed in the “Taxonomy” section.
Furthermore, we propose P. pachysporus as a new species and combine Lentinus campinensis Teixeira into the Panus genus, both of which are grounded in morphological data. Our study also revealed the presence of four endophytic samples located within Panus, spanning two distinct clades: KF496188 and KF496194 from Brazilian specimens in the “neostrigosus” clade, and the paratypes of Endopandanicola thailandica Tibpromma & K.D. Hyde from Thailand that we now treat as an independent species closely related to P. roseus. This scenario leads us to combine E. thailandica in Panus as P. thailandicus. We have also conducted a comprehensive revision of the list of Panus species found in Brazil and provide an identification key for the species that occur in the country with certainty.
Type revision and type localities
We morphologically analyzed the following holotypes that were loaned (the list is as follows: “species [type: type locality]”): Lentinus campinensis [IAC 4376: Brazil], Panus fasciatus (Berk.) Singer [K(M) 153553: Tasmania, Australia], P. hymenorhizus [LPS16800: Brazil], P. guaraniticus Speg. [LPS19295: Paraguay], P. parvus [URM80840: Brazil], P. strigellus [K(M) 153658: Cuba; syntype: K(M) 179354]. Additionally, we had access to photos of the holotypes of L. blepharodes Berk. & M. A. Curtis [K(M) 1436369: Cuba], L. pseudociliatus Raithelh. [BAFC52304: Argentina], L. thomensis Cout. [LISU63038: São Tomé and Príncipe], P. fulvus [K(M) 179387: Sri Lanka], P. similis [syntype: K(M) 179352: Sri Lanka], and P. velutinus [UPS F-012409: Brazil].
Furthermore, the following holotypes were not located or not confirmed at herbaria as cited by Pegler (1983) or confirmed as lost by the curatorships. Lentinus coelopus Lév. [PC: USA], L. natalensis Van der Byl [PREM: South Africa], Panus ciliatus [PC0093414: Indonesia], and P. tephroleucus [PC0737995: Suriname] were not confirmed by the curatorship at those herbaria as we have not received an answer from them. Panus conchatus [K(M) or UPS: Sweden] and P. neostrigosus [K(M): USA] are probably lost as K staff could not find them and we did not locate P. conchatus type in the UPS catalog (http://webdev.its.uu.se/evomus/botanik/home.php, accessed 02/10/2023). In addition, Lentinus castaneus Ellis & T. Macbr. [ISC: Nicaragua], L. fallax Speg. [LPS1271: Paraguay], and P. convivalis [K: Brazil] were confirmed as lost by the curatorship. Additionally, given the Panus circumscription the protologue of P. convivalis is inconclusive (Corner 1981).
The type of Lentinus velutinus Fr. was not found in UPS and was never studied by Pegler (1983) who, instead, provides a description based on Agaricus velutinus Fr. [= Panus velutinus (Fr.) Fr.]. The type of Lentinus velutinus Fr. should represent another lentinoid species and seems to be lost.
Taxonomy
Panus campinensis (Teixeira) Drechsler-Santos & Robledo, comb. nov. (Fig. 2).
Panus campinensis (holotype: IAC 4376 of Lentinus campinensis). a Basidioma overview, scale bar = 1 cm. b Detail of the lamellae and their insertion into the stipe, scale bar = 0.5 cm. c Surface detail and context of the stipe, scale bar = 0.2 cm. d Detail of lamellae and margin of incurvation, scale bar = 0.2 cm
MycoBank: 804004.
Basionym: Lentinus campinensis Teixeira, Bragantia 6:169. 1946.
Complete description: See Teixeira (1946).
Material examined: Brazil, São Paulo: Campinas, Bosque dos Jequitibas, 2 December 1943, F.P. Bastos s/n (IAC 4376!, holotype).
Notes: As outlined by Teixeira (1946), Lentinus campinensis displays distinctive characteristics, including a lateral to eccentric stipe and a reniform pileus [see Fig. b from plate III in Teixeira (1946)]. The upper or external surfaces of both the pileus and stipe exhibit a velutinate to villose texture (see Fig. 2c). Under microscopic examination, it has a dimitic hyphal system composed of hyaline to pale brown, unbranched skeletal hyphae and hyaline, clamped generative hyphae; it presents pleurocystidia and lacks hyphal pegs. This combination of features corresponds to the morphological circumscription of Panus (Corner 1981; Hibbett and Vilgalys 1993; Drechsler-Santos et al. 2012), and hence, the new combination is proposed.
This name had been previously regarded as a synonym of Lentinus velutinus due to the rather broad morphological concept of L. velutinus (Pegler 1983). However, upon conducting a detailed morphological analysis of the type specimen, significant distinctions were uncovered. Notably, the lateral to eccentric stipe, along with the reniform pileus that curves inwards from the margin should be regarded as additional diagnostic characteristics [see Fig. 2a, d and also refer to plate III in Teixeira (1946)]. Lentinus velutinus sensu Pegler (1983) is a species characterized by a more or less slender basidiomata, with an elongated stipe and a circular, infundibuliform pileus that typically folds in a distinctive manner when mature or dried (typically midway between the margin and the center of the pileus). Moreover, the holotype of L. campinensis has short ellipsoid basidiospores, 5.0–6.0 × 2.5–3.0 µm (n = 40, Qm = 1.50). In contrast, basidiospores of L. velutinus are oblong-cylindric to ellipsoid, 5.0–7.0(8.0) × 3.0–3.7 µm (Qm = 1.87) according to Pegler (1983), or 6.0–7.0 × 2.5–3.0 µm according to Teixeira (1946). It is worth to mention that the presence or absence of a pseudosclerotium could be considered a diagnostic character. However, it is important to note that the type specimen might have been collected without the pseudosclerotium, rendering this feature inconclusive for differentiation.
Panus capelariae Sousa-Guimarães, Menolli & Drechsler-Santos, sp. nov. (Figs. 1, 3a–c, and 4).
MycoBank: 850307.
Typification: Brazil, Santa Catarina: Florianópolis, Parque Municipal da Lagoa do Peri, 11 December 2016, Sousa-Guimarães, D. K. DG11 (holotype FLOR 73885). GenBank accession numbers: ITS = MT669126; nrLSU = MT669146.
Etymology: “capelariae”, named in honor of Dr. Marina Capelari, a Brazilian mycologist who contributed to the taxonomy of Brazilian mushrooms and also collected one of the specimens examined.
Diagnosis: Panus capelariae is recognized for the radially plicate-sulcate pileus surface, subdistant lamellae, and narrowly utriform and thin-walled pleurocystidia.
Description: Pileus 24–35 mm diam., infundibuliform to cyathiform, chestnut to dark brown (N80Y70M60), radially plicate-sulcate, almost glabrous to slightly velutinate, glabrous towards the center, margin curved downwards and slightly sulcate. Lamellae decurrent, beige (N40Y60M40), subdistant, margin entire, with lamellulae. Stipe central, 25–40 × 2–4 mm, mostly cylindric, villose to tomentose, concolorous with the pileus surface or slightly darker, slightly enlarged at the base, arising from a small to large pseudosclerotium.
Hyphal system dimitic. Generative hyphae 2.0–4.0 μm diam., hyaline, branched, clamped, thin-walled to rarely thick-walled. Skeletal hyphae 2.5–5.0 μm diam., hyaline to pale brown, unbranched, thick-walled. Basidiospores ellipsoid to cylindric, [70/7/7] 5.0–8.1 × 2.5–4.3 μm (Qm = 1.94; Q = 1.6–2.5; Lm = 6.87 μm; Wm = 3.12 μm), hyaline, thin-walled, smooth, rarely with guttulae, IKI − . Basidia cylindrical-clavate, 17–32 × 5.0–6.2 μm, hyaline, thin-walled, 4-sterigmated, clamped at the base. Pleurocystidia 20–37(47) × 3.7–7.5 of two types: (1) flexuous, thin-walled, smooth; (2) conical to narrowly conical, thick-walled (1.0–2.5 μm thick). Cheilocystidia 19–26 × 3.0–6.0 μm, flexuous, smooth, thin to thick-walled. Hymenophoral trama irregular, hyaline, similar in structure to the context. Pileipellis an epicutis composed of erect to loose fascicles of brown, thick-walled, and clamped generative hyphae.
Habit, habitat, and known distribution: Solitary, rarely gregarious, with a pseudosclerotium. Growing on fallen dead logs. Currently known from Southern South America, in Tropical Broadleaved Forest from Brazil and Argentina.
