Abstract
Nasopharyngeal colonization by Streptococcus pneumoniae is an important initial step for the subsequent development of pneumococcal infections. Pneumococci have many virulence factors that play a role in colonization. Pneumolysin (PLY), a pivotal pneumococcal virulence factor for invasive disease, causes severe tissue damage and inflammation with disruption of epithelial tight junctions. In this study, we evaluated the role of PLY in nasal colonization of S. pneumoniae using a mouse colonization model. A reduction of numbers of PLY-deficient pneumococci recovered from nasal tissue, as well as nasal wash, was observed at days 1 and 2 post-intranasal challenges, but not later. The findings strongly support an important role for PLY in the initial establishment nasal colonization. PLY-dependent invasion of local nasal mucosa may be required to establish nasal colonization with S. pneumoniae. The data help provide a rationale to explain why an organism that exists as an asymptomatic colonizer has evolved virulence factors that enable it to occasionally invade and kill its hosts. Thus, the same pneumococcal virulence factor, PLY that can contribute to killing the host, may also play a role early in the establishment of nasopharynx carriage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae is an important human pathogen causing a broad spectrum of diseases including acute otitis media, rhinosinusitis, pneumonia, meningitis, and sepsis. Acquisition of asymptomatic colonization of the human nasopharynx with S. pneumoniae occurs frequently and is necessary for subsequent disease (Faden et al. 1997; Gray et al. 1980). The nasopharynx is also an important reservoir for the horizontal spread of S. pneumoniae (Gray et al. 1980; Gwaltney et al. 1975). Although effective antibiotics and vaccines have been developed, the increasing development of antimicrobial resistance and spread of replacement non-vaccine serotypes emphasizes urgent demands for a better understanding of the pathogenesis of this pathogen (Weinberger et al. 2011; Mehr and Wood 2012).
It is well known that pneumococci have multiple virulence factors that provide pneumococci some protection against host defense systems (Jedrzejas 2001; Dave et al. 2004; Magee and Yother 2001; Ren et al. 2004). Pneumococcal virulence factors allow pneumococci to resist host defenses and invade from mucosal surfaces to the circulation (Rubins et al. 1996). However, despite the fact that adherence is an important initial step for establishment of nasopharyngeal colonization in humans (in mouse models defined as nasal colonization), contributions of the known virulence factors to nasopharyngeal colonization are still largely unknown. Why do pneumococci contain disease-permitting virulence factors? One probable interpretation is that nasal colonization involves not only simple adherence or planktonic existence on mucosal surfaces but also requires biofilm formation and/or minimal invasion (Gilley and Orihuela 2014; Marks et al. 2014; Perez et al. 2014; Shak et al. 2013; Briles et al. 2005).
Pneumolysin (PLY), a thiol-activated or cholesterol-dependent cytotoxin, is an important pneumococcal virulence factor and is implicated in multiple steps of pneumococcal pathogenesis (Boulnois et al. 1991; Paton 1996). The principal activity of PLY is its capacity to form pores in cholesterol-rich membranes, which causes severe tissue damage and inflammation including impaired cilia function, altered alveolar permeability, and disruption of epithelial tight junctions (Paton et al. 1984; Steinfort et al. 1989; Paton and Ferrante 1983; Nandoskar et al. 1986; Rubins et al. 1992, 1993). Inoculation of PLY into the lungs reproduces histological lung damage in a mouse pneumonia model (Feldman et al. 1991). Isogenic PLY-negative mutant strains of pneumococci were less virulent than their wild-type counterpart strains after intraperitoneal or intranasal injection in mice (Houldsworth et al. 1994; Berry et al. 1989).
The direct roles of PLY in establishing nasopharyngeal pneumococcal colonization were previously studied by Paton et al. and others (Paton and Ferrante 1983; Steinfort et al. 1989; Nandoskar et al. 1986). In this study, we confirm and extend knowledge on the relative contribution of PLY to nasopharyngeal colonization.
