Abstract
Microbial adhesion to surfaces and the subsequent biofilm formation may result in contamination in food industry and in healthcare-associated infections and may significantly affect postoperative care. Some plants produce substances with antioxidant and antimicrobial properties that are able to inhibit the growth of food-borne pathogens. The aim of our study was to evaluate antimicrobial and anti-biofilm effect of baicalein, resveratrol, and pterostilbene on Candida albicans, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Escherichia coli. We determined the minimum inhibitory concentrations (MIC), the minimum adhesion inhibitory concentration (MAIC), and the minimum biofilm eradication concentration (MBEC) by crystal violet and XTT determination. Resveratrol and pterostilbene have been shown to inhibit the formation of biofilms as well as to disrupt preformed biofilms. Our results suggest that resveratrol and pterostilbene appear potentially very useful to control and inhibit biofilm contaminations by Candida albicans, Staphylococcus epidermidis, and Escherichia coli in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilm is a surface-associated community of microorganisms that is embedded in exopolymeric matrix (Donlan 2001; Douglas 2002). Extracellular polymeric substances (EPS) form a barrier that impedes the penetration of antimicrobial agents into the biofilm structure (Tote et al. 2010). The phenotype of biofilm cells changes along with the up- and downregulation of many genes (Donlan and Costerton 2002; Haussler and Fuqua 2013). The ability to form tenacious biofilms results in protection against low and high temperatures and low water activity, i.e., conditions employed in food industry technologies for storage of products: (Chmielewski and Frank 2003). Sterilization eliminates the planktonic forms of microorganisms but biofilms are 1000× more resistant and may be responsible for secondary infections (Bridier et al. 2015; Singh et al. 2014). The recent realization of the emergence of antimicrobial resistance has emphasized the need for better understanding of biofilm-related behavior of food microflora and potentially pathogenic contaminants in food industry and lead to an increased interest in elucidating the role of surface-microorganism behavior (see Table 1).
Candida spp. are food pathogens and contaminants, as well as commensal microorganism without negative influence on healthy organisms. Candida species adhere readily to surfaces and form biofilms (Kuhn 2002); they also easily acquire resistance to host defense mechanisms and antifungal drugs (Kuhn et al. 2004; Ramage et al. 2005). Their pathogenicity manifests mainly in immunocompromised patients (Wang et al. 2014). The high resistance of Candida sp. biofilms is caused by the extracellular matrix (Donlan 2001; Rajasekharan et al. 2014).
Pseudomonas aeruginosa often forms part of natural microflora of vegetables, meat, milk, and other food products (Franzetti and Scarpellini 2007). It is considered to be the causative agent of off-flavors and unpleasant textural changes in finished food products (Martin et al. 2011; Van Tassell et al. 2012). P. aeruginosa is not only a food contaminant but also a pathogen responsible for nosocomial infections, bacteremia, pneumonia, and urinary tract infections. It belongs to pathogenic bacteria that may possess a broad resistance toward antibiotics and biocides (Leid et al. 2009; Page and Heim 2009). The natural resistance of P. aeruginosa to many antimicrobial drugs is usually connected with the ability to produce extracellular polymers and biofilm formation.
Staphylococcus spp. are one of the major etiological agents of food poisoning (Sievert et al. 2013) and a major milk pathogen. Besides contamination of foods, Staphylococcus sp. is also responsible for the increasing incidence of biomaterial infection, extended hospital stays, and patient mortality (Laverty et al. 2015).
Escherichia coli strains are among the common opportunistic pathogens found in humans; their pathogenicity is often connected with the biofilm formation (Paulo et al. 2010).
Synthetic additives are often used to lessen the probability of food microbial contamination and their side effects are one of the studied issues (Tepe et al. 2005). The importance of using nontoxic natural agents as replacements of synthetic ones is therefore evident. Some plants produce substances with pronounced antimicrobial properties that give them selective advantage in their natural environment (Zhao et al. 2005). Such compounds might be potentially used for food preservation (Delamare et al. 2007). Antimicrobial activity was demonstrated in essential oils, extracts from plants used in traditional Chinese medicine (Coenye et al. 2012; Marinas et al. 2015), but also in Ficus sp. (Awolola 2014) that contains substances such as resveratrol, pterostilbene, baicalein, and others (Huang 1999; Wu and Wen 2009).
Many procedures and biocidal agents such as benzalkonium chloride, and sodium hypochlorite are employed in food industry to eliminate microbial biofilms and suppress biofilm formation (Pagedar and Singh 2015). The efficacy of commonly applied biocides is dependent on many factors such as the cleaning regime, concentration, contact time, and properties of cleaned surface.
Plants are a rich source of biologically active compounds, many of which possess antimicrobial activity (Palombo 2011). We have focused on three such compounds: baicalein, resveratrol, and its structural analogue pterostilbene. Baicalein is found in high concentrations in medicinal plants, Scutellaria baicalensis and Oroxylum indicum cells (Dinda et al. 2017). Both resveratrol and pterostilbene are also found in plants, especially in fruits and vegetables. Resveratrol has a wide potential in medicinal applications due to its anti-aging, anti-carcinogenic, anti-inflammatory, and anti-oxidant properties (Cottart et al. 2010; Kolouchova et al. 2005). All three substances have long been studied due to their positive influence on human health, stemming from their presence as active compound in traditional medicine applications (baicalein) or their antioxidative properties (resveratrol, pterostilbene). Their antimicrobial activity was discovered much later and their antibiofilm properties are only now being discovered. The antibiofilm activity of natural substances is often strain or genera-specific, therefore it is necessary to study a wide range of microorganisms and experimental approaches to obtain comprehensive information leading to potential applications. The antimicrobial and antibiofilm activity of baicalein, resveratrol, and pterostilbene has been observed against selected microorganisms (Zeng et al. 2008; Chen et al. 2016; Hu et al. 2017; Cho et al. 2015; Lee et al. 2014b). For potential application of these substances, it is necessary to study their influence on both planktonic and biofilm populations, as it is known that their properties vary significantly. This is intensively studied in medicine, as pathogenic microorganisms freely switch between free and adhered form of life which diminishes the effectivity of standard antimicrobials. In this regard, the baicalein effectivity was studied (Serpa et al. 2012), as well as the stilbenes (Lee et al. 2014a; Li et al. 2014; Nimmy et al. 2014). The novelty of our study is the combination of the approaches of observing their effect on both initial adhesion and biofilm eradication, as well as comparison with substance efficiency toward the planktonic cells.