Additional specimens examined: Brazil, São Paulo: São Paulo, 2008, Capelari, M. 4365 (SP446152); Caraguatatuba, Parque Estadual Serra do Mar, 19 January 2016, Elias, S. G. SGE238 (FLOR 73887); ibid., 20 January 2016, Reck, M. A. MAR1154 (FLOR 73888); ibid., July 2016, Copini, E. EC72 (FLOR 73889); Rio Grande do Sul: Araricá, 23 November 2017, Palacio, M. MP296 (FLOR 73890); Santa Catarina: Florianópolis, UCAD, trilha principal, 2012, Drechsler-Santos, E. R., DS840 (FLOR73886).
Notes: Panus capelariae is primarily characterized by its radially plicate-sulcate pileus, utriform and thin-walled pleurocystidia, the presence of small to large pseudosclerotia, and its distribution in the Neotropical region. In terms of macroscopic features, it bears a striking resemblance to P. similis due to their shared radially plicate-sulcate pileus and subdistant lamellae. However, P. similis, typified by a collection from Sri Lanka, distinguished by smaller basidiospores 5.0–6.2 × 2.5–3.2 μm, conical to narrowly conical pleurocystidia with thick walls, and an Asian distribution with putative records in Africa, as documented by Pegler (1983).
Phylogenetically, P. capelariae was recovered as sister (Fig. 1: unsupported, SUPPL. Fig. 1: 71) to a clade with a new species described below and another Mexican phylogenetic species (Fig. 1, SUPPL. Fig. 1). For morphological comparison, see hereafter. Also, P. capelariae does not cluster with the P. similis sensu stricto subclade, which comprises sequences from collections in Sri Lanka (Ediriweera et al. 2021) and Vietnam. Corner (1981) considered P. similis as a variety of P. fulvus [Panus fulvus var. similis (Berk. & Broome) Corner]. However, based on morphology, the independence between the species P. similis and P. fulvus is supported. Panus fulvus can be distinguished from P. capelariae by its erect fasciculate hairs on the pileus and by moderately spaced lamellae, as shown by Corner (1981). Within the clade associated with P. capelariae, there are three sequences from Argentinean collections that were previously labeled as P. similis; however, these collections represent P. capelariae, as shown by our phylogenetic results, thus expanding the distribution of this species to Argentina.
Panus pachysporus Sousa-Guimarães, Menolli & Drechsler-Santos, sp. nov. (Figs. 3d, e and 5).
Microcharacters of Panus pachysporus (SP60914, holotype). a Generative hyphae, scale bar = 5 µm. b Basidia. c Pleurocystidia. d Cheilocystidia. e Basidiospores. f, g Light microscopy of basidiospores, showing the thickened wall; f basidiospore under Congo red reagent using standard light microscopy; g basidiospore under differential interference contrast (DIC) light microscopy in grayscale. Scale bars = 10 µm
MycoBank: 850309.
Typification: Brazil, Rio Grande do Sul: Gramado, Várzea Grande, 07 May 1961, Costa-Neto, J. P. (holotype SP60914).
Etymology: from the Greek “pachy” = thick and “sporus” = basidiospore, referring to the thick-walled basidiospores.
Diagnosis: Panus pachysporus is distinguished by its large, thick-walled basidiospores.
Description: Pileus 11–40 mm diam., infundibuliform to cyathiform, coriaceous, chestnut brown to dark brown (N60A60M50) when dry, slightly striate, villous to tomentose, margin thin. Lamellae decurrent, vinaceous brown (N60A30M40), moderately to densely crowded, margin entire, with lamellulae. Stipe central, 13–24 × 3–5 mm, cylindric, slightly enlarged at the base, surface villous to tomentose, chestnut brown, concolorous with the pileus surface.
Hyphal system dimitic. Generative hyphae 2.0–4.5 μm diam., hyaline, clamped, thin-walled, some thick-walled. Skeletal hyphae 2.5–6.0 μm diam., hyaline, thick-walled, IKI − . Basidiospores ellipsoid to cylindric, [30/1/1] 6.5–11.5(12.0) × 4.0–5.5(6.0) μm (Qm = 1.97, Q = 1.6–2.4, Lm = 10 μm, Wm = 5.0 μm), hyaline, thick-walled, smooth, some with guttulae, IKI − . Basidia cylindric-clavate, 17.0–26 × (5.5)6.0–8.0 μm, hyaline, thin-walled, 4-sterigmated, clamped at the base. Pleurocystidia of two types: (1) clavate, (22)25–32 × 5.5–7.0 μm, thin-walled, smooth; (2) narrowly conical 23–46 × 4.0–7.5 μm, narrowly thick-walled (1.0–2.0 μm thick), smooth. Cheilocystidia clavate (18.0)21–28 × 4.0–7.5 μm, smooth, thin-walled. Hymenophoral trama irregular, of radiate construction, consisting of a dimitic hyphal system with skeletal hyphae. Pileipellis a trichodermium composed of erect generative hyphae, with thick- and brown-walls, with clamp connections.
Habit, habitat, and known distribution: Gregarious, without a pseudosclerotium. Known only from the type locality in the Tropical Broadleaved Forest of the state of Rio Grande do Sul, Brazil.
Notes: The macromorphological description provided above is based on a dehydrated specimen dating back to 1961. Panus pachysporus is notably characterized by presenting the largest and thick-walled basidiospores of the genus. Panus velutinus and P. pachysporus share several similarities, including a coriaceous, chestnut brown, and velutinate pileus, which is marked with striations, and lamellae that are moderately to densely crowded. However, P. velutinus can be distinguished by presenting a pseudosclerotium, larger basidiomata (pilei up to 160 mm diam. and stipe 35–205 × 2.0–8.0 mm), larger cheilocystidia (22)25–36(40) × 5.0–8.5 μm, and thin-walled and smaller basidiospores that typically measure 4.5–7.0 × 3.0–4.0 μm.
Panus speciosus Sousa-Guimarães & Drechsler-Santos, sp. nov. (Figs. 6a–c and 7).
MycoBank: 850308.
Typification: Brazil, Santa Catarina: Florianópolis, Universidade Federal de Santa Catarina, 01 November 2013, Drechsler-Santos, E. R. DS1151 (holotype FLOR 68417). GenBank accession numbers: ITS = MT669130; nrLSU = MT669150.
Etymology: from Latin “speciosus” = handsome, referring to the beauty of this Panus species.
Diagnosis: Panus speciosus is distinguished by the soft, white to cream pileus, the mostly glabrous pileus surface and a fibrillose stipe.
Description: Pileus 18–50 mm diam., applanate when young, infundibuliform at maturity, surface white to cream (N10A20M10) when fresh and then slightly darkening to beige when dry (N10A30M20), hairy-squamose only at the center, margin involute, glabrous. Lamellae decurrent, beige to pinkish brown when fresh (A50M40C30) and then brownish (A80M50C30) when dry, crowded, anastomosed near the stipe, margin entire, with lamellulae. Stipe central to eccentric, 28–45 × 5 mm, flexuous, flattened, slightly enlarged towards the apex, beige (N10A30M20), surface glabrescent, fibrillose.
Hyphal system dimitic. Generative hyphae 2.0–5.0 μm diam., hyaline, clamped, thin- to thick-walled. Skeletal hyphae 2.5–5.5 μm diam., thick-walled. Basidiospores ellipsoid to cylindric, [60/3/3] (4.0)5.0–7.5 × 2.5–3.5 (Qm = 1.91; Q = 1.6–2.2; Lm = 6.0 µm; Wm = 3.0 µm), hyaline, thin-walled, smooth, rarely gutted, IKI − . Basidia cylindric-clavate, (18)21–35(49) × 4.5–9.0 μm, hyaline, thin-walled, 4-sterigmated, clamped at the base. Pleurocystidia of two types: (1) narrowly clavate to clavate, 30–45 × 6.0–9.0 μm, thin-walled; (2) narrowly utriform, 22–64 × 6.0–12.0 μm, thick-walled (1.5–3.0 μm thick), almost smooth. Cheilocystidia clavate, 22–39(42) × 6.5–12.0 μm, smooth, thin-walled. Hymenophoral trama irregular, of radiate construction, consisting of a dimitic hyphal system with skeletal hyphae. Pileipellis an epicutis composed of radially to parallel arranged generative hyphae, with slightly thick and brown walls, septated, clamp connections not observed.
Habit, habitat, and known distribution: Gregarious, without pseudosclerotium. On fallen dead logs. Currently known from Brazil, in the Tropical Broadleaved Forest in the urban area of Florianópolis, Santa Catarina state, and in Singapore.
Additional specimens examined: Brazil, Santa Catarina: Florianópolis, Universidade Federal de Santa Catarina, 18 October 2016, Sousa-Guimarães, D. K. DG25 (FLOR 68418); ibid., 02 December 2017 Sousa-Guimarães, D. K. DG68 (FLOR 68419).
