Abstract
The aim of this investigation was to determine the persistence of biofilm-associated antibiotic resistance developed by methicillin-sensitive Staphylococcus aureus (MSSA), of different capsular types, during biofilm formation. Because of superiority of the tissue culture plate (TCP) over the Congo Red Agar (CRA) method for measuring biofilm formation, it was used to determine the persistence of the antibiotic resistance developed by the isolates in biofilms. The antibiotic resistance was found to persist for 3–4 wk post-propagation as planktonic subcultures. Interestingly, some strains even developed resistance to vancomycin and/or teicoplanin. However, no association of either biofilm formation or persistent antibiotic resistance with the major capsular phenotype was observed. These observations highlight the potential significance of (a) determining the antibiograms of S. aureus subcultured from biofilms developed in vitro using the TCP method as well as from planktonic cultures for formulation of an optimal therapeutic strategy, and (b) continuing to identify predominant non-capsular antigens contributing to biofilm formation, regardless of the capsular phenotype for the development of an effective potentially broad-spectrum vaccine for prevention of bovine mastitis caused by S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common etiological agents of contagious mastitis (40.5 %) in cows is Staphylococcus aureus (Workineh et al. 2002). This presents a serious problem for the Australian dairy industry, the third largest rural industry, valued at $12 billion at wholesale and $2.9 billion for export, representing 9 % of world dairy trade (Dairy Australia 2011). Each case of mastitis is estimated to cost approximately $200 per head with an average of three clinical mastitis cases per 100 cows on farm, mainly caused by this pathogen.
The major difficulty encountered in treating and managing staphylococcal mastitis has been the versatility of S. aureus in developing antimicrobial resistance, also noted with coagulase-negative Staphylococcus species (Melchior et al. 2007). In chronic and localised infections like mastitis, this may be largely be dependent on the bacterium’s ability to form biofilms (Mah and O’Toole 2001) in which bacterial cells can evade the antibiotic and host defences employed to inhibit colonization and subsequent infections. Biofilm formation starts with interaction of surface anchored bacterial factors, particularly the MSCRAMMs such as autolysin (Pozzi et al. 2012), an extracellular adhesion protein, PNAG (Maira-Laitren et al. 2004), eDNA (Mann et al. 2009), protein SasG (Geoghegan et al. 2010), and other recently described factors such as fibronectin binding proteins and clumping factor (Szczuka et al. 2012). While the role played by PNAG, also alternatively referred to as PIA, in infections caused by S. aureus has been well documented (Maira-Laitren et al. 2004) and reviewed extensively elsewhere (Archer et al. 2011; Arciola et al. 2012), there are no reports, to the best of our knowledge, on either the development of persistence of biofilm-associated antibiotic resistance of S. aureus or the emergence of resistance to different antibiotics.
On the other hand, PNAG-negative strains forming biofilms in in vitro settings have been reported (Beenkeen et al. 2004) highlighting the importance of other MSCRAMMs such as Bap, predominantly associated with isolates from chronic bovine mastitis infections (Tormo et al. 2005). Whether this is the case for infections in vivo is not known, as most clinical isolates of S. aureus produce PNAG (Szczuka et al. 2012) demonstrated to participate in biofilm formation (Kropec et al. 2005). Given the identification of MSCRAMMs other than PNAG as contributors to biofilm formation as highlighted above, it is important to continue the identification of the most critical biofilm-forming antigens of S. aureus, and other staphylococcal species, because it may assist in the development of an effective broad-spectrum vaccine or other antimicrobial therapies for infections caused by this versatile pathogen.
Majority of human S. aureus clinical isolates are reported to express either CP5 or CP8, representing 70-80 % of all the isolates (Nemeth and Lee 1995; Fattom et al. 1996), although their percentage distribution in bovine mastitis has been reported to be variable (Han et al. 2000), with non-typeable strains expressing a different surface antigen 336, a cell wall ribitol teichoic acid substituted with an N-acetyl glucosamine monosaccharide (Verdier et al. 2007). Non-typeable S. aureus isolates do not have a functional genetic locus for either CP5 or CP8 synthesis (Cocchiaro et al. 2006). However, the potential role of capsules of S. aureus in the colonisation process, the first step in biofilm formation, has not been investigated. This perhaps is due to acceptance of the “golden rule” that the major function of capsule is protection of the pathogen from opsono-phagocytosis (Nemeth and Lee 1995; Gordon and Franklin 2008). Interestingly, the capsules of S. pneumonia, a different gram-positive coccus, were reported to enhance pneumococcal colonisation by limiting mucus-mediated clearance (Magee and Yother 2001; Nelson et al. 2007), hence our interest in determining the potential association of the major capsular phenotypes of S. aureus with biofilm formation, and as a corollary, antibiotic resistance.