One of the advantages of all three compounds is their low cytotoxicity. Baicalein was reported to show almost no toxicity to human normal epithelial, peripheral and myeloid cells (Dinda et al. 2017). Toxicity study of an herbal formulation containing Scutellaria baicalensis extract with 2 g/L of baicalein on nude mice showed no toxicities. The nude mice ingested the extract in a diet and were monitored for 13 weeks, showed no abnormalities in their behavior (Donald et al. 2012). The stilbenes such as resveratrol and pterostilbene also were reported to show low toxicity in normal hemopoietic stem cells (Tsai et al. 2017) and pterostilbene oral administration to nude mice (100 μg/kg per day) for 8 weeks did not produce signs of acute toxicity (Kosuru et al. 2016).
The present study was undertaken to evaluate the antibacterial/anti-biofilm properties of the natural compounds baicalein, resveratrol, and pterostilbene against selected microorganisms—Candida albicans, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Escherichia coli—that cause food and nosocomial infections.
Material and methods
Microbial strains and growth media
Candida albicans DBM 2164, Staphylococcus epidermidis DBM 3179, and Escherichia coli DBM 3125 were kindly provided by the Collection of Microorganisms at UCT Prague. Pseudomonas aeruginosa NRRL B-59189 was obtained from the ARS Culture Collection, Bacterial Foodborne Pathogens and Mycology Research Unit, National Center for Agricultural Utilization Research, USA.
C. albicans was pre-cultured (30 °C) before each experiment in yeast-peptone-dextrose medium (YPD), S. epidermidis (37 °C) in Tryptone Soya Broth medium (TSB), and P. aeruginosa and E. coli (30 °C) in Luria Broth medium (LB). All microbial strains were stored at − 70 °C. The microbial strains were pre-cultured for 24 h to achieve exponential phase of growth (100 ml in Erlenmeyer flasks, 100 rpm).
Biologically active agents
The natural substances (baicalein, resveratrol and pterostilbene) were purchased from Sigma-Aldrich and were dissolved in dimethylsulfoxid (final concentration of DMSO in the medium was 1% in all assays). This DMSO concentration was proven not to interfere with the growth and evaluation methods by independent control cultivations.
Determination of minimum inhibitory concentration of planktonic cells
The response of planktonic cells to various concentration of natural substances was characterized as the minimal inhibitory concentration. Minimum inhibitory concentrations (MICs) for planktonic cells by baicalein, resveratrol and pterostilbene were determined by microdilution method according to (Sharma et al. 2010). The cultivation of planktonic cells was carried out in flat-bottomed microtiter plates using Bioscreen C analyzer (Oy Growth Curves Ab Ltd., Finland). Aliquots of 30 μl of standard cell suspensions of the microorganisms (A600nm = 0.1) in growth medium were transferred into the microtiter plates with serially diluted antimicrobial agent and growth medium to a final volume of 320 μl, followed by incubation for 24 h at 30 or 37 °C (depending on microbial strain). The MICs were assigned as the lowest concentration that did not allow visible growth of more than 20% (MIC80) or 50% (MIC50) after overnight incubation according to the definition by Andrews (2001). Experiments were performed in triplicate. Control cultivations without the natural substances were included.
Determination of minimum adhesion inhibition concentration and the agent efficiency against biofilm formation
For the initial adhesion (biofilm formation) testing and the minimum adhesion inhibition concentration (MAIC) determination, the substances were added at the beginning of the cultivation.
The biofilm cultivation was performed in 96-well microtiter plates, into which aliquots of 200 μl of standard cell suspensions (A600nm = 0.6) in growth medium and the appropriate agent were transferred into each well. The microtiter plate was covered with a lid held by semipermeable parafilm and was placed into an orbital shaker (150 rpm) at 30 or 37 °C (according to microorganism) to allow for biofilm development and the cultivation was carried out for 24 h. After 24 h, each well was washed three times with saline and biofilm formation on the bottom of wells was evaluated by XTT reduction assay and the crystal violet staining method. Minimum adhesion inhibition concentration (MAIC) was determined by the XTT reduction assay—MAICs were assigned as the lowest concentration that did not exhibit more than 20% (MAIC80) or 50% (MAIC50) of metabolic activity of biofilm cells. The total biofilm biomass was determined by the crystal violet staining method. As control, cultivations without the natural substances were used. All experiments were performed in 8 parallels.
Determination of minimum biofilm eradication concentrations and the agent efficiency against pre-formed biofilms
For the biofilm susceptibility testing and minimum biofilm eradication concentration (MBEC) determination, the substances were added to a preformed, 24-h-old biofilm. The biofilm cultivation was performed in 96-well microtiter plates. Aliquots of 200 μl of standard cell suspensions (A600nm = 0.6) in growth medium were transferred into a 96-well microtiter plate, followed by cultivation for 24 h at 30 or 37 °C (according to microorganism). The microtiter plate was covered with a lid held by semipermeable parafilm and was placed into an orbital shaker (150 rpm) at 30 or 37 °C (according to microorganism) to allow for biofilm development and the cultivation was carried out for 24 h. After 24 h (biofilm development without agent), each well was washed three times with saline, followed by the addition of growth medium and serially diluted agents. The cultivation continued for the next 24 h (30 or 37 °C at 150 rpm in an orbital shaker), after which each well was washed three times with saline and biofilm on the bottom of wells was evaluated by XTT reduction assay and the crystal violet staining method. Minimum biofilm eradication concentrations (MBEC) were determined by the XTT reduction assay—MBECs were assigned as the lowest concentration that did not exhibit more than 20% (MBEC80) or 50% (MBEC50) of metabolic activity of biofilm cells. The total biofilm biomass was determined by the crystal violet staining method. Biofilm populations were quantified by XTT reduction assay and the crystal violet staining method. As control, cultivations without the natural substances were used. All experiments were performed in 8 parallels.