Notes: Panus speciosus is characterized by its whitish and glabrous pileus, featuring a squamose central area, and a stipe that varies from fibrillose to glabrescent. Panus hirtiformis (Murril) Drechsler-Santos & Wartchow, described from Belize, also has a glabrous pileus, which appears pinkish ochraceous when fresh (Pegler 1983). However, it distinguishes from P. speciosus by exhibiting a brown ochraceous pileus, smaller basidia 15–19 × 3.5–4.5 μm, and longer pleurocystidia 28–80 × 5.0–9.0 μm (Pegler 1983).
Panus caespiticola (Pat. & Har.) Drechsler-Santos & Wartchow also presents an applanate and pale ochraceous to cream-colored pileus. Nevertheless, it differs from P. speciosus in having slightly longer and differently shaped pleurocystidia (fusoid to lageniform, 35–54 μm long) and shorter cheilocystidia 24–33 × 7.0–9.0 μm, with a cylindrical-lanceolate to ventricose-fusoid morphology (Pegler 1983; Drechsler-Santos et al. 2012).
Phylogenetically, P. speciosus was recovered in the “speciosus” clade nested within at least four phylogenetic species that exhibit shared morphological features. These species are discussed hereafter in the “speciosus” clade.
Panus stiptonotatus Sousa-Guimarães & Drechsler-Santos, sp. nov. (Figs. 1, 6d–f, and 8).
MycoBank: 850310.
Typification: Brazil, Santa Catarina: Florianópolis, Parque Municipal do Córrego Grande, 04 June 2016, Sousa-Guimarães, D. K. DG06 (holotype FLOR 68422). GenBank accession numbers: ITS = MT669133; nrLSU = MT669153.
Etymology: “Stipto” from Latin = stipe; from Latin “notatus” = marked, notable, referring to the marked nodulose portions of the stipe.
Diagnosis: Panus stiptonotatus is distinguished by having nodular portions on the stipe and pleurocystidia that are partially immersed in the hymenium.
Description: Pileus 22–30 mm diam., infundibuliform, dark brown (N90A70M10) when dry, surface hispid strigose with short and erect hairs, margin involute, hairy. Lamellae decurrent, brown (N60A50M50) when dry, moderately crowded, anastomosed near the stipe, margin entire, with lamellulae. Stipe central to eccentric, 38–65 × 5 mm, sometimes with nodules extending over the entire surface of the stipe or disposed on the middle portion or on the base and then, nodulose portion up to 9 mm diam., cylindric to flexuous, surface pilose, velutinate and tomentose from base to apex, brown, concolorous with the pileus surface, arising from a pseudosclerotium.
Hyphal system dimitic. Generative hyphae 1.5–5.0 μm diam., hyaline, clamped, thin-walled, some thick-walled. Skeletal hyphae 2.5–5.0 μm diam., thick-walled. Basidiospores ellipsoid to oblong ellipsoid, [70/7/7] 4.0–5.5(6.0) × 2.0–3.0 μm (Qm = 1.82, Q = 1.6–2.0, Lm = 5.0 μm, Wm = 2.5 μm), hyaline, thin-walled, smooth, rarely gutted, IKI − . Basidia cylindrical-clavate, 17.0–22(24) × 4.5–7.0 μm, hyaline, thin-walled, 4-sterigmated, clamped at the base. Pleurocystidia of two types: (1) flexuous with subcapitate apex to clavate, 40–62 × 5.0–9.0 μm, thin-walled, smooth, almost totally immersed in the hymenium, projecting (3.5–8.0 μm) from the initial portion of the hymenophoral trama; (2) fusiform, 17.0–24 × 5.0–8.0 μm, narrowly thick-walled (1.0–1.5 μm thick). Cheilocystidia flexuous, (11.0)13.0–22.0 × 3.5–7.0 μm, smooth, thin-walled. Hymenophoral trama irregular, hyaline consisting of a dimitic hyphal system with skeletal hyphae. Pileipellis an epicutis composed of erect, loosely arranged fascicles of unbranched, brown, thick-walled, clamped generative hyphae.
Habit, habitat, and known distribution: Solitary, rarely gregarious, with pseudoesclerotium. Growing on fallen dead logs. Currently known from Southern Brazil, in Tropical Broadleaved Forest, in the states of Santa Catarina and Rio Grande do Sul.
Additional specimens examined: Brazil, Santa Catarina: Santo Amaro da Imperatriz, Plaza Caldas da Imperatriz, 21 March 2014, J. Menezes Prata JP0048 (FLOR 68424); ibid., Florianópolis, PBP0002 (FLOR 68425); ibid., Unidade de Conservação Ambiental Desterro (UCAD), 28 March 2017, Oliveira, C. A. T. CATO176 (FLOR 68423). Rio Grande do Sul: Porto Alegre, Morro Santana, 27 July 2016, Alves-Silva, G. GAS850 (FLOR 68420); ibid., GAS851 (FLOR 68421).
Notes: Panus stiptonotatus is characterized by the presence of nodular portions in the stipe and flexuous pleurocystidia, which are partially embedded in the hymenium, showcasing a visible subcapitate apex. Panus caespiticola, described from Mali (Africa), also presents a nodulose-like stipe (Pegler 1983), but it can be readily distinguished by its slightly larger basidiospores (5.5–7.0 × 3.0–4.5 μm) and longer cheilocystidia (24–33 μm long) that are cylindrical-lanceolate to ventricose-fusoid (Pegler 1983).
Panus velutinus and P. pachysporus also exhibit velutinate, chestnut brown pileus surfaces and lamellae that are moderately to densely crowded. However, P. velutinus is distinguished by smaller [(22)25–31 × 5.5–9.0 μm] and differently shaped pleurocystidia, which are clavate and thin-walled. Conversely, P. pachysporus differs mainly by larger basidiospores, 6.5–11.5(12) × 4.0–5.5(6.0) μm.
In a phylogenetic context (Fig. 1, SUPPL. Fig. 1), P. stiptonotatus forms a sister group (Fig. 1: 100/1, SUPPL. Fig. 1: 98) to a specimen from Mexico identified as Lentinus cf. velutinus and P. capelariae as sister to them (Fig. 1: unsupported, SUPPL. Fig. 1: 71). This specimen, Lentinus cf. velutinus, is not related to P. velutinus s.s., as we define it here (see below), and in a broad sense, P. velutinus is different to P. stiptonotus as discussed above. Panus capelariae differs from P. stiptonotatus by having radially plicate-sulcate pileus surface and subdistant lamellae.
Panus thailandicus (Tibpromma & K.D. Hyde) Menolli, Alves-Silva, Sousa-Guimarães & Drechsler-Santos, comb. nov. (Fig. 1).
MycoBank: 850311.
Basionym: Endopandanicola thailandica Tibpromma & K.D. Hyde, MycoKeys 33: 32 (2018).
Description: See Tibpromma et al. (2018).
Notes: Panus thailandicus was initially characterized based on cultures of endophytic fungi found in association with the leaves of Pandanaceae (Tibpromma et al. 2018). Hitherto, the morphology of its basidioma, or whether the species is capable of producing it, remains uncharted. In terms of phylogenetic positioning, it was recovered related to P. roseus (Fig. 1), within a distinct lineage (see SUPPL. Fig. 1) among other Panus species but not as a distinct genus. Consequently, we have formally combined Endopandanicola thailandica into Panus and Endopandanicola must be considered a synonym of Panus.
Panus velutinus (Fr.) Fr. Epicrisis: 398 (1838), non (Fr.) Sacc. 1887 (Figs. 1, 9, and 10).
Panus velutinus. a Basidioma overview [holotype UPS F-012409 of Agaricus (Omphalia) velutinus]. b–d Epitype FLOR 75655 of P. velutinus. b Basidioma overview, scale bar = 2 cm. c Detail of the lamellae and their insertion into the stipe, scale bar = 0.5 cm. d Detail of pileus surface and margin involute. Scale bars = 1 cm
≡ Agaricus (Omphalia) velutinus Fr. Linnaea 5: 508 (1830), non-Lentinus velutinus Fr. Linnaea 5: 510 (1830).
MycoBank: MB178816.
Holotype: Brazil: December 1830, Sic Beyrich (UPS F-012409) (Fig. 9a).
Epitype: Brazil: Rio Grande do Sul: Canela, 09 October 2016, Garcia, V. O. VOG30 (FLOR 75655). GenBank accession numbers: ITS = MT669139; nrLSU = MT669155 (Figs. 8b–d and 9).
