Abstract
The basidiomycetous yeast Cryptococcus humicola was shown to be tolerant to manganese, cobalt, nickel, zinc, lanthanum, and cadmium cations at a concentration of 2.5 mmol/L, which is toxic for many yeasts. The basidiomycetous yeast Cryptococcus terreus was sensitive to all these ions and did not grow at the above concentration. In the presence of heavy metal cations, С. humicola, as opposed to C. terreus, was characterized by the higher content of acid-soluble inorganic polyphosphates. In vivo 4′,6′-diamino-2-phenylindole dihydrochloride staining revealed polyphosphate accumulation in the cell wall and cytoplasmic inclusions of С. humicola in the presence of heavy metals. In C. terreus, polyphosphates in the presence of heavy metals accumulate mainly in vacuoles, which results in morphological changes in these organelles and, probably, disturbance of their function. The role of polyphosphate accumulation and cellular localization as factors of heavy metal tolerance of Cryptococcus humicola is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are widespread environmental pollutants hazardous to human health. Exposure to these toxic metal ions may result in growth cessation, apoptosis, and cell death of many microorganisms. Several mechanisms of heavy metal toxicity in microorganisms have been proposed: they replace the essential metal in metalloproteins, allosterically inhibit some enzymes by binding to their catalytic sites, indirectly cause oxidative stress, and exhibit membrane-damaging and chaotropic effects (Macomber and Hausinger 2011; Vagabov et al. 2008; Cray et al. 2013a). Cd2+ caused endoplasmic reticulum stress in Saccharomyces cerevisiae by inducing the unfolded protein response, and Cd2+ toxicity is believed to be the direct consequence of accumulation of this cation in the endoplasmic reticulum (Gardarin et al. 2010). This effect may be chaotropicity mediated. It should be noted that microbial cells are able to tolerate chaotropic stressors better at low temperatures (Chin et al. 2010).
The mechanisms of heavy metal tolerance in microorganisms are extensively studied (Spain 2003; Culotta et al. 2005; Thorsen et al. 2009; Wysocki and Tamás 2010). Multiple genes and signaling pathways are responsible for yeast viability under heavy metal excess. The two sets of metal-responsive genes were revealed under the exposure of the yeast S. cerevisiae to copper, silver, zinc, cadmium, mercury, and chromium (Jin et al. 2008). The mechanisms of heavy metal tolerance in yeasts include environmental sensing, sulfur and glutathione biosynthesis, vacuolar and endosomal transport, and sorting (Thorsen et al. 2009). Two hundred and thirty-seven genes involved in Сd2+ tolerance in Schizosaccharomyces pombe were revealed (Kennedy et al. 2008). These genes represent a number of pathways including sulfate assimilation, phytochelatin synthesis and transport, ubiquinone biosynthesis, stress signaling, cell wall biosynthesis and cell morphology, gene expression and chromatin remodeling, vacuole function, and intracellular transport of macromolecules (Kennedy et al. 2008). The concept of Mn2+ homeostasis in S. cerevisiae is based on the involvement of a considerable number of genes in this process (Culotta et al. 2005). Candida tropicalis isolated from wastewater was found to be resistant to the high concentrations of Zn2+, Ni2+, Hg2+, and Pb2+; the synthesis of glutathione increased, and its involvement in metal tolerance was suggested (Rehman and Anjum 2011). It should be noted that some stress responses and adaptations in yeast do not involve gene-mediated membrane processes (Permyakov et al. 2012; Mollinedo 2012).
The heavy metal tolerance of basidiomycetous yeasts has been observed in some species (Vadkertiová and Sláviková 2006; Vreulink et al. 2010; Singh et al. 2013) but little studied as yet. The study of heavy metal tolerance is of interest for understanding the changes in yeast biodiversity under conditions of environmental pollution.