XTT reduction assay
Minimum adhesion inhibitory concentrations (MAIC) and minimum biofilm eradication concentrations (MBEC) of natural substances were determined using XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H–tetrazoliumhydroxide) reduction assay (Zhou et al. 2012). For each assay, the wells were washed three times with saline to remove planktonic cells and into each well was added 155 μL of glucose solution (36.1 mg/mL), 40 μL of XTT solution (1 mg/mL), and 5 μL of menadione solution (0.11 mg/mL). The plate was incubated in dark at 30 or 37 °C for 3 h and then a 100 μl volume from each well was transferred into 96-well microtiter plate and the colorimetric change was determined using spectrophotometric reader (Tecan, Switzerland) at 492 nm. Experiments were performed in 8 parallels.
Crystal violet staining
The total biofilm biomass was determined by staining the biofilm with crystal violet (CV). In the quantification process, each well was washed three times with saline and into each well was added 100 μL of 0.1% filtered CV (Carl Roth, Germany) solution. The plate was incubated for 20 min at room temperature. Afterwards, each well was washed three times with saline. CV bound to the biofilm biomass was released by adding 200 μL of 96% ethanol (Penta, Czech Republic) and, after incubating for 10 min at room temperature, measured using spectrophotometric reader (Tecan, Switzerland) at 580 nm. Experiments were performed in 8 parallels.
Light microscopy—Cellavista device
In one representative sample from eight parallels, the area populated by biofilm was visualized by Cellavista device, as previously described (Kvasničková et al. 2015). Cellavista device (Synentech, Germany) is a fully automated cell imager that incorporates bright-field inverted microscope with high-resolution camera and software for image analysis.
Statistical analysis
The significance of difference between control and substance efficiency on adhesion inhibition and preformed biofilm was determined by one-way analysis of variance (ANOVA) and Tukey’s HSD test.
Results and discussion
Determination of minimum inhibitory concentrations of plant-derived agents
The MIC50 values (inhibition of planktonic cells) of baicalein, pterostilbene and resveratrol are shown in Table 2. The inhibitory properties of specific compounds were dependent on the microorganism type, but pterostilbene was found as the most effective substance with lowest MIC50 against all studied microorganisms. Baicalein had inhibitory properties only against planktonic cells of C. albicans. Inhibition of planktonic growth by resveratrol was observed in C. albicans, S. epidermidis, and E. coli.
The MIC50 of pterostilbene for C. albicans (5 mg/L) that we found was very low and was near that of amphotericin B (2 mg/L) reported by Melo et al. (2011). The MIC80 of the agent for C. albicans (10 mg/L) was lower than the value determined by Li et al. (2014) (32 mg/L). Resveratrol, a close analogue of pterostilbene, had a MIC50 value against C. albicans 160 mg/L, a value similar to the results reported by other articles (Jung et al. 2005; Jung et al. 2007; Okamoto-Shibayama 2010; Weber et al. 2011). Though it was described to decrease the mitochondrial activity of cells in some strains, it is also known to show no inhibitory effect against other C. albicans strains (Collado-Gonzalez et al. 2012; Weber et al. 2011). Baicalein MIC50 for C. albicans (13–26 mg/L) was similar to that reported by Serpa et al. (2012), but significantly lower than the 500 mg/L reported (as baicalein MIC) by Wang et al. (2014). The mechanisms of action of baicalein were reported as induction of programmed cell death in C. albicans cells (Dai et al. 2009), promotion of the formation of blastoconidia, and formation of filaments (Serpa et al. 2012).
We found the MIC50 (60 mg/L) against P. aeruginosa only for pterostilbene and no MIC80 was found for any of the agents in the studied concentration ranges. The MIC80 against another gram-negative bacterium, E. coli, were determined for both pterostilbene and resveratrol. MIC80 for resveratrol (250 mg/L) against E. coli was lower than the value reported by Paulo et al. (2010) who observed resveratrol activity toward E. coli (> 400 mg/L) but did not observe any inhibition for P. aeruginosa (Paulo et al. 2010).
P. aeruginosa exhibits resistance to many biocides such as isopropanol, peracetic acid, sodium hypochlorite, iodophore or hydrogen peroxide, which are able to inhibit only planktonic cells, albeit in high concentrations (Tote et al. 2010). The most usual cleaning cycle in dairy industry uses a 15-min contact time of the biocide with the surface. Like the usually employed concentrations, this may be insufficient for P. aeruginosa elimination. P. aeruginosa MIC (for planktonic cells) were determined at 300 mg/L for benzalkonium chloride and sodium hypochlorite and about 50-fold lower for iodophore (Pagedar and Singh 2015).
In S. epidermidis, we found MIC80 for both pterostilbene and resveratrol, but baicalein did not inhibit the growth below the concentration 100 mg/L and its MICs were not found. Available data confirm the high concentration of baicalein necessary to inhibit the growth of many bacterial strains, both gram positive and negative. Luo et al. (2016) reported baicalein MIC50 for P. aeruginosa at 1000 mg/L while Enterococcus isolates were found to be inhibited by concentrations in excess of 256 mg/L (Chang et al. 2007). Gram-positive bacteria are inhibited by resveratrol in the growth phase of the bacterial cell cycle (Paulo et al. 2010). According to Yun et al. (2012), the antibacterial activity of baicalein against S. aureus involved membrane permeabilization, protein synthesis inhibition and modification of the activity of succinate dehydrogenase, malate dehydrogenase and DNA topoisomerase I and II (Yun et al. 2012).