Therefore the major aims of this study were to (1) investigate the biofilm-forming potential and the impact on the emergence of persistent antibiotic resistance developed by methicillin-sensitive S. aureus (MSSA) clinical isolates in biofilms and (2) determine potential association of biofilm formation with the major capsular phenotypes.
Material and methods
Bacterial strains and culture conditions
One hundred and sixty (160) phenotypically characterised, S. aureus isolates from bovine mastitis cases in Victoria and Queensland were cultivated in nutrient broth supplemented with 1 % glucose. The accredited control S. aureus strains used in this investigation were strain M (CP1), Smith diffuse strain (CP2), USA 400 MW2 (CP8), Strain Newman and USA 100 NRS 648 (CP5), CP-negative isolates (USA 300 LAC and USA 300 NRS 648) and a strong biofilm-forming strain ATCC 29213 as a positive control.
Screening of S. aureus isolates for methicillin-resistance
Chromogenic MRSA ID plates (Biomerieux 2011) were used to check for bacterial growth in the presence of cefoxitin and amplification of the mecA gene product using polymerase chain reaction as reported elsewhere (Murakami et al. 1991).
Detection of biofilm production
Congo Red Agar method
Colony phenotypes on CRA were determined as described by Freeman et al. (1989), with the modification of incubating of the streaked plates for 96 h to ensure maximal biofilm formation (data not shown).
Tissue Culture Plate method
Aliquots (200μL) of nutrient broth, supplemented with 1 % glucose, were inoculated with S. aureus strains in 96-well sterile microtitre plates and incubated overnight at 37 °C to ensure adequate biofilm production. The two methods used to assess biofilm formation were (1) sodium metaperiodate treatment method (Kogan et al. 2006) and (2) crystal violet staining method (Christensen et al. 1985). The arbitrary cut-off point used for nonbiofilm-producing strains was 0.120 (Stepanovic et al. 2000). The arbitrary cut-off points used for weakly, intermediate or strongly adherent bacterial populations were 0.130–4.00 × 0.120 A 570nm, 4.10–5.90 × 0.120 A 570nm and greater than 6 × 0.120 A 570nm, respectively.
Capsular typing
DNA extracts of 160 strains were run against Cap5 and Cap8 primers (Moore and Lindsay 2001). Primers for Cap5 forward were 5′-ATGACGATGAGGATAGCG-3′ and reverse 5′-CTCGGATAACACCTGTTGC-3′ with a Tm of 60 °C. Primers for Cap8 forward were 5′-ATGACGATGAGGATAGCG-3′ and reverse 5′-CACCTAACATAAGGCAAG-3′ with a Tm of 53 °C. Expected band size for Cap5 and Cap8 were 881 and 1,148 bp, respectively. The PCR products were separated on a 1.5 % agarose gel in 1× TAE buffer, stained with SYBR® Safe DNA Gel Stain (Invitrogen) and visualized using a UV trans-illuminator.
Amplification of the mecA gene
Detection of the mecA gene (Murakami et al. 1991) was carried out using the forward primer 5′-AAAATCGATGGTAAAGGTTGGC-3′ and reverse primer 5′-AGTTCTGCAGTACCGGATTTGC-3′ with a Tm of 52 °C. The PCR products were separated on 1.5 % agarose gel with 1× TAE buffer, stained with SYBR® Safe DNA Gel Stain (Invitrogen) and visualised the expected band size of 533 bp using a UV trans-illuminator.