Description based on Brazilian specimens, including the epitype designated here and the holotype of Agaricus velutinus Fr.: Pileus 90–160 mm diam., deeply umbilicate to broadly infundibuliform to cyathiform, coriaceous, pale brown or chestnut to cinnamon brown (N70M50C40), uniformly velutinous to short hispid, margin thin and involute. Lamellae decurrent, buff pale brown (N60M50C30), moderately to densely crowded, margin entire, with lamellulae. Stipe central, 35–205 × 2.0–8.0 mm, cylindrical, uniformly velutinous, slender, elongate, concolorous with the pileus surface, expanding slightly at both apex and base, arising from a hard pseudosclerotium.
Hyphal system dimitic. Generative hyphae 2.0–4.5 μm diam., hyaline, clamped, thin- to rarely thick-walled. Skeletal hyphae 2.5–5.0 μm diam., hyaline, thick-walled. Basidiospores ellipsoid to cylindrical, [80/1/4] 4.5–7.0 × 3.0–4.0 μm (Qm = 1.78; Q = 1.5–2.1; Lm = 6.0 μm; Wm = 3.25 μm), hyaline, thin-walled, smooth, rarely gutted, IKI − . Basidia cylindrical-clavate, 22–25 × 4.0–5.5 μm, hyaline, thin-walled, 4-sterigmated, clamped at the base. Pleurocystidia of two types: (1) clavate (22)25–31 × 5.5–9.0 μm thin-walled, smooth; (2) clavate to fusiform, 28–50 × 4.0–7.0 μm, narrowly thick-walled (1.5–2.0 μm thick). Cheilocystidia clavate to fusiform, (22)25–36(40) × 5.0–8.5 μm, smooth, thin-walled. Hymenophoral trama irregular, hyaline consisting of a dimitic hyphal system with skeletal hyphae. Pileipellis a trichodermium composed of erect, clamped generative hyphae, with thick- and brown-walls.
Habit, habitat, and known distribution: Solitary, rarely 2–3 basidiomata together, with pseudosclerotium. Dead fallen trunk. Hitherto and based on our results, it is considered a Neotropical species. Paleotropical records must be confronted by molecular analyses.
Additional specimens examined: Brazil, Paraná: Piraí do Sul, localidade Corpo Seco, 30 July 2016, Comin, M., CM10 (FLOR 75657); ibid., Foz do Iguaçu, Parque Nacional Iguaçu, Trilha do Hidrômetro, 7 November 2010, Karstedt, F. FK1659 (SP446146). São Paulo: Santo André, Estação Biológica de Paranapiacaba, November 1959, Gomes, A. (SP46390); São Paulo, Parque Estadual das Fontes do Ipiranga, 30 May 1984, Romaniuc-Neto, S. (SP193686). Santa Catarina: Florianópolis, 27 August 2014, Freire, F. M. FMF261 (FLOR 75658); ibid., 19 April 2016 Freire, F. M. DG01 (FLOR 75659); ibid., 5 November 2017, Drechsler-Santos, E. R. DS2116 (FLOR 75660); ibid., Lagoa do Peri, Início trilha da Gurita, Neves, 18 August 2018, Neves, M.A., MAN1270 (FLOR 75661). Rio Grande do Sul: Entre Ijuís, 03 August 2019, Palacio, M., MP446 (FLOR 75656).
Notes: Panus velutinus stands out due to its distinctive macromorphology (Fig. 9), characterized by a uniformly velutinate to short hispid upper surface, a deeply umbilicate to broadly infundibuliform or cyathiform pileus, and a cylindrical, slender, and elongated stipe featuring a characteristic pseudosclerotium. Morphologically, the most closely related species within the “velutinus” clade is P. stiptonotatus, which possesses velutinate, chestnut brown pileus surfaces and lamellae that are moderately to densely crowded. However, the stipe of P. stiptonotatus is distinctly different, characterized by nodules and a flexuous appearance. Other species within the same phylogenetic group exhibit significant differences in both macro and micro features, e.g., P. similis (see Pegler 1983; Ediriweera et al. 2021), P. tephroleucus (see Pegler 1983), and P. capelariae (this study).
The epithet “velutinus” within the genus Panus is associated with three names based on different authorities: P. velutinus (Fr.) Fr. (Fries 1838), P. velutinus (Fr.) Sacc. (Saccardo 1887), and P. velutinus (Fr.) Overh. (Overholts 1930). Panus velutinus (Fr.) Overh., as cited by Pegler (1983), does not exist. Overholts (1930) merely mentioned “Panus velutinus Fr.” in his work, referencing the principal study “Epicr. Syst. Myc. 398. 1838” by Fries (1838). In this context, Fries (1838) validly combined Agaricus (Omphalia) velutinus Fr. (Fries 1830, on page 508) into Panus, leading to the legitimate P. velutinus (Fr.) Fr. Panus velutinus (Fr.) Sacc. was also based on Agaricus velutinus Fr. by Saccardo (1887); however, this name is illegitimate (Art. 53.1; Turland et al. 2018) due to being a posterior homonym of Panus velutinus (Fr.) Fr. Pegler (1983) incorrectly addressed the name Lentinus velutinus Fr. in his description based on the type specimen of Agaricus (Omphalia) velutinus Fr., leading to further confusion of what species he was referring to. This is further confirmed by comparing the morphological description made by Pegler (1983) and the holotype of Agaricus velutinus (UPS F-012409), as well as the collection date (December). Thus, the combination made by Fries (1838), P. velutinus (Fr.) Fr. is the correct name for this species.
Furthermore, it is essential to address heterotypic synonyms of P. velutinus (Pegler 1983). Some of these synonyms, upon our examination of type specimens and protologues, appear morphologically distinct and should be recognized as independent species. For instance, Lentinus campinensis, now combined into Panus, is one such case. In addition, we do not agree with the synonymy of L. blepharodes from Cuba (Berkeley and Curtis 1869), L. fastuosus Kalchbr. & MacOwan from South Africa (Kalchbrenner 1881), L. fissus Henn. from Togo (Hennings 1897), L. holumbrinus De Seynes from Dem. Rep. of Congo (Seynes 1897), L. nepalensis Berk. from Nepal (Berkeley 1854), and L. thomensis from São Tomé and Príncipe (Coutinho 1925) with Panus velutinus. In general, all these species, based on our revision (from protologue and/or type specimen, see “Results”), present whitish basidiomata and anastomosed lamellae, which is completely different from the sensu stricto circumscription of P. velutinus proposed in this study. Additionally, none of those species present neither a deeply umbilicate to broadly infundibuliform or cyathiform pileus, nor a slender and elongated stipe.
Regarding other heterotypic synonyms, we found challenges in our attempts to study them. It has been confirmed that the types of L. fallax from Paraguay and L. castaneus from Nicaragua are missing (pers. comm. with curators of LPS and ISC herbaria, respectively). Additionally, L. pseudociliatus from Argentina (Raithelhuber 1974) has only fragments of the stipe, rendering it impossible to analyze. Lastly, for L. natalensis from South Africa, we were unable to ascertain its existence at PREM herbarium, as our inquiries were not answered by the curatorship.
To prevent further ambiguity surrounding P. velutinus, maintain a nomenclatural stability, and establish a phylogenetic concept, we have chosen an epitype (FLOR 75655) from a recently collected Brazilian specimen found in the type locality with DNA sequences.
Discussion
Global phylogeny of Panus and the taxonomic/nomenclatural implications
Our study addressed critical gaps in the phylogenetic understanding of Panus and highlights the need for taxonomic revisions within the group. By incorporating newly collected Panus specimens from Brazil and encompassing all available Panus sequences from the GenBank database, we have assembled the most comprehensive phylogenetic analysis of this genus to date. Our investigations have unveiled several noteworthy findings, including the identification of previously unknown lineages, some of which have been proposed as new species.
Previous Panus phylogenetic analyses have recovered three to five main clades (Grand 2004; Douanla-Meli and Langer 2010; Zmitrovich and Malysheva 2013; Vargas-Isla et al. 2015; Zmitrovich and Kovalenko 2016; Vinjusha and Kumar 2022). In contrast, our analyses revealed the existence of at least 11 well-supported main clades within the genus. In particular, below we have focused on discussing several of these clades, as follows.
“conchatus” clade
This clade encompasses two distinct groups of specimens labeled as Panus conchatus (see Fig. 1 and SUPPL. Fig. 1). One group (Panus aff. conchatus) was retrieved with specimens pantropically distributed (including Colombia, Honduras, India, Mexico, Tanzania, China, and the USA), while the other (Panus conchatus s.s.) comprises specimens particularly found in temperate regions of the Northern Hemisphere (including China, Finland, Germany, Russia, Sweden, USA, and the UK) (Fig. 1, SUPPL. Fig. 1 and Table S1). Moreover, we observed that certain specimens previously named as P. conchatus are also located within the “ciliatus” clade, demonstrating a lack of clear morphological criteria in the circumscription of P. conchatus.