Inorganic polyphosphates (PolyP) are an important biopolymer participating in many regulatory processes in microbial cells (Kulaev et al. 2004; Rao et al. 2009; Hirota et al. 2010; Achbergerová and Nahálka 2011). The interrelationship between phosphorus metabolism, PolyP, and heavy metal resistance was observed in Escherichia coli (Keasling et al. 2000), in the archaea Sulfolobus (Remonsellez et al. 2006), in Trichoderma harzianum (De Lima Freitas et al. 2011), and in insects (Gomes et al. 2012). Disruption of the pho84 gene encoding the phosphate transporter of the S. cerevisiae plasma membrane, PHO84, which is responsible for Mn2+ uptake via manganese-phosphate complexes, results in a manganese-resistant phenotype (Jensen et al. 2003). The pho80 mutants of S. cerevisiae defective in phosphate uptake, storage, and metabolism exhibit a wide range of defects in metal homeostasis (Rosenfeld et al. 2010). The ability of S. cerevisiae to adapt to toxic Mn2+ concentrations after an unusually long lag phase has been demonstrated. The adaptation correlated with the triggering of PolyP metabolism: the drastic increase in the rate and chain length of acid-soluble PolyP (Andreeva et al. 2013).
We have chosen the following two basidiomycetous yeast species as objects of our research: Cryptococcus humicola isolated from plant surface and Cryptococcus terreus isolated from soil (Golubev and Shabalin 1994). The cells of these two species substantially differed in the ability to take up phosphate from the medium under growth limitation: C. humicola, in contrast to C. terreus, proved to be an effective phosphate-accumulating organism (Breus et al. 2012). The aim of this study was to compare the two basidiomycetous yeasts, C. humicola and C. terreus, with respect to heavy metal tolerance and to determine whether these two species have any peculiarities of their PolyP pools.
Materials and methods
Strains and growth conditions
The yeasts were obtained from the All-Russian Collection of Microorganisms (Russian Academy of Sciences) and maintained on malt slants. C. terreus VKM Y-2253 and C. humicola 9-6 were grown on a shaker (140 rpm) at 29 °C in 200 mL of the medium containing (g/L) glucose, 10; yeast extract, 4; and peptone, 5.
The culture growth was estimated by absorbance measured in a 1-cm cuvette at 600 nm with a SF-26 spectrophotometer (Russia) using appropriate dilutions. After 72-h cultivation (the stationary phase), the cells were placed in fresh media. The high initial absorbance (A 600 ~1.4) was used to obtain sufficient biomass even in case of growth suppression.
The concentrations of heavy metal cations and phosphates in the media are indicated in the tables and figure legends. The salts (MnSO4⋅4Н2O, CoSO4⋅7H2O, NiSO4⋅7H2O, ZnSO4⋅7H2O, Ca(NO3)2⋅4H2O, La(NO3)3⋅6H2O, Cd(CH3COO)2⋅2H2O) were of analytical grade. The glucose peptone medium contained 1.6 mmol/L phosphate (Pi) derived from yeast extract and peptone. KH2PO4 was added to the media with enhanced Pi concentration (11.5 mmol/L).
Polyphosphate and manganese assay
For PolyP extraction and assay, the cells were harvested by centrifugation at 5,000 g for 10 min and washed twice with 0.1 mol/L citrate buffer, pH 4.5. PolyP fractions were extracted and assayed as described earlier (Vagabov et al. 2000). The PolyP1 fraction obtained by extraction with 5 % HClO4 (Vagabov et al. 2000) is indicated in this work as acid-soluble PolyP. Other fractions, PolyP2, PolyP3, PolyP4, and PolyP5, were obtained and assayed as described earlier (Vagabov et al. 2000). The total content of these fractions is presented in the tables and figures as acid-insoluble PolyP. Manganese concentration in the samples was determined by atomic emission spectroscopy after the exposure at 180 °C in 32 % HClO4 for 24 h (Lichko and Okorokov 1976).
Fluorescence microscopy
Fluorescence microscopy with the fluorochrome 4′,6′-diamino-2-phenylindole dihydrochloride (DAPI) is one of the methods for determining PolyP localization in living cells (Serafim et al. 2002; Pavlov et al. 2010; Puchkov 2010; Martin and Van Mooy 2013). For DAPI staining, 10 μg/mL of DAPI (Sigma, USA) was added to cell cultures followed by incubation at room temperature for 30 min. The samples were analyzed under fluorescent and phase contrast microscopes (AXIO Imager A1, ZEISS, Germany), filter set 49 (ZEISS), with the excitation maximum at 359 nm and the emission maximum at 460 nm.