Antibiotic susceptibility tests
Antibiotic susceptibility tests were performed using the CDS method (Bell et al. 2009) on biofilm-producing S. aureus isolates. The antibiotic sensitivities of bacteria subcultured from the biofilms were compared with the planktonic/free-floating bacteria using Sensitest plates (PathWest). The antibiotics used for susceptibility were benzylpenicillin (P 0.5), 0.5 μg per disc, cefoxitin (FOX 10), 10 μg per disc, cephalexin (CL 100), 100 μg per disc, ciprofloxacin (CIP 2.5), 2.5 μg per disc, co-trimoxazole (SXT 25), 25 μg per disc, erythromycin (E 5), 5 μg, linezolid (LZD 10), 10 μg, mupirocin (MUP 200), 200 μg, rifampicin (RD 1), 1 μg, teicoplanin (TEC 15), 15 μg/disc, tetracycline (TE 10), 10 μg per disc, and vancomycin (VAN 5), 5 μg per disc (Oxoid). Zones of inhibition of 6 mm or greater in diameter were interpreted as susceptible. Exceptions to this rule were vancomycin (VA5) and teicoplanin (TEC 15), for which a 2-mm diameter was used as the cut-off point.
Results
Using the CRA method, only 54 of the 160 isolates (33.8 %) were positive for biofilm formation. However, all the 160 MSSA isolates were positive for biofilm formation, graded as weak, moderate or intermediate and strong, by the TCP method (Table 1). This demonstrated the superiority of the TCP over CRA method for detection of biofilm formation. It was also observed that there was no association of the capsular phenotype of S. aureus and biofilm formation because all the encapsulated as well as the even the non-typeable strains that do not express the capsular antigens (58/160 strains by the TCP method; 14/160 strains by the CRA method) were found to produce biofilms (Table 1).
The accredited control S. aureus used in this investigation were found to range from nonbiofilm-producing (Strain M—CP1, Smith diffuse—CP2), weak biofilm producers (USA 300LAC—CP Neg and USA 100 NRS 64—CP5), moderate biofilm-producing (USA 300 NRS 680—CP Neg) to strong biofilm-producing strains (Strain Newman—CP5, USA 400 MW2—CP5 and the ATCC2913—CP8) (data not shown).
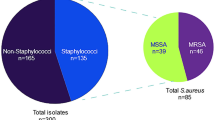
None of the 160 Australian bovine mastitis clinical isolates were found to be MRSA using the Chrom ID MRSA plates [Biomerieux] and absence of the mecA gene product by specific PCR. However, out of the accredited CP types, USA NRS 648 (CP5), USA 400 MW2 (CP8), USA 300 NRS 680 (CP Neg) and USA 300 LAC (CP Neg) were confirmed to be MRSA. Of the 160 Australian MSSA isolates, 63 % were encapsulated (44/160—CP5 and 58/160—CP8 type), the remaining 36.25 % being non-typeable as CP5 or CP8.
Antibiotic susceptibility tests performed on 34 strong biofilm-forming S. aureus isolates, including CP type 8-USA 400 Strain MW2, revealed multiple antibiotic resistances when grown as biofilms, with two Australian isolates (BoAISRF S.aur40 and BoAISRF S.aur41) and the USA 400 Strain MW2 developing resistance to vancomycin. The isolate BoAISRF S.aur41 also developed resistance to teicoplanin (Table 2).
The fact that 12 % of the Australian clinical mastitis MSSA isolates developed resistance to more than one antibiotic, as did the USA 400 MW2 strain, including vancomycin and/or teicoplanin, represents a matter of serious concern.
In order to determine whether the developed antibiotic resistance was transient or persistent, S. aureus scooped from the biofilms of the above-mentioned 5 isolates stored at 4 °C and re-tested for susceptibility against the same antibiotic after subculturing at days 7, 14, 21 and 30. Antibiotic resistance was found to persist until 3–4 weeks, reverting back to susceptibility by week 4 post-propagation as planktonic subcultures.