The systematics of P. conchatus, originally proposed based on a specimen from Sweden by Bulliard (1792), holds particular significance as it is the type species for the genus Panus. Based on the geographic distribution of both subclades within the “conchatus” clade and the topotype of P. conchatus, we recognize the clade consisting of specimens distributed in the north temperate regions to represent the sensu stricto status for P. conchatus. This clarification could significantly contribute to stabilizing the taxonomy of the species. However, it is crucial to verify the existence and current location of the original P. conchatus type specimen.
In the event that the type specimen is confirmed as lost, the designation of a lectotype or neotype may become necessary. Additionally, an epitype could be considered to establish a phylogenetic concept for the entity, thereby linking it to a nomenclatural type. Until these steps are taken, specimens identified as P. conchatus that cluster within the “ciliatus” clade (discussed below) and the Pantropics distributed clade named here as Panus aff. conchatus should be treated as distinct species different from P. conchatus s.s. It is possible that some of these specimens represent new species, while others may correspond to previously named ones, given the extensive list of synonyms associated with P. conchatus (https://www.mycobank.org/, accessed 01/05/2023). Future studies are required for P. conchatus, since we do not have access to their respective type specimens and DNA sequences from reference specimens dot not exist.
“roseus” clade
The “roseus” clade comprises exclusively species described or recorded from Asia, consisting of specimens previously identified as Lentinus roseus Karun., K.D. Hyde & Zhu L. Yang along with the paratypes of Endopandanicola thailandica. Notably, while L. roseus had previously been combined in Panus by Vinjusha and Kumar (2022), E. thailandica has now been integrated into the genus Panus as P. thailandicus (see “Taxonomy”). It is worth mentioning that Endopandanicola was originally described as a monospecific genus based on cultured endophytic specimens recovered from Pandanaceae leaves (Tibpromma et al. 2018). Based on our phylogenetic analyses (Fig. 1, SUPPL. Fig. 1), we propose the synonymization of Endopandanicola with Panus, with P. thailandicus identified as the sister taxon of P. roseus.
Additionally, it is essential to draw attention to the case of P. roseus because there are neither DNA sequences of its type specimen nor sequences from specimens from the type locality in Thailand. Furthermore, L. roseus specimens from China did not form a single phylogenetic species (see SUPPL. Fig. 1), highlighting the complexity surrounding the taxonomic status of this species.
“strigellus” clade
The “strigellus” clade groups American specimens identified as P. strigellus, Panus sp., and P. parvus, including the holotype of the latter. Interestingly, P. parvus did not emerge clearly as a distinct lineage in our analyses; instead, it weakly clustered (Fig. 1: 41/0.87, SUPPL. Fig. 1: 51) together with South American sequences named as P. strigellus (except those from Colombia).
In accordance with the original description, P. parvus could be distinguished from P. strigellus primarily by its smaller and more slender basidiomata, the presence of more abundant and irregularly distributed pileal squamules (in contrast to the scarce and concentric distribution of them in P. strigellus), and the pale buff pileus color of dried specimens (as opposed to the dark reddish-brown color in P. strigellus) (Drechsler-Santos et al. 2012). While not explicitly mentioned in Pegler (1983), the presence of elongate-clavate cheilocystidia was observed in the holotype of P. striguellus (K-M 153658!) by Drechsler-Santos et al. (2012), and similar pleurocystidia were also recorded by Vargas-Isla et al. (2015) in specimens from Brazil and the USA. Vargas-Isla et al. (2015) noted the villose to hispid pileus surface in young basidiomata, a feature not cited by Pegler (1983), even though he examined young specimens of its synonym L. tubarius Lloyd (Pegler 1983). Additionally, we examined the holotype of P. guaraniticus (LPS19295!), one of the seven heterotypic synonyms of P. strigellus listed by Pegler (1983), which displayed a similar pileus surface to P. parvus (Drechsler-Santos et al. 2012), although we did not observe cylindrical, thick-walled pleurocystidia.
In light of these findings, we consider two possible scenarios. One with the whole “strigellus” clade potentially being treated as a single species, P. strigellus, with P. parvus regarded as an additional synonym. This interpretation, which we are assuming conservatively at the moment, suggests that P. strigellus encompasses a broad distribution across the American continent, featuring considerable macro and micromorphological variation (Fig. 11). This variation encompasses differences in basidioma size, pileus color, distribution of squamules on the pileus surface, and the presence or absence of cylindrical, thick-walled pleurocystidia and cheilocystidia.
Panus strigellus. a Specimen FLOR 75673. b Specimen FLOR 75675. c Specimen FLOR 75674. d, e Holotype of Panus parvus (URM80840), scale bar e = 0.5 cm. f Specimen FLOR 75676. g Specimen TENN-F-055993. h Holotype of Panus strigellus (K-M 153658). i, j Holotype of Panus guaraniticus (LPS19295), scale bar i = 5 cm. Scale bars = 1 cm
The other scenario recognizes that the morphological and phylogenetic evidence presented in our analyses hints the existence of a much more intricate lineage within it, likely consisting of multiple species with distinct distribution patterns and morphological characteristics. To fully elucidate this complexity, additional sampling, morphological assessments, phylogenetic analyses (including the use of additional DNA markers and sequences from type localities specimens), and a comprehensive taxonomic revision of the names listed as synonyms of P. strigellus by Pegler (1983) are necessary.
“speciosus” clade
Phylogenetically, P. speciosus was recovered in a clade moderately supported (57/1) nested within at least four phylogenetic species in the “bambusinus,” “purpuratus,” “asiaticus,” and “paraibensis” clades, including two species unequivocally identified as P. purpuratus G. Stev. and P. paraibensis (Stevenson 1964; Galvão et al. 2023). Additionally, when including those specimens labeled as P. bambusinus (T.K.A. Kumar & Manim.) N. Vinjusha & T.K.A. Kumar and treating them as P. bambusinus, all these species share common characteristics, such as pinkish lamellae and white, pale cream to pinkish basidiomata. The P. lecomtei specimen recovered in the “asiaticus” clade is a misidentification, as we discuss below. Panus bambusinus stands out due to its 15–200 mm diam. and striated pileus, tomentose to strigose stipe, and versiform cheilocystidia (Kumar and Manimohan 2005; Vinjusha and Kumar 2022). Panus paraibensis differs by having pleurotoid basidiomata and smaller basidiospores (4.4–5.5 × 2–2.9) and P. purpuratus by its smaller basidiospores (5–6 × 2–2.5).
Our phylogenetic analyses (Fig. 1) evidence that P. speciosus may have a pantropical distribution as sequences from Singapore specimens grouped as conspecific with those from Brazil. Therefore, those specimens from Singapore should be studied to better understand the morphological concept of the species.
“velutinus” clade
The “velutinus” clade exhibits a complex composition, encompassing a diverse array of species. Many specimens named as P. similis and P. velutinus could lead to interpret these taxa as polyphyletic. It also includes P. capelariae and P. stiptonotatus, which are independent lineages, proposed here as new species (see “Taxonomy”). And, additionally, it also includes several other unsupported lineages in the phylogeny and without taxonomic stability based on the different names attributed to the sampled sequences. One of those lineages could confirm the occurrence of P. tephroleucus (type is from Suriname) or P. fulvus (type is from Sri Lanka) in Brazil. However, the unsupported subclade named as Panus cf. tephroleucus (Fig. 1, SUPPL. Fig. 1) exhibits significant morphological variation (Fig. 12). Due to the unavailability of sequences from the types or from specimens at the type locality associated with the names within this clade, confidently assigning a name to the Brazilian specimens under consideration becomes impossible. In this case, future studies are required for P. tephroleucus, since we do not have access to the type specimen or DNA sequences of any reference specimen. Our attempts to gather information from PC curatorship yielded no results, without feedback from PC herbarium.
The position of P. similis in the phylogeny (Fig. 1) raises noteworthy considerations. Specimens identified as P. similis from Argentina, Cameroon, India, Sri Lanka, and Vietnam were recovered as four phylogenetic species. Given that the type locality of P. similis is Sri Lanka (Berkeley and Broome 1873), we propose that the core represented by the specimens from Sri Lanka (UOC SIGWI S38) and Vietnam (LE-BIN 3011) should be treated as P. similis s.s. (Fig. 1). Additionally, morphological and molecular data provided by Ediriweera et al. (2021) also support the Sri Lanka specimen to be a representative of P. similis s.s. Moreover, sequences of TENN59008, TENN58995, and TENN59829 from Argentina, previously identified as P. similis, correspond to the new species, P. capelariae. In conclusion, P. similis is indeed an Asian species, and morphologically similar Neotropical specimens represent P. capelariae.