Results
The effects of heavy metals on yeast growth
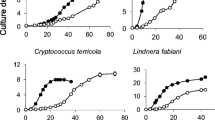
C. humicola and C. terreus differ in heavy metal tolerance (Fig. 1). The growth of C. terreus was inhibited by all of the tested cations except for calcium at a concentration of 2.5 mmol/L. Ca2+ had no effect on the growth of both cultures. The yeast C. humicola proved to be tolerant to the excess of heavy metal cations. The Co2+ and Сd2+ ions inhibited the growth of this yeast but partially.
The effects of Mn2+ on the growth of both yeasts were investigated more thoroughly (Fig. 2). The cells of C. humicola grew even at 10 mmol/L of Mn2+, whereas the cells of C. terreus stopped to grow at 1.25 mmol/L of the cation. The increase in phosphate concentration from 1.6 to 11.5 mmol/L had no effect on the growth of both cultures in the presence and absence of Mn2+ (data not shown). The C. terreus was cultivated for 7 days in the media containing manganese (2.5 mmol/L) and phosphate (1.6 or 11.5 mmol/L), but no growth was observed.
Both yeast species were jointly cultivated in the media with and without Mn2+. They are well differentiated by light microscopy: C. humicola cells are rod-shaped and C. terreus cells are round. The number of cells of each type was counted under a light microscope. The media were inoculated with a suspension containing the cells of C. humicola (0.5 %) and C. terreus (99.5 %). After 48-h cultivation without manganese, the culture contained 40 % of C. terreus cells. C. humicola was predominant (97.4 %) in the medium with 2.5 mmol/L of Mn2+. This result suggests the possibility of replacement of sensitive species by resistant species under heavy metal pollution of the environment.
Manganese in the medium and biomasses
We determined the content of manganese after 48-h cultivation in the culture liquid, in the extracts from biomass samples with 0.1 mol/L citrate buffer (pH 3.0), and in the rest of the biomass (Table 1). Under the exposure of С. terreus in the presence of 2.5 mmol/L Mn2+, the decrease of manganese in the medium was insignificant (Table 1). It was not surprising as no culture growth was observed. However, the biomass contained 12 μmol manganese per g wet biomass. Under the cultivation of С. humicola, the content of manganese in the culture medium decreased depending on Pi concentration. After washing with citrate buffer, the content of manganese in the biomass was 33 μmol per g wet biomass independent of Pi concentration. The difference in manganese content in the culture medium at 1.6 and 11.5 mmol/L Pi can be explained by manganese absorption on the cell surface at high Pi concentration. This manganese can be easily extracted by citrate buffer (Table 1). Thus, the tolerance of С. humicola to excess manganese cannot be explained by the lower uptake of the cation.
The effects of heavy metals on PolyP content in C. humicola and C. terreus
Figure 3 shows the content of acid-soluble and acid-insoluble PolyP in the cells of C. humicola and C. terreus in the presence of heavy metal cations in the medium with 1.6 mmol/L phosphate. The content of PolyP without metals was similar in the cells of both species.
The content of PolyP in yeast cells at 48-h cultivation in the media containing 2.5 mmol/L metal cations and 1.6 mmol/L Pi: 1 acid-soluble PolyP, Cryptococcus humicola; 2 acid-insoluble PolyP, Cryptococcus humicola; 3 acid-soluble PolyP, Cryptococcus terreus; 4 acid-insoluble PolyP, Cryptococcus terreus
The effects of metal cations on PolyP content in both species were different. The presence of Сa2+, Cd2+, and La3+ had a little influence on PolyP content in the cells of C. terreus. The content of PolyP in C. terreus cells increased 3.5-fold in the presence of Mn2+. The content of PolyP in C. humicola cells increased 1.8-, 3.9-, and 3.4-fold in the presence of Сa2+, Cd2+, and Mn2+, respectively. The content of PolyP in C. humicola cells decreased 2-fold in the presence of La2+.