Materials and methods
Bacterial strains and growth conditions
The pneumococcal strains used in this study were TIGR4 (serotype 4), EF3030 (serotype 19 F), BG7322 (serotype 6A), and their isogenic ply deletion mutants. All pneumococcal strains were grown in Todd-Hewitt broth with 0.5 % yeast extract (THY) or on blood agar plates at 37 °C with 5 % CO2. To maintain the inactivating insertion, ply deletion mutants were grown in THY broth containing 0.3 μg/mL erythromycin.
The strains were confirmed as S. pneumoniae by optochin sensitivity and production of a serotype-specific capsule evaluated by Quellung reaction using diagnostic antiserum obtained from Statens Serumin Institut (Copenhagen, Denmark), as described previously (Berry et al. 1989).
Bacteria for intranasal challenge were grown at 37 °C to mid-exponential phase (absorbance at 600 nm (A600) of 0.5) in THY broth containing the appropriate antibiotics and were stored at −80 °C until use. Inocula were then resuspended in sterile phosphate-buffered saline (PBS) at appropriate infection dosages.
Construction of pneumolysin-negative mutants
A PLY-negative mutant of S. pneumoniae D39 strain, PLY-A, originally constructed by insertion-duplication mutagenesis as described (Berry et al. 1989; Berry & Paton 2000). This mutant was characterized by Southern blot hybridization and by its lack of pneumolysin activity in a functional lytic assay (Berry et al. 1989; Yother et al. 1992). In the present study, genomic DNA of PLY-A was used to transform three pneumococcal strains, TIGR4, EF3030, and BG7322, to construct PLY-negative mutants using procedures described by Yother et al. (Yother et al. 1986). Briefly, strains to be transformed were grown in THY to an absorbance at 600 nm of 0.5 and diluted 1:50 in competence medium (CTM) (THY with 0.2 % bovine serum albumin, 0.2 % glucose, and 0.02 % CaCl2) with 500 ng/mL competence-stimulating peptides CSP1 and CSP2 to induce competency. Bacteria were incubated at 37 °C for 2 h, followed by plating on blood agar plates containing 0.3 μg/mL erythromycin (Havarstein et al. 1995). After repeating the procedure five times, the PLY-negative mutants were finally obtained. The PLY-negative mutants were MH7 (a PLY-negative BG7322 mutant), MH8 (a PLY-negative TIGR4 mutant), and MH12 (a PLY-negative EF030 mutant). These new mutants were each characterized by polymerase chain reaction (PCR) and by an absence of lytic activity in the assay for pneumolysin induced hemolysis, as described previously (Berry et al. 1989; Yother et al. 1992). Mutants showed similar rates of growth to the original wild type mutants.
Mice
A six-week-old female CBA/NSlc (CBA/N) mice were obtained from Japan SLC, Inc. (Shizuoka, Japan). CBA/N mice lack the ability to produce efficient antibodies to polysaccharides and lack serum antibodies to the phosphocholine determinant of pneumococcal teichoic acids because of abnormal B cell maturation (Briles et al. 1981). Mice were maintained in a specific pathogen-free environment fully accredited by the Japanese Association for the Accreditation of Laboratory Animal Care. The experimental protocol was approved by the DNA Recombination Experiment Committee and the Animal Care and Use Committee at Wakayama Medical University (No. 551 and 728). All mouse experiments were performed according to the guidelines of the Laboratory Animal Center for Biomedical Research, Wakayama Medical University.
Nasal colonization model
Mice were inoculated intranasally, as indicated, with 1 × 104 to 1 × 106 colony-forming units (CFUs) per mouse of viable pneumococcal stains or PLY-negative mutants in 10 μL of sterile PBS without anesthesia. One to ten days later, the mice were euthanized with CO2 and nasal washes and washed nasal tissue were obtained. Briefly, the nasal cavity of each mouse was washed with 1 mL sterile PBS after tracheotomy. The nasal tissue including nasal conchae, olfactory epithelium, and sinus mucosa was collected after removing the brain in the cranium. The nasal tissues were homogenized individually in 1 mL PBS.