Panus fulvus could be a similar case as P. similis, occurring exclusively in Asia or instead, being pantropical. But there are no available sequences from specimens from Asia, and as presented above, all the specimens labeled as P. fulvus are from the American continent, raising questions about the pantropical distribution of P. fulvus. Further investigation is needed regarding P. tephroleucus vs. P. fulvus in America.
Regarding the systematics of P. velutinus, several critical points warrant attention and some of them have been presented in the taxonomic notes of the species (see “Taxonomy”). Based on the morphology of holotype UPS F-012409 (see “Taxonomy” and Figs. 9 and 10) and the acceptance of Agaricus (Omphalia) velutinus (Fries 1830, on page 508) as the correct basionym for the species, contrary to what was traditionally assumed by Pegler (1983), we realized the necessity of choosing an epitype (FLOR 75655) from a new Brazilian specimen collected in the type locality (Fries 1830), in order to have also a phylogenetic stability.
“neostrigosus” clade
The “neostrigosus” clade stands as the sister group to the “ciliatus” clade and consists of a single species, P. neostrigosus, notable for its extensive distribution across different continents, with records from Brazil, Canada, China, India, Japan, Mexico, Philippines, Russia, the USA (including Hawaii and Puerto Rico), and Turkey (Fig. 1; SUPPL. Fig. 1; Table S1). Among P. neostrigosus synonyms, P. lecomtei (Fr.) Corner and P. rudis Fr. are the labels associated with the majority of specimens recovered in this clade. It is noteworthy the type of Lentinus martianoffianus Kalchbr., as already synonymized in P. neostrigosus by Psurtseva et al. (2021).
A certain degree of confusion between P. neostrigosus and the morphologically similar species P. strigellus (as discussed earlier) prompted further investigation by Vargas-Isla et al. (2015). They displayed a combination of morphological, molecular, and mating compatibility test data to clarify this situation. Their phylogenetic analysis unequivocally established the distinctiveness of the two species. Nonetheless, concerns arose about the validity of the name P. neostrigosus vs. P. lecomtei.
To address those concerns, P. neostrigosus was proposed as a new name, allowing for the proper combination of Lentinus strigosus Fr. into Panus and avoiding a homonym (Drechsler-Santos et al. 2012). Lentinus strigosus and L. lecomtei are synonyms and share equal naming priority because both were described by Fries (1825). Vargas-Isla et al. (2015) argued that, within Panus, P. lecomtei (Fr.) Corner should be the correct name for this taxon because the epithet strigosus was already occupied within Panus by another species, viz., Panus strigosus Berk. & M.A. Curtis. However, as pointed out by Sanuma et al. (2016), the International Code of Nomenclature for Algae, Fungi, and Plants (Turland et al. 2018) in Art. 11.5 states that when a choice exists between legitimate names of equal priority, the first such choice to be effectively published establishes the priority of the selected name. In the case where L. strigosus was intentionally preferred over the synonym L. lecomtei by Murrill (1915) and later by Pegler (1972), the priority between these two names was set when they were still classified under Lentinus. Consequently, the correct name for this species is P. neostrigosus.
“ciliatus” clade
The “ciliatus” clade is characterized by the presence of at least three distinct phylogenetic species (Fig. 1, SUPPL. Fig. 1). One of these species remains unnamed and hails from a sequence (MK184520) from Madagascar, while the other two consist of specimens previously identified as P. conchatus, P. subfasciatus, P. fasciatus, and P. ciliatus (Fig. 1, SUPPL. Fig. 1). Panus conchatus actually belongs to a separate and unrelated lineage (see above). These P. conchatus specimens are misidentifications. Panus subfasciatus holotype, in turn, grouped together specimens assigned to P. fasciatus. It suggests further investigation needed in order to elucidate whether both taxa are conspecific or distinct species, as proposed by Tibpromma et al. (2017). Furthermore, concerning specimens labeled as P. ciliatus, one from Thailand was recovered in the “Panus subfasciatus” clade and another two from Brazil and USA in the “Panus ciliatus” clade. The Brazilian specimen (SP446150 = FK1890), the unique specimen we had access to the morphology, resembles P. ciliatus morphologically.
As Pegler’s (1983) description of Panus ciliatus, the Brazilian specimen (SP446150, Fig. 13) presents a densely crowded, vinaceous-colored lamellae, a radially striated but not sulcate pileus that is finely hispid, velutinate, and displays concentric zoning, ellipsoid to cylindric basidiospores 5.5–7.0 × 2.5–3.5 μm, and pleurocystidia measuring 22–36 × 5.0–8.0 μm, which are clavate and thin- to rarely thick-walled (1.0–2.0 μm thick).
This evidence also support us to indicate P. ciliatus is pantropically distributed. It was described based on an Indonesian specimen by Léveillé (1844), and the type was already phylogenetically tested and confirmed in previous analysis by Dr. Jaya Seelan (Univ. of Malaysia Sabah) as part of the subclade here named “Panus ciliatus,” together with Brazilian specimens as well (J. Seelan. pers. comm., unpublished data).
Brazilian diversity of Panus
In our comprehensive evaluation, we considered various sources of data, including morphological and molecular information, to assess the occurrences of Panus species in Brazil. We have confirmed the occurrence of P. ciliatus (pantropical distribution), P. neostrigosus (widespread), P. paraibensis (endemic to Brazil), P. strigellus (American distribution with P. parvus as a synonym), and P. velutinus (Neotropical distribution with pantropical distribution to be confirmed), and we introduced P. capelariae (southern South America distribution), P. speciosus (pantropical distribution), and P. campinensis, P. pachysporus, and P. stiptonotatus, all three as endemic species to Brazil, comprising ten species confirmed in the Brazilian ecosystems. Additionally, we rejected the occurrence in Brazil of P. conchatus (putatively restricted to the North temperate region), P. convivalis (no quality data is available), P. fulvus (Asiatic distribution but with pantropical occurrences to be confirmed), P. hymenorhizus (not a Panus species, since it was described as growing on small twigs and having free lamellae; Spegazzini 1889; LPS16800!), P. similis (an Asiatic species), and P. tephroleucus (probably with Neotropical distribution, but quality data is lacking).
In conclusion, our comprehensive assessment of Panus in Brazil has led to the confirmation of ten species supported by morphological and/or molecular data. However, we acknowledge that further research, particularly involving type specimens and additional DNA sequencing, is important to continue the studies in order to determine the presence or absence of other Panus species in Brazil. To aid in the accurate identification of these confirmed species, we present below an identification key.
Identification key for Panus species occurring in Brazil
1. Basidioma with pseudosclerotium ……………… 2
1’. Basidioma without pseudosclerotium or without distinct information on pseudosclerotium presence ……………… 5
2 Basidioma large and robust, pileus up to 160 mm diam., stipe slender up to 205 mm long ………………Panus velutinus
2’ Basidioma smaller, pileus up to 80 mm diam., stipe up to 90 mm ……………… 3
3 Pileus almost glabrous, radially plicate-sulcate; lamellae spaced ………………P. capelariae
3’ Pileus velutinate to hispid-strigose with short hairs, not sulcate; lamellae moderately to densely crowded …………….. 4
4 Lamellae moderately crowded, anastomosed at the stipe, brown; stipe cylindric with nodules extending over the entire surface of the stipe or present on the middle portion or on the base ………………P. stiptonotatus.
4’ Lamellae densely crowded, not anastomosed at the stipe, with a vinaceous hue; stipe cylindric without nodules…………………….P. ciliatus.
5 Basidioma pleurotoid, convex plane …………………….P. paraibensis
5’ Basidioma agaricoid, cyathiform or infundibuliform …………….. 6
6 Pileus and stipe equally velutinate to villose ……………..P. campinensis
6’ Pileus glabrous or densely hairy …………….. 7
7 Pileus glabrous, squamules restricted to the center …………………….P. speciosus
7’ Pileus with few isolated hairs to densely hairy ……………………. 8
8’ Basidiospores 6.5–12 μm long, thick-walled …………………….P. pachysporus
8’ Basidiospores up to 7.0 μm long, thin-walled ……………………. 9
9 Pileus densely villous to hispid-strigose; gloeocystidia absent ……………..P. neostrigosus
9’ Pileus glabrous, with blackish spiniform squamules, to finely striate; gloeocystidia present ………………P. strigellus
Availability of data and material
All data used in this study are either directly cited (nomenclature) or are available through the cited references (underlying phylogeny and sequence data) or through the cited repositories (MycoBank registration numbers, molecular data at Dataverse).