Inhibition of biofilm formation by plant-derived agents
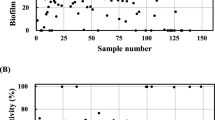
In Table 3 is shown the effect of substances on the biofilm formation determined by the XTT method as the minimal adhesion inhibiting concentration (MAIC). In Fig. 1, the influence of a series of concentrations of agents on total biofilm biomass determined by the crystal violet is shown. Crystal violet staining (CV) and determination of metabolic activity of cells by the XTT assay are two of the most often used methods used for the evaluation of biofilm development, but there is an obvious distinction due to the difference in their principle. Crystal violet staining determines total biofilm biomass—both metabolically active and inactive cells and EPS (Hawser et al. 1998; Ramage et al. 2001) while the XTT assay is based on the reduction of XTT sodium salt by dehydrogenases of metabolically active cells and reports on actual metabolic activity of the cells (Bizerra et al. 2008; O'Toole et al. 2000).
Inhibition of total biofilm biomass (determined by the crystal violet staining) in initial adhesion of C. albicans DBM 2164, S. epidermidis DBM 3179 and E. coli DBM 3125 by baicalein, resveratrol, and pterostilbene. Error bars represent standard deviation. Control (no agent, 100%), *p < 0.05; **p < 0.01; ***p < 0.001
A significant inhibition of biofilm formation was observed in E. coli. Pterostilbene had the lowest MAIC50 (40 mg/L) followed by baicalein (80 mg/L). Similar values for baicalein and resveratrol were reported by Bakkiyaraj et al. (2013). Resveratrol was reported to reduce swimming and swarming motilities and repress several key motility and flagellar genes (flhD, fimA, FimH, motB), without inhibiting the growth of planktonic cells (Lee et al. 2013). The degree of resistance may be strain dependent; thus, the E. coli DBM 3125 we studied showed higher resistance to resveratrol than the E. coli strain O157:H7 studied by Lee et al. (2013) whose biofilm was significantly reduced by a resveratrol concentration of 10 mg/L.
In S. epidermidis, baicalein at 80 mg/L concentration inhibited the biofilm formation of by 50%, pterostilbene exhibited a similar effect at 50 mg/L, and resveratrol at 170 mg/L. Among gram-positive bacteria, high concentrations of resveratrol are necessary for their inhibition, e.g., Propionibacterium acnes biofilm formation was found to be inhibited by 80% by resveratrol in concentration 3.2 g/L (Coenye et al. 2012).
In our work, pterostilbene at the highest concentration of 20 mg/L decreased Candida biofilm formation only by 30% (data not shown), resveratrol inhibited it with MAIC50 88 mg/L and baicalein, which induces apoptosis of C. albicans cells by suppressing extrusion of the drug due to inhibition of efflux pumps (Dai et al. 2009; Huang et al. 2008), at 200 mg/L. The resistance of Candida biofilms is an issue in both food industry and medicine (Mah and O’Toole 2001).
Beside the MAIC and MBEC determined by the XTT reduction assay, the total biofilm biomass during biofilm formation was determined by the crystal violet staining (Fig. 1).
P. aeruginosa was found to be the most resistant to the agents. The anti-biofilm activity of the agents that influenced the total biofilm biomass of P. aeruginosa (determined by crystal violet staining), was omitted, because none of the substances tested had any significant effect on the inhibition of P. aeruginosa. The highest adhesion inhibition which was obtained under the experimental conditions corresponded to a 10% decrease in metabolic activity of cells during biofilm formation in comparison with control (data not shown). Cho et al. (2013) showed 50% inhibition of P. aeruginosa biofilm formation by resveratrol (100 mg/L) while the highest concentration of resveratrol (170 mg/L) used in our study did not significantly inhibit the P. aeruginosa biofilm formation. P. aeruginosa exhibits a high resistance toward a variety of antimicrobials such as aminoglycosides and macrolide antibiotics (Pompilio et al. 2015). Pathogenesis and biofilm formation by P. aeruginosa are associated with the production of extracellular virulence factors and cell motility (Wahman et al. 2015). The weak penetration of antimicrobials into biofilm is the result of increased production of EPS in the biofilm and consequent decrease of diffusion of antibiotics and biocides (Dynes et al. 2009; Pompilio et al. 2015).
Baicalein inhibited the total biofilm biomass formation (Fig. 1a) in S. epidermidis, but was not active against E. coli. In C. albicans, baicalein even led to 50% increase higher total biofilm biomass, even though the metabolic activity of cells was suppressed (MAIC50 400 mg/L was found—see Table 3). Similar results were reported by Cao et al. (2008) who observed the inhibitory effect of baicalein on the metabolic activity of C. albicans cells and determined a MAIC50 of 200 mg/L. A twofold increase in total biofilm biomass was observed at the baicalein concentration of 200 mg/L. Baicalein therefore decreases the metabolic activity of C. albicans cells but promotes the total biofilm biomass production.
The results obtained by methods observing the viability of sessile cells (e.g., XTT reduction assay) and the total biofilm biomass (crystal violet staining) must therefore be carefully compared when used for the determination of the inhibitory effect of substances on the biofilm, especially for certain genera of microorganisms.
Resveratrol (Fig. 1b) had a concentration-dependent inhibitory effect on both bacteria, S. epidermidis and E. coli, as well as on the yeast C. albicans. Similar results were observed by Selma et al. (2012), and resveratrol at high concentration (3.2 g/L) also inhibited the adhesion of E. coli O15:H17 and the biofilm formation of gram-positive Propionibacterium acnes (Coenye et al. 2012).