PolyP content increased in the cells of both yeasts only in the presence of Mn2+. This increase was more marked at a higher phosphate concentration (11.5 mmol/L) (Table 2). It was probably due to the stimulation of PolyP synthesis in the presence of Mn2+. It is known that Mn2+ stimulates PolyP synthesis by the Vtc4 protein in S. cerevisiae (Hothorn et al. 2009).
Enhanced contribution of acid-soluble PolyP in the presence of Cd2+ and Mn2+ is a distinctive feature of C. humicola (Table 3). The contribution of this fraction increased to 30–40 % in C. humicola during the cultivation with Co2+, Ni2+, and Zn2+ (data not shown). A correlation was revealed between heavy metal tolerance and the accumulation of acid-soluble PolyP, which is considered to be localized in the cytosol (Kulaev et al. 2004).
The effects of heavy metals on cell morphology and DAPI staining
The cells of C. humicola grown in the medium with 1.6 mmol/L Pi and without metals had numerous small inclusions in the cytoplasm (Fig. 4a). Their DAPI fluorescence was in the orange, yellow, and green region of the spectrum (Fig. 4b), which is typical for DAPI-PolyP complexes (Serafim et al. 2002; Puchkov 2010). The periphery of vacuoles was stained with DAPI and looked like a bright-colored ring. C. terreus in the same medium showed green fluorescence of the cell envelope and diffuse fluorescence of the cytoplasm, but no marked PolyP inclusions were found in the cytoplasm (Fig. 5b).
The micrographs of the cells of Cryptococcus humicola (48 h of cultivation). a, c, e phase contrast microscopy; b, d, f fluorescence microscopy of DAPI-stained cells. Scale bar = 5 μm. a, b Control cultivation in the medium containing 1.6 mmol/L Pi; c, d cultivation in the presence of 2.5 mmol/L Mn2+ and 1.6 mmol/L Pi; e, f cultivation in the presence of 2.5 mmol/L Mn2+ and 11.5 mmol/L Pi. 1 Vacuole; 2 cell wall; 3 PolyP containing cytoplasmic inclusions; 4 light-absorbing component associated with the cell wall
The micrographs of Cryptococcus terreus cells (48 h of cultivation). a, c Phase contrast microscopy; b, d fluorescence microscopy of DAPI-stained cells. Scale bar = 5 μm. a, b Control cultivation in the medium containing 1.6 mmol/L Pi; c, d cultivation in the presence of 2.5 mmol/L Mn2+ and 1.6 mmol/L Pi. 1 Vacuole; 2 cell wall
The cultivation with excess Mn2+ ions resulted in the changes in cell morphology revealed by phase contrast and fluorescent microscopy after DAPI staining. The C. humicola cells grown in the presence of Mn2+ were larger than the control cells (Fig. 4c). In the medium with 11.5 mmol/L Pi in the presence of Mn2+, a light-absorbing component was associated with the cell wall (Fig. 4e). This component did not fluoresce after DAPI staining. Probably, it may be insoluble manganese salts which are washed with citrate (Table 1). The intensity of cell wall fluorescence in the presence of Mn2+ was much higher than in the control, independent of Pi concentration (Fig. 4d, f). Intensively fluorescing cytosolic inclusions look larger than in the control (Fig. 4f). However, these inclusions are visible not in all cells. We suppose that this effect is due to culture heterogeneity in PolyP content and to disturbance of the PolyP/DAPI complex by Mn2+.
In the presence of Mn2+, the cells of C. terreus have the enlarged bright-orange fluorescent vacuole containing numerous small inclusions (Fig. 5d). It seems that the essential part of PolyP accumulating in the presence of Mn2+ is localized in the vacuoles of this species. It correlates with the accumulation of acid-insoluble PolyP (Fig. 3). The intensity of cell wall fluorescence in the presence of Mn2+ increased similar to C. humicola cells. The two yeast species under study are substantially different in PolyP localization under manganese excess: the tolerant C. humicola cells accumulate PolyP in cytosolic inclusions, while the sensitive C. terreus accumulates PolyP in vacuoles.