References
Batista AC, Falcão RGS, Peres GEP, Moura NR (1966) Fungi Paraenses (Revisão da Coleção de Paul C. Hennings, do Museu Paraense Emílio Goeldi). Inst Micol 506:10–290
Berkeley MA (1843) Notices of fungi in the herbarium of the British Museum. Ann Mag Nat Hist 10:369–385
Berkeley MJ (1854) Decades of fungi. Decades XLI- XLIII. Indian fungi. Hooker’s J Bot Kew Gard Miscellany 6:129–143
Berkeley MJ, Broome CE (1873) Enumeration of the fungi of Ceylon. Part II., containing the remainder of the Hymenomycetes, with the remaining established tribes of fungi. J Linn Soc, Bot 14(73):29–140. https://doi.org/10.1111/j.1095-8339.1873.tb00301.x
Berkeley MJ, Curtis MA (1869) Fungi Cubenses (Hymenomycetes). J Linn Soc Bot 10:280–392
Bulliard P (1792) Herbier de la France; ou, Collection complette des plantes indigenes de ce royaume; avec leurs propriétés, et leurs usages en medecine, vol 12. Bulliard, Didot, Debure, and Belin, Paris
Cavalcante FSA, Campos MCC, de Lima JPS (2021) New occurrences of macrofungi (Basidiomycota) in southern Amazonas, Brazil. Ci e Nat 43:e46. https://doi.org/10.5902/2179460X44026
Corner EJH (1981) The agaric genera Lentinus, Panus and Pleurotus with particular reference to Malaysian species. Beih Nova Hedw 69:1–169
Coutinho AXP (1925) Florae mycologicae Insulae St. Thomae. Anais do Instituto Superior de Agronomia da Universidade Técnica de Lisboa, vol 2. Instituto Superior de Agronomia, Lisboa, pp 1–29
Dayarathne MC, Boonmee S, Braun U, Crous PW, Daranagama DA, Dissanayake AJ, Ekanayaka H, Jayawardena R, Jones EB, Maharachchikumbura SS, Perera RH (2016) Taxonomic utility of old names in current fungal classification and nomenclature: Conflicts, confusion & clarifications. Mycosphere 7:1622–1648. https://doi.org/10.5943/mycosphere/7/11/2
Douanla-Meli C, Langer E (2010) Reassessment of phylogenetic species relationship of some lentinoid fungi with velutinate basidiomes based on partial 28S ribosomal RNA gene sequencing. Sydowia 62(1):23–35
Drechsler-Santos ER, Wartchow F, Coimbra VRM, Gibertoni TB, Cavalcanti MAQ (2012) Studies on lentinoid fungi (Lentinus and Panus) from the semi-arid region of Brazil. J Torrey Bot 139:437–446
Ediriweera SS, Nanayakkara CM, Weerasena OV, Karunarathna SC, Wijesundera RL, Piyatissa MA (2021) Morphology and phylogeny reveal nine new records of polypores from dry zone of Sri Lanka. Chiang Mai J Sci 48(3):893–908
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Fries EM (1821) Systema mycologicum, sistens fungorum ordines, genera et species, vol 1. Sumptibus Ernesti Mauritii, Gryphiswaldia, Gryphiswaldiae, p 520
Fries EM (1825) Systema orbis vegetabilis. Primas lineas novae constructionis periclitatur Elias Fries. Pars I. Plantae homonemeae. Typographia Academica, Lund, pp 369
Fries EM (1830) Eclogae fungorum, praecipue ex herbarus germanorum de scriptorum. Linnaea 5:497–553
Fries E (1838) Epicrisis systematis mycologici seu synopsis Hymenomycetum. Typographia Academica, Uppsala. https://doi.org/10.1080/00222934009512452
Galvão VIP, Koroiva R, Wartchow F (2023) A new species of Panus (Panaceae, Polyporales) from Paraíba, Brazil. Phytotaxa 514(3):17. https://doi.org/10.11646/phytotaxa.619.2.5
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Góes-Neto A, Loguercio-Leite C, Guerrero RT (2005) DNA extraction from frozen field-collected and dehydrated herbarium fungal basidiomata: performance of SDS and CTAB-based methods. Biotemas 18(2):19–32
Grand EA (2004) Systematics and species concepts in the genera Lentinus Fr. and Panus Fr., with emphasis on the Lentinus tigrinus, L. crinitus and Panus lecomtei complexes. Thesis, University of Tennessee
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321. https://doi.org/10.1093/sysbio/syq010
Hennings P (1897) Fungi camerunenses II. Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 23:537-558
Hibbett DS, Vilgalys R (1993) Phylogenetic relationships of Lentinus (Basidiomycotina) inferred from molecular and morphological characters. Syst Bot 18:409–433. https://doi.org/10.2307/2419417
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42(2):182–192. https://doi.org/10.2307/2992540
Huelsenbeck JP, Rannala B (2004) Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst Biol 53(6):904–913. https://doi.org/10.1080/10635150490522629
Justo A, Miettinen O, Floudas D, Ortiz-Santana B, Sjökvist E, Lindner D, Nakasone K, Niemelä T, Larsson KH, Ryvarden L, Hibbett DS (2017) A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol 121(9):798–824. https://doi.org/10.1016/j.funbio.2017.05.010
Kalchbrenner C (1881) Fungi Macowaniani. Grevillea 9(52):131–137
Katoh S (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. https://doi.org/10.1093/bioinformatics%2Fbts199
Kumar TA, Manimohan P (2005) A new species of Lentinus from India. Mycotaxon 92:119–123
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol and Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Küppers H (1994) Atlas de los colores. Naturart, Barcelona
Lanfear R, Calcott B, Ho SY, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol and Evol 29(6):1695–1701. https://doi.org/10.1093/molbev/mss020
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B (2017) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol and Evol 34(3):772–773. https://doi.org/10.1093/molbev/msw260
Largent DL, Johnson D, Watling R (1977) How to identify mushrooms to genus III: microscopic features. Mad River Press, Eureka, California, p 148
Léveillé JH (1844) Champignons exotiques. Ann Nat Sci, Bot Ser 3(2):167–221
Liu S, Shen LL, Wang Y, Xu TM, Gates G, Cui BK (2021) Species diversity and molecular phylogeny of Cyanosporus (Polyporales, Basidiomycota). Front Microbiol 12:631166. https://doi.org/10.3389/fmicb.2021.631166
Maia LC et al (2015) Diversity of Brazilian Fungi. Rodriguésia 66(4):1033–1045. https://doi.org/10.1590/2175-7860201566407
Meijer AAR (2006) Preliminary list of the macromycetes from the Brazilian State of Paraná. Bol Mus Bot Mun 68:1–59
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE). IEEE Press, New Orleans, Louisiana. https://doi.org/10.1109/GCE.2010.5676129
Motato-Vásquez V, Gugliotta AM, Rajchenberg M, Catania M, Urcelay C, Robledo G (2020) New insights on Bjerkandera (Phanerochaetaceae, Polyporales) in the Neotropics with description of Bjerkandera albocinerea based on morphological and molecular evidence. Plant Ecol Evol 153(2):229–245. https://doi.org/10.5091/plecevo.2020.1667
Murrill WA (1915) (Agaricales) Polyporaceae. North Amer Fl 9:201–296
Oliveira-Filho AT (2015) Um sistema de classificação fisionômico-ecológico da vegetação neotropical: segunda aproximação. In: Eisenlohr PV, Felfili JM, de Melo MMRF, de Andrade LA, Meira-Neto JAA (eds) Fitossociologia no Brasil: métodos e estudos de casos, v2. UFV, Viçosa, pp 385−411
Olou BA, Krah FS, Piepenbring M, Yorou NS, Langer E (2020) Diversity of Trametes (Polyporales, Basidiomycota) in tropical Benin and description of new species Trametes parvispora. MycoKeys 65:25. https://doi.org/10.3897/mycokeys.65.47574
Overholts LO (1930) Eu-Basidiomycetes. In: Chardon CE, BA Toro. Mycological explorations of Colombia. J Agric Univ P R 14:195−353. https://doi.org/10.46429/jaupr.v14i4.14223
Palacio M, Robledo GL, Reck MA, Grassi E, Góes-Neto A, Drechsler-Santos ER (2017) Decrypting the Polyporus dictyopus complex: recovery of Atroporus Ryvarden and segregation of Neodictyopus gen. nov. (Polyporales, Basidiomycota). PLoS ONE 12(10):0186183. https://doi.org/10.1371/journal.pone.0186183
Pattengale ND, Alipour M, Bininda-Emonds ORP, Moret BME, Stamatakis A (2009) How many bootstrap replicates are necessary?. In: Batzoglou, S. (eds) Research in Computational Molecular Biology. RECOMB 2009. Lecture Notes in Computer Science(LNBI), vol 5541. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-02008-7_13