Increasing concentrations of pterostilbene (Fig. 1c) were found to inhibit biofilm formation of C. albicans and E. coli. The effect of pterostilbene S. epidermidis biofilm biomass was found to be opposite; with increasing concentration of the agent the total biofilm biomass increased, but did not match the detected metabolic activity of the cells. At 20 mg/L, the adhesion monitored as the total biofilm biomass increased by 133%, while the metabolic activity of cells in biofilm decreased by 20%. The anti-biofilm effect of pterostilbene is based on its antiadhesive and anti-morphological transition activities (Li et al. 2014). Li et al. (2014) also observed inhibition of C. albicans biofilm formation by 60% (4 mg/L pterostilbene) and 90% (32 mg/L pterostilbene) and a decrease of the cellular surface hydrophobicity of C. albicans (Li et al. 2014).
Eradication of pre-formed biofilm by plant-derived agents
Minimum biofilm eradication concentrations (determined by the XTT reduction assay) are shown in Table 3. The influence of a series of concentrations of agents on total biofilm biomass determined by the crystal violet is shown in Fig. 2.
Inhibition of total biofilm biomass (determined by the crystal violet staining) of pre-formed biofilm of C. albicans DBM 2164, S. epidermidis DBM 3179 and E. coli DBM 3125 by baicalein, resveratrol, and pterostilbene. Error bars represent standard deviation. Control (no agent, 100%), *p < 0.05; **p < 0.01; ***p < 0.001
Resveratrol and pterostilbene displayed similar effect on E. coli biofilm eradication as on inhibition of biofilm formation, MBEC50 was found for both agents.
S. epidermidis pre-formed biofilm metabolic activity was effectively inhibited by both resveratrol and pterostilbene. Both substances were more efficient in biofilm eradication than in the inhibition of biofilm formation. The MBEC50 values (100 and 25 mg/L, respectively) are almost half the MAIC50 values (170 and 50 mg/L, respectively).
Baicalein exhibited less significant influence on S. epidermidis biofilm eradication, MBEC was not found and the total biofilm biomass was not significantly affected. The concentration of 400 mg/L decreased the amount of mature biofilm by a mere 20%. Cui et al. (2016), who studied the anti-biofilm effect of sage oil on S. aureus, found significant biofilm eradication only above the concentration of 2000 mg/L.
The resveratrol MBEC50 value we found for the yeast C. albicans was 88 mg/L. Pterostilbene at a concentration of 20 mg/L reduced the total biofilm biomass by 15%. These agents thus may contribute to the eradication of C. albicans biofilm, which is often found very resistant against the commonly used antibiotics. It should be noted that Melo et al. (2011) did not find fluconazole and amphotericin B MBEC50 and MBEC80 values for C. albicans, even though the monitored concentrations were greater than 2000 mg/L.
As in biofilm formation, the pre-formed biofilms of the gram-negative bacterium P. aeruginosa were not significantly inhibited by the agents and therefore the results of total biofilm biomass are not shown. Only the highest concentration of baicalein (400 mg/L) reduced the amount of P. aeruginosa biofilm by 10% (data not shown).
Resveratrol eradicated the total biofilm biomass of preformed biofilms of C. albicans, S. epidermidis, and E. coli with a similar intensity and was the most effective—with increasing concentration of resveratrol the total biofilm biomass was reduced, the reduction at 170 mg/L being almost 35%. This effect was visualized by light microscopy in Fig. 3.
The studied plant-derived compounds may be an advantageous alternative to the commonly used biocides such as EDTA, benzalkonium chloride, sodium hypochlorite, or iodophore that have often high MICs (hundreds of mg/L) and MBECs (up to thousands of mg/L) (El-Sharif and Hussain 2011; Pagedar and Singh 2015). Although antibiotics have lower MICs, their MBECs are high (up to hundreds and thousands of mg/L) (Barchiesi et al. 1998; Huang et al. 2008; Pompilio et al. 2015; Serpa et al. 2012; Zhou et al. 2012) and nevertheless their application in food industry is impossible.
Conclusion
Overuse or misuse of antibiotics for the control of infections and the difficulties in eradication of biofilm formation and resistance of microorganisms increases the pressure on finding new techniques for CIP processes. Our study demonstrates the ability of natural substances derived from plants to inhibit microbial biofilms that occur in both the food industry and medicine. Baicalein, resveratrol and pterostilbene, all originally isolated from plants, have been shown to inhibit the formation of biofilms and disrupt preformed biofilms. In particular, resveratrol and pterostilbene displayed high inhibition of biofilm cell viability for the opportunistic pathogens under study. The highest resistance to these substances was found in Pseudomonas aeruginosa. In Escherichia coli, all three compounds caused a significant drop of cell viability in biofilm formation and eradication of pre-formed biofilm. Our results show that these new antimicrobials can be used with good effect in food industry and medicine and can contribute to an improvement of strategies designed to ensure better food safety and quality.