During cultivation in the presence of 2.5 mmol/L Ca2+, the fluorescence of DAPI-stained C. humicola cells was similar to the control (Figs. 4b and 6a). During the cultivation of C. terreus cells in the presence of 2.5 mmol/L Ca2+, numerous DAPI-fluorescent granules were observed in the cytoplasm but not in vacuoles (Fig. 6b).
In the presence of Cd2+ (Fig. 6c), the cell wall fluorescence of C. humicola cells becomes more intense, and the number of fully fluorescent cells increases. Cd2+ causes the lysis of some part of C. terreus cells. A considerable amount of extracellular material is formed in the C. terreus culture in the presence of Cd2+; this material is associated with the cell walls and fluoresces after DAPI staining (Fig. 6d). In the presence of La3+ (Fig. 6e), the cell wall of C. humicola fluoresces most brightly compared to other cations under study. The fluorescence of the C. terreus cell wall also increases; however, many cells are damaged and fluoresce entirely (Fig. 6f).
Discussion
In this work, we have studied two basidiomycetous yeast species with different resistance to heavy metal cations. C. humicola and C. terreus showed tolerance and sensitivity, respectively, to all of the cations used. Since PolyP is believed to be an important factor of microbial resistance to heavy metal cations (Kulaev et al. 2004), we have attempted to reveal the differences in the state of these polymers in the cells of both yeast species under heavy metal excess. The content of acid-soluble PolyP increased in the tolerant С. humicola but not in the sensitive С. terreus. DAPI fluorescence shows that the PolyP accumulating in C. humicola in response to excess heavy metal cations are localized in cytoplasmic inclusions and in the cell wall, while in С. terreus, they are localized in vacuoles. Moreover, the morphology of vacuoles in С. terreus changed and their function was probably disturbed. It is supposed that acid-soluble PolyP is presumably localized in the cytoplasm (Kulaev et al. 2004). The increase in the content of this PolyP and the enlargement of DAPI-stained PolyP inclusions in the cytoplasm of C. humicola confirm this suggestion. The PolyP-synthesizing enzymes in yeast are little studied as yet. Two of them have been revealed in S. cerevisiae: dolychylpyrophosphate:polyphosphate transferase responsible for the synthesis of the minor part of yeast cell polyP localized mainly in the cell wall (Kulaev et al. 2004) and Vtc4 protein, a transport chaperone localized in the vacuolar membrane (Hothorn et al. 2009).
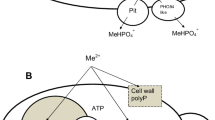
DAPI fluorescence data (Fig. 7) suggest that the significant portion of PolyP in the cells of С. terreus and С. humicola is associated with vacuoles. The hypothetic scheme of PolyP accumulation in the cells of both species is given in Fig. 7. In C. humicola cells, some part of PolyP is agglomerated close to the vacuolar membrane on the cytoplasmic side, and then, the formed PolyP granules are released into the cytoplasm. In C. terreus cells, DAPI staining revealed a different situation: the accumulated PolyP is localized inside the vacuole. The mechanism of Vtc4 functioning revealed in S. cerevisiae provides the translocation of the growing PolyP chain across the membrane (Hothorn et al. 2009). It suggests the possibility of PolyP translocation mainly into the vacuoles or cytoplasm, depending on the peculiarities of Vtc4 proteins in different yeasts. The suggested differences in Vtc4 functioning in the cells of С. humicola and С. terreus may cause the difference in PolyP localization.
The results suggest that the accumulation of PolyP in cytoplasmic inclusions may be one of the factors providing the heavy metal tolerance of С. humicola cells by forming cation/PolyP complexes. It seems that C. humicola realizes many pathways of cell protection from excess toxic cations. The cultivation of C. humicola in the media with 2.5 mmol/L Mn2+ and 11.5 mmol/L Pi for more than 72-h results in pH alkalization from the initial value 5.8 to 7.2. The poorly soluble Mn(OH)2 formed in the alkaline medium is easily oxidized in the air to MnO(OH)2, since the cultivation takes place under aeration. As a result, the medium and the biomass become brown, which is typical of MnO(OH)2. Alkalization of the medium to 8.0 during long-term cultivation is typical of C. humicola and does not depend on the presence of excess manganese. This alkalization, resulting in the formation of low-soluble Mn2+ compounds, seems to be an additional factor of manganese resistance. As regards C. terreus, during its growth without manganese, the medium is alkalized from pH 5.8 to 7.0–7.5. In the presence of excess manganese, when the culture does not grow, pH of the medium remains 5.8.