All nasal washes and homogenates of nasal tissue were serially diluted, and 25 μL aliquots were spread on blood agar plates, blood agar plates supplemented with 4 μg/mL gentamicin, and blood agar plates supplemented with 4 μg/mL gentamicin and 5 μg/mL optochin for wild type strains. Gentamicin prevented growth of most contaminants in the nasal wash (Converse and Dillon 1977). Blood agar plates supplemented with 0.3 μg/mL erythromycin, and blood agar plates supplemented with 0.3 μg/mL erythromycin and 5 μg/mL optochin were used when PLY-negative mutants were evaluated.
Using these two types of agar plates, we determined that the organisms observed on the gentamicin plates were pneumococci. Maintenance of the mutants on erythromycin plates prevented the loss of their mutations and the erythromycin resistance gene.
Statistics
All experiments were conducted two or more times under the same conditions. For quantitative data, statistical comparisons of numbers of CFUs of pneumococci recovered in nasal washes and nasal tissues between PLY wild type and PLY-negative mutant CFUs were performed using the Mann–Whitney U test. For quantitative values, medians and statistical calculations were analyzed for only samples with detectable bacteria. We performed the statistical analysis using Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). A p value of <0.05 was considered statistically significant.
Results
Numbers of CFUs recovered from nasal wash
Nonanesthetized CBA/N mice were inoculated intranasally with 5 × 105 to 1 × 106 CFUs of either S. pneumonia wild type or their isogenic mutants lacking PLY. All mice were alive at the time of the assay except a small number of mice inoculated with TIGR4 and BG7322 or their isogenic PLY-negative mutants MH8/MH7, which were dead or bacteremic. These rare dead or bacteremic mice were excluded from analysis of the nasal colonization. All mice challenged with EF3030 or their isogenic PLY-negative mutants MH12/MH7 were alive and non-bacteremic at the time of the assay.
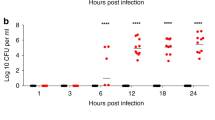
The numbers of CFUs recovered from nasal washes are shown in Fig. 1. The numbers of CFUs of EF3030 and its isogenic PLY-negative mutant MH12 in nasal washes were evaluated on days 2, 6, and 10 after intranasal inoculation (Fig. 1a). The number of CFUs of EF3030 recovered from nasal washes on day 1 was significantly higher than those of isogenic PLY-negative mutant MH12 (median 4.58 vs. 3.59, p = 0.001). Likewise, nasal colonization with TIGR4 or its isogenic PLY-negative mutant MH8 was evaluated on days 1, 5, and 7 (Fig. 1b). Nasal colonization with BG7322 or its isogenic PLY-negative mutant MH7 was evaluated on days 1, 7, and 10 (Fig. 1c). The numbers of CFUs of TIGR4 and BG7322 recovered from nasal washes on day 1 were significantly higher than those of PLY-negative mutants MH8 or MH7 (median 5.09 vs. 3.77, p = 0.030 for TIGR4/MH8; median 3.37 vs. 2.20, p = 0.001 for BG7322/MH7). There was no difference in colonization between S. pneumoniae wild type and its isogenic PLY-negative mutant after a day (day 1) from intranasal inoculation.