References
Alakomi HL, Puupponen-Pimia R, Aura AM, Helander IM, Nohynek L, Oksman-Caldentey KM, Saarela M (2007) Weakening of Salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J Agric Food Chem 55:3905–3912
Al-Shabib NA, Husain FM, Ahmad I, Khan MS, Khan RA, Khan JM (2017) Rutin inhibits mono and multi-species biofilm formation by foodborne drug resistant Escherichia coli and Staphylococcus aureus. Food Control 79:325–332
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16
Awolola VG (2014) Antibacterial and anti-biofilm activity of Ficus sansibarica Warb. subsp Sansibarica (Moraceae) extracts. Planta Med 80:779–779
Bakkiyaraj D, Nandhini JR, Malathy B, Pandian SK (2013) The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling 29:929–937
Balagopal A, Kothandapani S, Prathapkumar HS (2017) Study on E. coli and Salmonella biofilms from fresh fruits and vegetables. J Food Sci Technol 54:1091–1097
Barchiesi F, Di Francesco LF, Compagnucci P, Arzeni D, Giacometti A, Scalise G (1998) In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J Antimicrob Chemother 41:59–65
Bizerra FC, Nakamura CV, de Poersch C, Estivalet Svidzinski TI, Borsato Quesada RM, Goldenberg S, Krieger MA, Yamada-Ogatta SF (2008) Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res 8:442–450
Bridier A, Sanchez-Vizuete P, Guilbaud M, Piard JC, Naitali M, Briandet R (2015) Biofilm-associated persistence of food-borne pathogens. Food Microbiol 45:167–178
Cao Y, Dai B, Wang Y, Huang S, Xu Y, Cao Y, Gao P, Zhu Z, Jiang Y (2008) In vitro activity of baicalein against Candida albicans biofilms. Int J Antimicrob Agents 32:73–77
Chan MM-Y (2002) Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem Pharmacol 63:99–104
Chang P-C, Li H-Y, Tang H-J, Liu J-W, Wang J-J, Chuang Y-C (2007) In vitro synergy of baicalein and gentamicin against vancomycin-resistant Enterococcus. J Microbiol Immunol Infect 40:56–61
Chen Y, Liu TJ, Wang K, Hou CC, Cai SQ, Huang YY, Hu ZY, Huang H, Kong JL, Chen YQ (2016) Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One 11:e0153468
Chmielewski RAN, Frank JF (2003) Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf 2:22–32
Cho HS, Lee J-H, Ryu SY, Joo SW, Cho MH, Lee J (2013) Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 biofilm formation by plant metabolite ε-viniferin. J Agric Food Chem 61:7120–7126
Cho HS, Lee J-H, Cho MH, Lee J (2015) Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 31:1–11
Coenye T, Nelis HJ (2010) In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods 83:89–105
Coenye T, Brackman G, Rigole P, De Witte E, Honrae K, Rossel B, Nelis HJ (2012) Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine 19:409–412
Collado-Gonzalez M, Guirao-Abad JP, Sanchez-Fresneda R, Belchi-Navarro S, Arguelles JC (2012) Resveratrol lacks antifungal activity against Candida albicans. World J Microbiol Biotechnol 28:2441–2446
Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL (2010) Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res 54:7–16
Cui H, Zhou H, Lin L (2016) The specific antibacterial effect of the Salvia oil nanoliposomes against Staphylococcus aureus biofilms on milk container. Food Control 61:92–98
da Silva RA, Bernardo LP, Lopes JM, Moreno LVS, Porto VC (2017) Equisetum giganteum influences the ability of Candida albicans in forming biofilms over the denture acrylic resin surface. Pharm Biol 55:1698–1702
Dai B-D, Cao Y-Y, Huang S, Xu Y-G, Gao P-H, Wang Y, Jiang Y-Y (2009) Baicalein induces programmed cell death in Candida albicans. J Microbiol Biotechnol 19:803–809
Das A, Das MC, Sandhu P, Das N, Tribedi P, De UC, Akhterb Y, Bhattacharjee S (2017) Antibiofilm activity of Parkia javanica against Pseudomonas aeruginosa: a study with fruit extract. RSC Adv 7:5497–5513
Delamare APL, Moschen-Pistorello IT, Artico L, Atti-Serafini L, Echeverrigaray S (2007) Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem 100:603–608
Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M (2017) Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem 131:68–80
Donald G, Hertzer K, Eibl G (2012) Baicalein—an intriguing therapeutic phytochemical in pancreatic cancer. Curr Drug Targ 13:1772–1776
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Douglas LJ (2002) Medical importance of biofilms in Candida infections. Rev Iberoam Micol 19:139–143
Dynes JJ, Lawrence JR, Korber DR, Swerhone GD, Leppard GG, Hitchcock AP (2009) Morphological and biochemical changes in Pseudomonas fluorescens biofilms induced by sub-inhibitory exposure to antimicrobial agents. Can J Microbiol 55:163–178
El-Sharif AA, Hussain MHM (2011) Chitosan-EDTA new combination is a promising candidate for treatment of bacterial and fungal infections. Curr Microbiol 62:739–745
Franzetti L, Scarpellini M (2007) Characterisation of Pseudomonas spp. isolated from foods. Ann Microbiol 57:39–47
Gonzales-Siles L, Sjoling A (2016) The different ecological niches of enterotoxigenic Escherichia coli. Environ Microbiol 18:741–751
Gowrishankar S, Pandian SK (2017) Modulation of Staphylococcus epidermidis (RP62A) extracellular polymeric layer by marine cyclic dipeptide-cyclo(L-leucyl-L-prolyl) thwarts biofilm formation. Biochim Biophys Acta 1859:1254–1262
Gupta P, Sarkar A, Sandhu P, Daware A, Das MC, Akhter Y, Bhattacharjee S (2017) Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: a study with plumbagin and gentamicin. J Appl Microbiol 123:246–261
Haussler S, Fuqua C (2013) Biofilms 2012: new discoveries and significant wrinkles in a dynamic field. J Bacteriol 195:2947–2958