Pegler DN (1971) Lentinus Fr. and related genera from Congo-Kinshasa (Fungi). Bull Jard Bot Natl Belg 41:273–281
Pegler DN (1972) Lentineae (Polyporaceae), Schizophyllaceae et especes lentinoides et pleurotoides des Tricholomataceae. Fl Illust Champ Afr Centre Fasc 1:1–26
Pegler DN (1983) The genus Lentinus: a world monograph. Kew Bull Addit Ser 10:1–281
Pegler DN (1997) The Agarics of São Paulo, Brazil: an account of the agaricoid fungi (Holobasidiomycetes) of São Paulo State, Brazil. Royal Botanic Gardens, UK
Peintner U, Kuhnert-Finkernagel R, Wille V, Biasioli F, Shiryaev A, Perini C (2019) How to resolve cryptic species of polypores: an example in Fomes. IMA Fungus 10:1–21. https://doi.org/10.1186/s43008-019-0016-4
Psurtseva NV, Zmitrovich IV, Seelan JS, Bulakh EM, Hughes KW, Petersen RH (2021) New data on morphology, physiology, and geographical distribution of Lignomyces vetlinianus, its identity with Lentinus pilososquamulosus, and sufficient phylogenetic distance from Le. martianoffianus. Mycol Progress 20:809–821. https://doi.org/10.1007/s11557-021-01701-z
Putzke J (1994) Lista dos fungos Agaricales (Hymenomycetes, Basidiomycotina) referidos para o Brasil. Cad Pesq Univ Fed Santa Cruz Do Sul, Ser Bot 6:3–186
Putzke J, Putzke MTL (2002) Os reinos dos fungos, vol 2. Santa Cruz do Sul, Edunisc, p 212
Raithelhuber J (1974) Hongos argentinos I. Compañía Impresora Argentina, Buenos Aires
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67(5):901–4
Rick J (1907) Fungi austro-americani Fasc. V U VI in: Ann Mycol 5:28–31
Rick J (1930) Contributio ad Monographiam Polyporacearum et Agaricacearum Brasiliensium IV. Brotéria, Sér Bot 24:27–118
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542. https://doi.org/10.1093/sysbio/sys029
Saccardo PA (1887) Sylloge Fungorum Omnium Hucusque Cognitorum, vol. V. R. Friedländer & Sohn, Berlin. https://doi.org/10.5962/bhl.title.5371
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Sanuma OI, Tokimoto K, Sanuma C, Autuori J, Sanuma LR, Martins MS, Junior NM, Ishikawa NK, Apiamö RM (2016) Sanöma samakönö sama tökö nii pewö oa wi ĩ tökö waheta: Ana amopö= Enciclopédia dos Alimentos Yanomami (Sanöma): Cogumelos. Hutukara Associação Yanomami e Instituto Socioambiental, São Paulo, pp 108
Seynes J de (1897) Recherches pour servir à l’Histoire Naturelle et à la Flore des Champignons du Congo Français, vol 1. Masson & Cie, Paris
Soltis PS, Soltis DE (2003) Applying the bootstrap in phylogeny reconstruction. Statist Sci 18(2):256–267. https://doi.org/10.1214/ss/1063994980
Sousa-Guimarães DK, Alves-Silva G, Camacho O, Menolli Jr N, Góes-Neto A, Souza JF, Robledo RL, Neves MA, Drechsler-Santos ER (2022) Data for: Studies on Panus (Panaceae, Polyporales): morphology and phylogeny assist new species descriptions. Harvard Dataverse, V2. 10.7910/DVN/DZVFKL
Spegazzini C (1889) Fungi Puiggariani: Pugillus I. Bol Acad Nac Ci Cordoba 11:1–381
Stamatakis A (2014) RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1312. https://doi.org/10.1093/bioinformatics/btu033
Stevenson G (1964) The Agaricales of New Zealand V. Kew Bull 19(1):1–59
Teixeira AR (1946) Himenomicetos brasileiros III. Bragantia 6:165–188
Teixeira AR (1995) Método para estudo das hifas do basidiocarpo de fungos poliporáceos. Manual nº 6, Instituto de Botánica, São Paulo, pp 22
Thiers B [continuously updated] Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Home page at: http://sweetgum.nybg.org/ih/
Tibpromma S et al (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261. https://doi.org/10.1007/s13225-017-0378-0
Tibpromma S, Hyde KD, Bhat JD, Mortimer PE, Xu J, Promputtha I, Doilom M, Yang JB, Tang AMC, Karunarathna SC (2018) Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 33:25–67. https://doi.org/10.3897/mycokeys.33.23670
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. https://doi.org/10.12705/Code.2018
Vargas-Isla R, Capelari M, Meloni N, Nagasawa E, Tokimoto K, Ishikawa NK (2015) Relationship between Panus lecomtei and P. strigellus inferred from their morphological, molecular and biological characteristics. Mycoscience 56(6):561–571. https://doi.org/10.1016/j.myc.2015.05.004
Vellinga EC, Noordeloos ME (2001) Glossary. In: Noordeloos ME, Kuyper ThW, Vellinga EC (eds) Flora agaricina neerlandica, vol 5. CRC Press, Boca Raton, pp 6–11
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
Vinjusha N, Kumar TA (2022) Validation of Panus bambusinus and P. roseus (Panaceae, Polyporales). Phytotaxa 533(4):235–236. https://doi.org/10.11646/phytotaxa.533.4.7
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press Inc, New York, pp 315–322
Zmitrovich IV, Kovalenko AE (2016) Lentinoid and polyporoid fungi, two generic conglomerates containing important medicinal mushrooms in molecular perspective. Int J Med Mushrooms 18:23–38. https://doi.org/10.1615/intjmedmushrooms.v18.i1.40
Zmitrovich IV, Malysheva VF (2013) Towards a phylogeny of Trametes alliance (Basidiomycota, Polyporales). Mikol Fitopatol 47(6):358–380
Acknowledgements
We are grateful to all the protected areas and their directors for permission to sample collections; the curators of mentioned fungaria for the loan of specimens (FLOR, IAC, SP, URM); the fungaria curatorship that readily sent photos and support us with type specimen data (BAFC, ISC, LISU, LPS, K, TENN, UPS); the Laboratório Multiusuário de Estudos em Biologia (LAMEB/UFSC) for providing infrastructure to carry out the molecular studies; J. Prado, C. Bicudo, and F. Wartchow for nomenclatural discussion on Panus neostrigosus versus Panus lecomtei; Dra. Fernanda Karstedt for pictures of Panus ciliatus; Dra. Viviane de Oliveira Garcia and colleagues from MICOLAB-UFSC for specimen collections; Dr. Diogo Henrique Costa-Rezende and Kelmer Cunha for assistance in analyses and discussion of species and pictures, respectively; and Dr. Jaya Seelan Sathiya Seelan (Univ. of Malaysia Sabah) for generating ITS and nrLSU sequences from some Brazilian specimens loaned from the herbarium SP. This research is part of the MIND.Funga research group: https://mindfunga.ufsc.br/.
Funding
The authors thank Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) for providing master and PhD scholarships to DKSG and FB; the PPGFAP/UFSC; the FONCYT (PICT 0830 to GR) and Fundación FungiCosmos for partial financing of the research; the Society of Systematic Biologists for the Mini-ARTS award to FB, allowing type revisions at LPS. NMJr. thanks the “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP) (grant #18/15677–0). AGN, ERDS, NMJr., and GAS are supported by CNPq (Grant Nos. 308880/2022–6, 310150/2022–1, 314236/2021–0, and 153025/2022–0, respectively).
Author information
Authors and Affiliations
Contributions
Conceptualization: Denyse K. Sousa-Guimarães, Elisandro R. Drechsler-Santos; methodology: Denyse K. Sousa-Guimarães, Felipe Bittencourt; formal analysis and investigation: Denyse K. Sousa-Guimarães, Olga Camacho, Genivaldo Alves-Silva, Elisandro R. Drechsler-Santos; writing—original draft preparation: Denyse K. Sousa-Guimarães, Genivaldo Alves-Silva, Elisandro R. Drechsler-Santos; writing—review and editing: Felipe Bittencourt, Gerardo L. Robledo, Nelson Menolli Jr, Aristóteles Góes-Neto; funding acquisition: Elisandro R. Drechsler-Santos, Aristóteles Góes-Neto; resources: Elisandro R. Drechsler-Santos, Aristóteles Góes-Neto; supervision: Genivaldo Alves-Silva, Nelson Menolli Jr, Gerardo L. Robledo; Elisandro R. Drechsler-Santos.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Zhu-Liang Yang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sousa-Guimarães, D.K., Alves-Silva, G., Bittencourt, F. et al. A comprehensive phylogeny of Panus (Panaceae, Polyporales) and revisited Brazilian diversity. Mycol Progress 23, 19 (2024). https://doi.org/10.1007/s11557-024-01955-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01955-3