Thus, C. humicola, like C. tropicalis (Rehman and Anjum 2011), is a heavy metal-resistant yeast. The study of such microorganisms from natural ecotopes is of interest for understanding the changes in the species set under technogenic pollutions of soil and water (Liu et al. 2012).
The study of the yeasts from extreme environments has revealed that membrane fluidity fluctuation is an important factor of stress tolerance: the high absolute fluidity fluctuation is associated with the lower survival. Fluidity and its variations therefore reflect the survival strategy and fitness in extreme environments and are good indicators of adaptability of microorganisms (Turka et al. 2011). The C. humicola strain used in this study seems to have adaptive mechanisms for overcoming cellular membrane disorder, because this yeast strain produces a membrane-damaging extracellular glycolipid (Puchkov et al. 2002). C. terreus is highly sensitive to this glycolipid, indicating the lower ability of its membranes to overcome stress factors. It may be one of the causes of the high sensitivity of C. terreus to heavy metal stress. The resistance to membrane-damaging and chaotrophic agents and long-chain polyphosphate accumulation may be the factors enhancing the competitive ability of some Cryptococcus species in some ecological niches (Kachalkin and Yurkov 2012; Cray et al. 2013b).
It should be noted that the fungi tolerant to heavy metals may be a protective factor for other organisms. For example, mycorrhization protects roots from Cd-induced injury by preventing the access of cadmium to plant cells (Schützendübel and Polle 2002).
Back to PolyP, this widespread biopolymer is the key stress-overcoming factor in microorganisms. It participates in induction of the synthesis of RpoS, the RNA-polymerase subunit in bacteria responsible for stress and stationary phase gene expression and in the regulation of the level of the stringent response factor, guanosine 5′-diphosphate 3′-diphosphate (ppGpp) (Rao et al. 2009). They may be a source for ATP synthesis under stresses (Castro et al. 1999) and protect from environmental stresses under phosphate limitation conditions (Jahid et al. 2006). Finally, numerous data suggest the potential involvement of polyphosphates in detoxification of heavy metal cations by their complexation and sequestration (Kulaev et al. 2004). C. humicola may be a promising model for studying the mechanisms of heavy metal tolerance.
References
Achbergerová L, Nahálka J (2011) Polyphosphate—an ancient energy source and active metabolic regulator. Microb Cell Factories 10:63–70
Andreeva NA, Ryazanova LP, Dmitriev VV, Kulakovskaya TV, Kulaev IS (2013) Adaptation of Saccharomyces cerevisiae to toxic manganese concentration triggers changes in inorganic polyphosphates. FEMS Yeast Res 13:463–470
Breus NA, Ryazanova LP, Dmitriev VV, Kulakovskaya TV, Kulaev IS (2012) Accumulation of phosphate and polyphosphate by Cryptococcus humicola and Saccharomyces cerevisiae in the absence of nitrogen. FEMS Yeast Res 12:617–624
Castro CD, Koretsky AP, Domach MM (1999) NMR-observed phosphate trafficking and polyphosphate dynamics in wild-type and vph1-1 mutant Saccharomyces cerevisiae in response to stresses. Biotechnol Prog 15:65–73
Chin JP, Megaw J, Magill CL, Nowotarski K, Williams JP, Bhaganna P, Linton M, Patterson MF, Underwood GJC, Mswaka AY, Hallsworth JE (2010) Solutes determine the temperature windows for microbial survival and growth. PNAS 107:7835–7840
Cray JA, Russell JT, Timson DJ, Singhal RS, Hallsworth JE (2013a) A universal measure of chaotropicity and kosmotropicity. Environ Microbiol 15:287–296