Hawser SP, Norris H, Jessup CJ, Ghannoum MA (1998) Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2h-tetrazolium hydroxide (xtt) colorimetric method with the standardized national committee for clinical laboratory standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol 36:1450–1452
Hirschfeld J, Akinoglu E, Wirtz DC, Hoerauf A, Bekeredjian-Ding I, Jepsen S, Haddouti EM, Limmer A, Giersig M (2017) Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine 13:1587–1593
Hu DD, Zhang RL, Zou Y, Zhong H, Zhang ES, Luo X, Wang Y, Jiang YY (2017) The structure-activity relationship of pterostilbene against Candida albicans biofilms. Molecules 22:360
Huang KC (1999) Antibacterial, antiviral, and antifungal herbst, the pharmacology of chinese herbst. In C. Press (Ed.): Florida
Huang S, Cao YY, Di Dai B, Sun XR, Zhu ZY, Cao YB, Wang Y, Gao PH, Jiang YY (2008) In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull 31:2234–2236
Jung HJ, Hwang IA, Sung WS, Kang H, Kang BS, Seu YB, Lee DG (2005) Fungicidal effect of resveratrol on human infectious fungi. Arch Pharm Res 28:557–560
Jung HJ, Seu YB, Lee DG (2007) Candicidal action of resveratrol isolated from grapes on human pathogenic yeast C. albicans. J Microbiol Biotechnol 17:1324–1329
Kolouchova I, Melzoch K, Smidrkal J, Filip V (2005) The content of resveratrol in vegetables and fruit. Chem List 99:492–495
Kosuru R, Rai U, Prakash S, Singh A, Singh S (2016) Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. Eur J Pharmacol 789:229–243
Kuhn DM (2002) Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun 70:878–888
Kuhn DM, Mukherjee PK, Clark TA, Pujol C, CHandra J, Hajjeh RA, Warnock D, Soll DR, Ghannoum MA (2004) Candida parapsilosis characterization in a outbreak setting. Emerg Infect Dis 10:1074–1081
Kvasničková E, Maťátková O, Čejková A, Masák J (2015) Evaluation of baicalein, chitosan and usnic acid effect on Candida parapsilosis and Candida krusei biofilm using a Cellavista device. J Microbiol Methods 118:106–112
Laverty G, McCloskey AP, Gorman SP, Gilmore BF (2015) Anti-biofilm activity of ultrashort cinnamic acid peptide derivatives against medical device-related pathogens. J Pept Sci 21:770–778
Lee JH, Cho HS, Joo SW, Regm SC, Kim JA, Ryu CM, Ryu SY, Cho MH, Lee J (2013) Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157: H7. Biofouling 29:1189–1203
Lee JH, Kim YG, Ryu SY, Cho MH, Lee J (2014a) Resveratrol oligomers inhibit biofilm formation of Escherichia coli O157:H7 and Pseudomonas aeruginosa. J Nat Prod 77:168–172
Lee K, Lee JH, Ryu SY, Cho MH, Lee J (2014b) Stilbenes reduce Staphylococcus aureus hemolysis, biofilm formation, and virulence. Foodborne Pathog Dis 11:710–717
Leid JG, Kerr M, Selgado C, Johnson C, Moreno G, Smith A, Shirtliff ME, O'Toole GA, Cope EK (2009) Flagellum-mediated biofilm defense mechanisms of Pseudomonas aeruginosa against host-derived lactoferrin. Infect Immun 77:4559–4566
Li DD, Zhao LX, Mylonakis E, Hu GH, Zou Y, Huang TK, Yan L, Wang Y, Jiang YY (2014) In vitro and in vivo activities of pterostilbene against Candida albicans biofilms. Antimicrob Agents Chemother 58:2344–2355
Luo J, Kong JL, Dong BY, Huang H, Wang K, Wu LH, Hou CC, Liang Y, Li B, Chen YQ (2016) Baicalein attenuates the quorum sensing-controlled virulence factors of Pseudomonas aeruginosa and relieves the inflammatory response in P. aeruginosa-infected macrophages by downregulating the MAPK and NFkappaB signal-transduction pathways. Drug Des Devel Ther 10:183–203
Mah T-FC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Marinas IC, Oprea E, Chifiriuc MC, Badea IA, Buleandra M, Lazara V (2015) Chemical composition and antipathogenic activity of Artemisia annua essential oil from romania. Chem Biodivers 12:1554–1564
Martin NH, Murphy SC, Ralyea RD, Wiedmann M, Boor KJ (2011) When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. J Dairy Sci 94:3176–3183
Melo AS, Bizerra FC, Freymuller E, Arthington-Skaggs BA, Colombo AL (2011) Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med Mycol 49:253–262
Nguyen TH, Park MD, Otto M (2017) Host response to Staphylococcus Epidermidis colonization and infections. Front Cell Infect Microbiol 7: online
Nimmy A, Goel AK, Sivakumar KC, Kumar RA, Sabu T (2014) Resveratrol—a potential inhibitor of biofilm formation in Vibrio cholerae. Phytomed 21:286–289
Nohynek LJ, Alakomi HL, Kahkonen MP, Heinonen M, Helander IM, Oksman-Caldentey KM, Puupponen-Pimia RH (2006) Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer 54:18–32
Nostro A, Guerrini A, Marino A, Tacchini M, Di Giulio M, Grandini A, Akin M, Cellini L, Bisignano G, Saraçoğlu HT (2016) In vitro activity of plant extracts against biofilm-producing food-related bacteria. Int J Food Microbiol 238:33–39
Okamoto-Shibayama K (2010) Resveratrol impaired the morphological transition of Candida albicans under various hyphae-inducing conditions. J Microbiol Biotechnol 20:942–945
O'Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Ann Rev Microbiol 54:49–79
Page MG, Heim J (2009) Prospects for the next anti-pseudomonas drug. Curr Opin Pharmacol 9:558–565
Pagedar A, Singh J (2015) Evaluation of antibiofilm effect of benzalkonium chloride, iodophore and sodium hypochlorite against biofilm of Pseudomonas aeruginosa of dairy origin. J Food Sci Technol 52:5317–5322
Palombo EA (2011) Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Alternat Med 1–15
Paulo L, Ferreira S, Gallardo E, Queiroz JA, Domingues F (2010) Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J Microbiol Biotechnol 26:1533–1538