Numbers of pneumococcal CFUs recovered from nasal washes. a Mice challenged with EF3030 or its PLY-negative mutant MH12 (serotype 19 F) at 1 × 106 CFUs per mouse in 10 μL PBS. b Mice challenged with TIGR4 or its PLY-negative mutant MH8 (serotype 4) at 5 × 105 CFUs per mouse in 10 μL PBS. c Mice challenged with BG7322 or its PLY-negative mutant MH7 (serotype 6A) at 1 × 105 CFUs per mouse in 10 μL PBS. **p < 0.01, *p < 0.05. The dotted line indicates the lower limit of detection
Numbers of CFUs recovered from nasal tissue
To better understand the role of PLY for nasal colonization, the numbers of CFUs in washed nasal tissues were evaluated separately from those in nasal washes after intranasal inoculation of pneumococci. Like the results of nasal washes, the numbers of CFUs recovered from washed nasal tissues of TIGR4 on day 2 and EF3030 on day 1 were significantly higher than those of their isogenic PLY-negative mutants MH8/MH12 (median 5.98 vs. 4.35, p = 0.011 for EF3030; median 5.41 vs. 4.94, p = 0.017 for TIGR4) (Fig. 2a, b). There was no difference in numbers of wild-type or PLY-negative mutant bacteria CFUs recovered from the nasal tissues on days 5, 6, 7, or 10 post inoculation. In contrast, when CFUs of strain BG7322 were examined, significantly higher numbers of CFUs from wild type BG7322 and the PLY-negative mutant MH7 were recovered on both days 1 and 7 (median 4.68 vs. 3.72, p = 0.022 for day 1; median 4.69 vs. 3.96, p = 0.021 for day 7) (Fig. 2c).
Numbers of pneumococcal CFUs recovered from washed nasal tissue. a Mice challenged with EF3030 or its PLY-negative mutant MH12 (serotype 19F) at 1 × 106 CFUs per mouse in 10 μL PBS. b Mice challenged with TIGR4 or its PLY-negative mutant MH8 (serotype 4) at 5 × 105 CFUs per mouse in 10 μL PBS. c Mice challenged with BG7322 or its PLY-negative mutant MH7 (serotype 6A) at 1 × 105 CFUs per mouse in 10 μL PBS. **p < 0.01, *p < 0.05. The dotted line indicates the detection limit. These data are from the same mice shown in Fig. 1
Effect of inoculation dose on nasal colonization
The effect of different inoculation doses were examined by intranasally inoculating mice with 1 × 104 to 1 × 106 CFUs of S. pneumoniae strain BG7322 and enumerating CFUs in nasal washes and washed nasal tissues on day 1 (Fig 3a and b). All mice survived the challenge. One mouse intranasally inoculated with BG7322 at 1 × 104 CFUs did not show colonization (this mouse was excluded from analysis). All other mice exhibited colonization. In this experiment, numbers of CFUs in washed nasal tissues were evaluated separately from those in nasal washes to better understand the roles of PLY for nasal colonization.
Numbers of pneumococcal CFUs recovered from nasal washes or washed nasal tissue at various inoculation doses. Mice were challenged with BG7322 (serotype 6A) or its PLY-negative mutant MH7 (serotype 6A) at 1 × 104, 1×105, or 1 × 106 CFUs per mouse in 10 μL PBS. CFU in their nasal washes (a) and nasal tissue (b) were determined after 24 h. **p < 0.01, *p < 0.05. The dotted line indicates the lower limit of detection
There was no significant difference in the numbers of CFUs recovered from nasal washes between BG7322 wild type and its isogenic PLY-negative mutant MH7 when mice were intranasally challenged with 1 × 106 CFUs (Fig. 3a). In contrast, the numbers of CFUs recovered from washed nasal tissue were significantly higher in mice inoculated with BG7322 wild type than its isogenic PLY-negative mutant MH7 at this inoculation dose (median log10 5.08 vs. 4.22, p = 0.002) (Fig. 3b). The numbers of CFUs recovered from both nasal washes and washed nasal tissue after intranasal inoculation with 1 × 105 CFUs were significantly higher in mice challenged with BG7322 wild type than in mice with its isogenic PLY-negative mutant MH7 (median 3.37 vs. 2.20, p = 0.001 for nasal wash; median 4.68 vs. 3.72, p = 0.003 for nasal tissue). The numbers of CFUs recovered from both nasal washes and washed nasal tissue after intranasal inoculation with 1 × 105 CFUs was also significantly higher in mice challenged with BG7322 wild type than in mice challenged with its isogenic PLY-negative mutant (median 3.38 vs. 2.29, p = 0.001 for nasal wash; median 4.35 vs. 3.25, p = 0.001 for nasal tissue). When mice were inoculated intranasally with BG7322 or its PLY-negative mutant at 1 × 104, there were slightly more than a log more pneumococci in the nasal wash and nasal tissue of mice inoculated with the wild type vs. mutant bacteria (p < 0.01). So, at all doses we observed more CFU with wild type than PLY-negative mutant at one or both of the tissues studied.