Cray JA, Bell ANW, Bhaganna P, Mswaka AY, Timson DJ, Hallsworth JE (2013b) The biology of habitat dominance; can microbes behave as weeds? Microb Biotechnol 6:453–492
Culotta VC, Yang M, Hall MD (2005) Manganese transport and trafficking: lesson learned from Saccharomyces cerevisiae. Eukaryot Cell 4:1159–1165
De Lima Freitas A, Ferreira de Moura G, Barbosa de Lima MA, Mendes de Souza P, Alves da Silva CA, de Campos Takaki GM, do Nascimento AE (2011) Role of the morphology and polyphosphate in Trichoderma harzianum related to cadmium removal. Molecules 16:2486–2500
Gardarin A, Chédin S, Lagniel G, Aude JC, Godat E, Catty P, Labarre J (2010) Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol Microbiol 76:1034–1048
Golubev W, Shabalin Y (1994) Microcin production in Cryptococcus humicola. FEMS Microbiol Lett 119:105–110
Gomes FM, Carvalho DB, Peron AC, Saito K, Miranda K, Machado EA (2012) Inorganic polyphosphates are stored in spherites within the midgut of Anticarsia gemmatalis and play a role in copper detoxification. J Insect Physiol 58:211–219
Hirota R, Kuroda A, Kato J, Ohtake H (2010) Bacterial phosphate metabolism and its application to phosphorus recovery and industrial bioprocess. J Biosci Bioeng 109:423–432
Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, Mayer A (2009) Catalytic core of a membrane-associated eucaryotic polyphosphate polymerase. Science 324:513–516
Jahid IK, Silva AJ, Benitez JA (2006) Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low-phosphate environment. Appl Environ Microbiol 72:7043–7049
Jensen LT, Ajua-Alemandji M, Culotta VC (2003) The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem 278:42036–42040
Jin YH, Dunlap PE, McBride SJ, Al-Refai H, Bushel PR, Freedman JH (2008) Global transcriptome and deletome profiles of yeast exposed to transition metals. PLoS Genet 4(4):e1000053
Kachalkin AV, Yurkov AM (2012) Yeast communities in Sphagnum phyllosphere along the temperature-moisture ecocline in the boreal forest-swamp ecosystem and description of Candida sphagnicola sp. nov. Antonie Van Leeuwenhoek 102:29–43
Keasling JD, Van Dien SJ, Trelstad P, Renninger N, McMahon K (2000) Application of polyphosphate metabolism to environmental and biotechnological problems. Biochem Mosc 65:324–331
Kennedy PJ, Vashisht AA, Hoe KL, Kim DU, Park HO, Hayles J, Russell P (2008) A genome-wide screen of genes involved in cadmium tolerance in Schizosaccharomyces pombe. Toxicol Sci 106:124–139
Kulaev IS, Vagabov VM, Kulakovskaya TV (2004) The biochemistry of inorganic polyphosphates. Wiley, Chichester
Lichko LP, Okorokov LA (1976) The compartmentalization of magnesium and phosphate ions in Saccharomyces carlsbergensis cells. Dokl Akad Nauk SSSR (in Russian) 227:756–758
Liu Y, Zhou T, Crowley D, Li L, Liu D, Zheng J, Yu X, Pan G, Hussain Q, Zhang X, Zheng J (2012) Decline in topsoil microbial quotient, fungal abundance and C utilization efficiency of rice paddies under heavy metal pollution across South China. PLoS One 7(6):e38858
Macomber L, Hausinger RP (2011) Mechanisms of nickel toxicity in microorganisms. Metallomics 3:1153–1162
Martin P, Van Mooy BA (2013) Fluorometric quantification of polyphosphate in environmental plankton samples: extraction protocols, matrix effects, and nucleic acid interference. Appl Environ Microbiol 79:273–281
Mollinedo F (2012) Lipid raft involvement in yeast cell growth and death. Front Oncol 10(2):140. doi:10.3389/fonc.2012.00140, eCollection 2012
Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gómez-García MR, Abramov AY (2010) Inorganic polyphosphate and energy metabolism in mammalian cells. J Biol Chem 285:9420–9428