Pekmezovic M, Aleksic I, Barac A, Arsic-Arsenijevic V, Vasiljevic B, Nikodinovic-Runic J, Senerovic L (2016) Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected citrus species. Pathog Dis 74:ftw102
Pompilio A, Crocetta V, Pomponio S, Fiscarelli E, Di Bonaventura G (2015) In vitro activity of colistin against biofilm by Pseudomonas aeruginosa is significantly improved under “cystic fibrosis-like” physicochemical conditions. Diagn Microbiol Infect Dis 82:318–325
Puligundla P, Mok C (2017) Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J Appl Microbiol 122:1134–1148
Rahman MRT, Lou Z, Yu F, Wang P, Wang H (2017) Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J Biol Sci 24:324–330
Rajasekharan SK, Ramesh S, Bakkiyaraj D (2014) Synergy of flavonoids with HDAC inhibitor: new approach to target Candida tropicalis biofilms. J Chemother 8:1–8
Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL (2001) Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45:2475–2479
Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL (2005) Candida biofilms: an update. Eukaryot Cell 4:633–638
Ribic U, Klancnik A, Jersek B (2017) Characterization of Staphylococcus epidermidis strains isolated from industrial cleanrooms under regular routine disinfection. J Appl Microbiol 122:1186–1196
Ruzica T, Raspor P (2017) Influence of growth conditions on adhesion of yeast Candida spp. and Pichia spp. to stainless steel surfaces. Food Microbiol 65:179–184
Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options—review. J Med Microbiol 62:10–24
Selma MV, Larrosa M, Beltran D, Lucas R, Morales JC, Tomas-Barberan F, Espin JC (2012) Resveratrol and some glucosyl, glucosylacyl, and glucuronide derivatives reduce Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes Scott a adhesion to colonic epithelial cell lines. J Agric Food Chem 60:7367–7374
Serpa R, Franca EJ, Furlaneto-Maia L, Andrade CG, Diniz A, Furlaneto MC (2012) In vitro antifungal activity of the flavonoid baicalein against Candida species. J Med Microbiol 61:1704–1708
Sharma M, Manoharlal R, Negi AS, Prasad R (2010) Synergistic anticandidal activityof pure polyphenol curcuminI in combinationwith azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res 10:570–578
Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S (2013) Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009-2010. Infect Cont Hosp Ep 34:1–14
Singh B, Vuddanda PR, Vijayakumar MR, Kumar V, Saxena PS, Singh S (2014) Cefuroxime axetil loaded solid lipid nanoparticles for enhanced activity against S. aureus biofilm. Colloids Surf B 121:92–98
Szczuka E, Jabłonska L, Kaznowski A (2017) Effect of subinhibitory concentrations of tigecycline and ciprofloxacin on the expression of biofilm-associated genes and biofilm structure of Staphylococcus epidermidis. Microbiology-SGM 163:712–718
Tepe B, Daferera D, Sokmen A, Sokmen M, Polissio M (2005) Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90:333–340
Tote K, Horemans T, Vanden Berghe D, Mae L, Cos P (2010) Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 76:3135–3142
Tsai HY, Ho CT, Chen YK (2017) Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J Food Drug Anal 25:134–147
Van Houdt R, Michiels CW (2010) Biofilm formation and the food industry, a focus on the bacterial outer surface—review. J Appl Microbiol 109:1117–1131
Van Tassell JA, Martin NH, Murphy SC, Wiedmann M, Boor KJ, Ivy RA (2012) Evaluation of various selective media for the detection of Pseudomonas species in pasteurized milk. J Dairy Sci 95:1568–1574
Wahman S, Emara M, Shawky RM, El-Domany RA, Aboulwafa MM (2015) Inhibition of quorum sensing-mediated biofilm formation in Pseudomonas aeruginosa by a locally isolated Bacillus cereus. J Basic Microbiol 55:1406–1416
Wang K, Yan J, Dan W, Xie J, Yan B, Yan W, Sun M, Zhang B, Ma M, Zhao Y, Jia F, Zhu R, Chen W, Wang R (2014) Dual antifungal properties of cationic antimicrobial peptides polybia-MPI: membrane integrity disruption and inhibition of biofilm formation. Peptides 56:22–29
Weber K, Schulz B, Ruhnke M (2011) Resveratrol and its antifungal activity against Candida species. Mycoses 54:30–33
Wu J, Wen H (2009) Antifungal susceptibility analysis of berberine, baicalin, eugenol and curcumin on Candida albicans. J Med Coll PLA 24:142–147
Yun BY, Zhou L, Xie KP, Wang YJ, Xie MJ (2012) Antibacterial activity and mechanism of baicalein. Acta Pharm Sin 47:1587–1592
Zeng Z, Qian L, Cao L, Tan H, Huang Y, Xue X, Shen Y, Zhou S (2008) Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl Microbiol Biotechnol 79:119–126
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Zhou Y, Wang G, Li Y, Liu Y, Song Y, Zheng W, Zhang N, Hu X, Yan S, Jia J (2012) In vitro interactions between aspirin and amphotericinB against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother 56:3250–3260
Zida A, Bamba S, Yacouba A, Ouedraogo-Traore R, Guiguemdé RT (2017) Anti-Candida albicans natural products, sources of new antifungal drugs: a review. J Mycol Med 27:1–19
Acknowledgements
This work was supported by the Czech Science Foundation (GA CR) [grant number 17-15936S] and the “Operational Programme Prague – Competitiveness” (CZ.2.16/3.1.00/24503) and the “National Program of Sustainability I”—NPU I (LO1601 - No.: MSMT-43760/2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolouchová, I., Maťátková, O., Paldrychová, M. et al. Resveratrol, pterostilbene, and baicalein: plant-derived anti-biofilm agents. Folia Microbiol 63, 261–272 (2018). https://doi.org/10.1007/s12223-017-0549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0549-0