Discussion
Despite numerous studies focused on the role of virulence factors of S. pneumoniae against host defense systems, their roles in nasal colonization have not yet been completely elucidated. We hypothesize that the tissue associated pneumococci may be required to maintain long-lasting colonization. These pneumococci may be present as surface-attached biofilms or minimally invasive bacteria in the tissue (Marks et al. 2014; Briles et al. 2005). To invade local tissue, S. pneumoniae need to be able to break through epithelial barriers. In this study, we focused on the roles of PLY in nasal colonization.
Our previous study and others revealed that asymptomatic nasal colonization with S. pneumoniae involves two subpopulations of weakly attached and strongly tissue-associated pneumococci (Briles et al. 2005; Kim and Weiser 1998; Ogunniyi et al. 2007). The transparent phase is loosely attached to the mucosal surface, and the opaque phase is more intimately associated with washed nasal tissue (Briles et al. 2005; Kim and Weiser 1998). Although we did not evaluate phase variations in this study, we believe that it may be important in the acquisition and maintenance of nasal colonization.
The role of PLY in nasal colonization is controversial (Ogunniyi et al. 2007; Kadioglu et al. 2000, 2002; Rayner et al. 1995). It was considered that PLY has a relatively minor role in nasopharyngeal colonization compared to other pneumococcal virulence factors such as PspA and PspC (Rubins et al. 1996; Steinfort et al. 1989; Rubins et al. 1998; Brown et al. 1983; Briles et al. 2000; Jarva et al. 2002; Ren et al. 2004). However, numerous publications have shown the critical role of PLY in contributing to pneumococcal pneumonia (Rayner et al. 1995; Berry et al. 1989). Mice infected with PLY-negative mutants showed increased bacterial clearance from the lung (Ogunniyi et al. 2007). In contrast to these previous results, numbers of PLY-negative mutants were significantly lower than those of PLY wild type on day 1 after inoculation in this study. Ogunniyi et al. reported that the numbers of PLY-negative D39 serotype 2 strain recovered from the nose up to 7 days after intranasal inoculation were not significantly different from those of the PLY wild-type parent (Ogunniyi et al. 2007). Orihulea et al. also showed no role for PLY in enhanced nasal colonization (Orihuela et al. 2004). While these two reports discussed the relatively minor roles of PLY in nasal colonization, their actual results demonstrated the reduction in nasal colonization at very early time points (approximately 24 h) after intranasal inoculation. Our findings reevaluate their results and further suggest that PLY plays a role in colonization at days 1 and 2 postinfection and does so for both pneumococci in washed tissue, and in nasal washes, which are the endpoints studied by other investigators. At later time points, PLY played no role. Therefore, it appears to influence the early establishment, but not the later maintenance, of colonization.
PLY may contribute to pneumococcal colonization not only via various inhibitory effects on both adaptive and innate immune responses but also by directly damaging epithelial surfaces. Production of PLY has been reported to be associated with increased bacterial adherence to human respiratory epithelial cells in vitro. Rayner et al. reported that PLY is produced earlier and causes more severe damage to human respiratory mucosa in organ culture and is associated with increased pneumococcal adherence (Berry et al. 1989). In contrast, several studies showed that mutants deficient in PLY could still colonize the nasopharynx as well as the wild type. Weiser et al. investigated PLY-triggered inflammatory responses that contributed to the clearance of colonies (Nelson et al. 2005; Ratner et al. 2005). Rubins et al. also reported that PLY-negative serotype 14 mutants colonize the murine nose as efficiently as PLY wild-type pneumococci, both at early and late time points (Rubins et al. 1995). These discrepancies could be explained by differences in inoculation dose because our data indicated that the maximum effect of PLY on colonization was not seen at the highest dose tested.