Permyakov S, Suzina N, Valiakhmetov A (2012) Activation of H+-ATPase of the plasma membrane of Saccharomyces cerevisiae by glucose: the role of sphingolipid and lateral enzyme mobility. PLoS One 7:e30966. doi:10.1371/journal.pone.0030966
Puchkov EO (2010) Brownian motion of polyphosphate complexes in yeast vacuoles: characterization by fluorescence microscopy with image analysis. Yeast 27:309–315
Puchkov EO, Zahringer U, Lindner B, Kulakovskaya TV, Seydel U, Wiese A (2002) Mycocidal, membrane-active complex of Cryptococcus humicola, is a new type of cellobiose lipid with detergent features. Biochim Biophys Acta (Biomembranes) 1558:161–170
Rao NN, Gómez-García MR, Kornberg A (2009) Inorganic polyphosphate: essential for growth and survival. Ann Rev Biochem 78:605–647
Rehman A, Anjum MS (2011) Multiple metal tolerance and biosorption of cadmium by Candida tropicalis isolated from industrial effluents: glutathione as detoxifying agent. Environ Monit Assess 174:585–595
Remonsellez F, Orell A, Jerez CA (2006) Copper tolerance of the thermoacidophilic archaeon Sulfolobus metallicus: possible role of polyphosphate metabolism. Microbiology 152:59–66
Rosenfeld L, Reddi AR, Leung E, Aranda K, Jensen LT, Culotta CV (2010) The effect of phosphate accumulation on metal ion homeostasis in Saccharomyces cerevisiae. J Biol Inorg Chem 15:1051–1062
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Serafim LS, Lemos OC, Levantesi C, Tandoi V, Santos H, Reis MA (2002) Methods for detection and visualization of intracellular polymers stored by polyphosphate-accumulating microorganisms. J Microbiol Methods 51:1–18
Singh P, Raghukumar C, Parvatkar RR, Mascarenhas-Pereira MB (2013) Heavy metal tolerance in the psychrotolerant Cryptococcus sp. isolated from deep-see sediments of Central Indian Basin. Yeast 30:93–101
Spain A (2003) Implication of microbial heavy metal tolerance in the environment. Rev Undergrad Res 2:1–6
Thorsen M, Perrone GG, Kristiansson E, Traini M, Ye T, Dawes IW, Nerman O, Tamás MJ (2009) Genetic basis of arsenite and cadmium tolerance in Saccharomyces cerevisiae. MC Genomics 12:100–105
Turka M, Plemenitašb A, Gunde-Cimermana N (2011) Extremophilic yeasts: plasma-membrane fluidity as determinant of stress tolerance. Fungal Biol 115:950–958
Vadkertiová R, Sláviková E (2006) Metal tolerance of yeasts isolated from water, soil and plant environments. J Basic Microbiol 46:145–152
Vagabov VM, Trilisenko LV, Kulaev IS (2000) Dependence of inorganic polyphosphate chain length on the orthophosphate content in the culture medium of the yeast Saccharomyces cerevisiae. Biochem Mosc 65:349–355
Vagabov VM, Ivanov AY, Kulakovskaya TV, Kulakovskaya EV, Petrov VV, Kulaev IS (2008) Efflux of potassium ions from cells and spheroplasts of Saccharomyces cerevisiae yeast treated with silver and copper ions. Biochemistry (Mosc) 73:1224–1227
Vreulink JM, Stone W, Botha A (2010) Effects of small increases in copper levels on culturable basidiomycetous yeasts in low-nutrient soils. J Appl Microbiol 109:1411–1421
Wysocki R, Tamás MJ (2010) How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev 34:925–951
Acknowledgments
The authors were supported by the Program of Presidium of Russian Academy of Sciences “The Problems of Life Origin and Biosphere Formation.” They are grateful to Dr. W.I. Golubev for kindly providing yeast strains and to E. Makeeva for assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreeva, N., Ryazanova, L., Dmitriev, V. et al. Cytoplasmic inorganic polyphosphate participates in the heavy metal tolerance of Cryptococcus humicola . Folia Microbiol 59, 381–389 (2014). https://doi.org/10.1007/s12223-014-0310-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-014-0310-x