Our current in vivo evidence strongly supports an important role for PLY in the early pathogenesis of nasopharyngeal colonization. The data help provide a rationale to explain why an organism that exists as an asymptomatic colonizer has also evolved a virulence factor that enables it to occasionally invade and kill its host. PLY may elicit sufficient host inflammatory (Witzenrath et al. 2011) responses to actually enhance colonization. It has been reported that PLY is downregulated once biofilms develop (Sanchez et al. 2011), which may explain why it took a few days for the effect of pneumolysin on carriage to go away in our studies. Other virulence factors such as PspA and capsule are also important in colonization as well as invasive disease (Briles et al. 2005). It is conceivable that carriage involves a careful balance between having sufficient virulence to establish and maintain stable colonization and yet being not so virulent as to provoke significant local inflammation. Thus, the virulence factors of S. pneumoniae that are necessary for killing humans may primarily exist to facilitate nasopharynx carriage.
References
Berry AM, Paton JC (2000) Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun 68:133–140
Berry AM, Yother J, Briles DE, Hansman D, Paton JC (1989) Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun 57:2037–2042
Boulnois GJ, Paton JC, Mitchell TJ, Andrew PW (1991) Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol 5:2611–2616
Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R (1981) Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med 153:694–705
Briles DE, Hollingshead SK, Nabors GS, Paton JC, Brooks-Walter A (2000) The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine 19(1):S87–S95
Briles DE, Novak L, Hotomi M, van Ginkel FW, King J (2005) Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect Immun 73:6945–6951
Brown EJ, Hosea SW, Frank MM (1983) The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev Infect Dis 5(4):S797–S805
Converse GM III, Dillon HC Jr (1977) Epidemiological studies of Streptococcus pneumoniae in infants: methods of isolating pneumococci. J Clin Microbiol 5:293–296
Dave S, Carmicle S, Hammerschmidt S, Pangburn MK, McDaniel LS (2004) Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J Immunol 173:471–477
Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y (1997) Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis 175:1440–1445
Feldman C, Munro NC, Jeffery PK, Mitchell TJ, Andrew PW, Boulnois GJ, Guerreiro D, Rohde JAL, Todd HC, Cole PJ, Wilson R (1991) Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am J Respir Cell Mol Biol 5:416–423
Gilley RP, Orihuela CJ (2014) Pneumococci in biofilms are non-invasive: implications on nasopharyngeal colonization. Front Cell Infect Microbiol 4:163
Gray BM, Converse GM III, Dillon HC Jr (1980) Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142:923–933
Gwaltney JM Jr, Sande MA, Austrian R, Hendley JO (1975) Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J Infect Dis 132:62–68
Havarstein LS, Coomaraswamy G, Morrison DA (1995) An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci U S A 92:11140–11144
Houldsworth S, Andrew PW, Mitchell TJ (1994) Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1/3 by human mononuclear phagocytes. Infect Immun 62:1501–1503
Jarva H, Janulczyk R, Hellwage J, Zipfel PF, Bjorck L, Meri S (2002) Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J Immunol 168:1886–1894
Jedrzejas MJ (2001) Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev 65:187–207
Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, Andrew PW (2000) Host cellular immune response to pneumococcal lung infection in mice. Infect Immun 68:492–501
Kadioglu A, Taylor S, Iannelli F, Pozzi G, Mitchell TJ, Andrew PW (2002) Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect Immun 70:2886–2890
Kim JO, Weiser JN (1998) Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis 177:368–377
Magee AD, Yother J (2001) Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun 69:3755–3761
Marks LR, Mashburn-Warren L, Federle MJ, Hakansson AP (2014) Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. J Infect Dis 210:25–34
Mehr S, Wood N (2012) Streptococcus pneumoniae—a review of carriage, infection, serotype replacement and vaccination. Paediatr Respir Rev 13:258–264
Nandoskar M, Ferrante A, Bates EJ, Hurst N, Paton JC (1986) Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology 59:515–520
Nelson AL, Barasch JM, Bunte RM, Weiser JN (2005) Bacterial colonization of nasal mucosa induces local expression of siderocalin, aniron-sequestering component of innate immunity. Cell Microbiol 7:1404–1417
Ogunniyi AD, LeMessurier KS, Graham RM, Watt JM, Briles DE, Stroeher UH, Paton JC (2007) Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun 75:1843–1851
Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI (2004) Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis 190:1661–1669
Paton JC (1996) The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol 4:103–106
Paton JC, Ferrante A (1983) Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun 41:1212–1216
Paton JC, Rowan-Kelly B, Ferrante A (1984) Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun 43:1085–1087
Perez AC, Pang B, King LB, Tan L, Murrah KA, Reimche JL, Wren JT, Richardson SH, Ghandi U, Swords WE (2014) Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathog Dis 70:280–288
Ratner AJ, Lysenko ES, Paul MN, Weiser JN (2005) Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proc Natl Acad Sci U S A 102:3429–3434
Rayner CF, Jackson AD, Rutman A, Dewar A, Mitchell TJ, Andrew PW, Cole PJ, Wilson R (1995) Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun 63:442–447
Ren B, Szalai AJ, Hollingshead SK, Briles DE (2004) Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun 72:114–122
Rubins JB, Duane PG, Charboneau D, Janoff EN (1992) Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun 60:1740–1746
Rubins JB, Duane PG, Clawson D, Charboneau D, Young J, Niewoehner DE (1993) Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun 61:1352–1358
Rubins JB, Charboneau D, Paton JC, Mitchell TJ, Andrew PW, Janoff EN (1995) Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Invest 95:142–150
Rubins JB, Charboneau D, Fasching C, Berry AM, Paton JC, Alexander JE, Andrew PW, Mitchell TJ, Janoff EN (1996) Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med 153:1339–1346
Rubins JB, Paddock AH, Charboneau D, Berry AM, Paton JC, Janoff EN (1998) Pneumolysin in pneumococcal adherence and colonization. Microb Pathog 25:337–342
Sanchez CJ, Kumar N, Lizcano A, Shivshankar P, Dunning Hotopp JC, Jorgensen JH, Tettelin H, Orihuela CJ (2011) Streptococcus pneumoniae in biofilms are unable to cause invasive disease due to altered virulence determinant production. PLoS One 6, e28738
Shak JR, Ludewick HP, Howery KE, Sakai F, Harvey RM, Paton JC, Klugman KP, Vidal JE (2013) Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. MBio 10:e00655–13
Steinfort C, Wilson R, Mitchell T, Feldman C, Rutman A, Todd H, Sykes D, Walker J, Saunders K, Andrew PW, Boulnois GJ, Cole PJ (1989) Effects of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun 57:2006–2013
Weinberger DM, Malley R, Lipsitch M (2011) Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973
Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B (2011) The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J Immunol 187:434–440
Yother J, McDaniel LS, Briles DE (1986) Transformation of encapsulated Streptococcus pneumoniae. J Bacteriol 168:1463–1465
Yother J, Handsome GL, Briles DE (1992) Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol 174:610–618
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
The Briles lab is supported by NIH grant R01AI118805.
Additional information
Muneki Hotomi and Jun Yuasa contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hotomi, M., Yuasa, J., Briles, D.E. et al. Pneumolysin plays a key role at the initial step of establishing pneumococcal nasal colonization. Folia Microbiol 61, 375–383 (2016). https://doi.org/10.1007/s12223-016-0445-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-016-0445-z