Discussion
Comparison of the TCP with the CRA method for detection of biofilm formation, using four accredited CP types, three non-encapsulated strains and 160 MSSA isolates from clinical bovine mastitis cases in Australia, revealed that substantial number (66.25 %) of biofilm-producing isolates remained undetected when the CRA method was used to detect biofilm production. On the other hand, 100 % of the MSSA isolates were found to produce biofilms using the TCP method. This conclusion is in contrast to previous reports by other investigators (Oliviera et al. 2006; Begum et al. 2007). Our decision to use the incubation period of 96 h is validated by the report by Begum et al. (2007) who, using fluorescent in situ hybridisation, reported that the biofilm-forming ability of S. aureus increased with an increase in the incubation time (34.6 % at 24 h, 69.2 % at 48 h and 80.8 % at 72 h post-incubation). Our conclusion is based on the assumption that the antigens precipitating biofilm formation by the CRA plate and TCP methods are the same, an area needing further investigations. However, regardless of the method adopted for detection of biofilm-producing S. aureus, there appeared to be no association of the capsular phenotype with biofilm formation since many non-encapsulated S. aureus strains were also found to form weak, moderate, or strong biofilms, as reported recently for S. aureus strains of human origin (Szczuka et al. 2012).
In addition to confirming a multitude of the previous reports on development of antibiotic resistance in biofilms (Christensen et al. 1985; Stepanovic et al. 2000; Mah and O’Toole 2001; Melchior et al. 2007; Kogan et al. 2006; Tormo et al. 2005; Oliveira et al. 2006; Begum et al. 2007), our investigation is the first one to demonstrate that antibiotic resistance, developed by MSSA strains during biofilm formation and became persistent as judged by retention of antibiotic resistance in planktonic subcultures until days 21 to 30. Subsequently, these strains reverted back to antibiotic sensitivity. The mechanism underpinning persistence of antibiotic resistance of S. aureus in biofilms was not investigated in this study but warrants further investigation(s).
Emergence of persistent antibiotic resistance by S. aureus is a matter of great concern in the therapy of staphylococcal local and systemic infections, particularly given the change in the resistance phenotype, as demonstrated for select S. aureus strains in this investigation, to vancomycin or teicoplanin, the last resort antibiotics. It is hypothesised that selection of antibiotics for treatment of mastitis, based on the susceptibility of S. aureus in both the planktonic and the biofilm-embedded/associated states, may yield better therapeutic outcomes particularly in stubborn cases of bovine mastitis as recently suggested for human MRSA infections (Khan et al. 2011). Furthermore, the lack of association of the capsular phenotypes of S. aureus with biofilm formation ruled out a potential role of one of the three major polysaccharide MSCRAMMs in colonisation of the host by S. aureus, hence warranting comparative studies on the protective potential of capsular versus the non-capsular biofilm- forming antigen(s)-based vaccine(s).
Abbreviations
- BAP:
-
Biofilm-associated protein
- MSSA:
-
Methicillin-sensitive Staphylococcus aureus
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- TCP:
-
Tissue Culture Plate
- CRA:
-
Congo Red Agar
- MSCRAMMs:
-
Microbial surface components recognizing adhesive matrix molecules
- PIA:
-
Polysaccharide intercellular adhesin
- PNAG:
-
Poly-N-acetyl glucosamine
- CP:
-
Capsular polysaccharide
References
Archer NK, Mazaitis MJ, Costerton JW, Leid GL, Powers EM, Shirtliff ME (2011) Staphylococcus aureus biofilms. Virulence 2:445–459
Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials Biomaterials 33:5967–5982
Beenkeen KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS (2004) Global gene expression in Staphylococcus aureus biofilms. J Bact 186:4665–4684
Begum HA, Uddin MS, Islam MJ, Nazir KHMNH, Islam MA, Rahman MT (2007) Detection of biofilm producing coagulase positive Staphylococcus aureus from bovine mastitis, their pigment production, hemolytic activity and antibiotic sensitivity pattern. J Bangladesh Soc Agric Sci Technol 4:97–100
Bell SM, Pham JN, Fisher GT (2009) Antibiotic susceptibility testing by the CDS method: a manual for medical and veterinary laboratories. Fifth Edition. South Eastern Area Laboratory Services, NSW
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006
Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC (2006) Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol Microbiol 59:948–960
Dairy Australia (2011) Dairy Australia Strategic Plan 2011–2015. http://www.dairyaustralia.com.au/. Accessed December 2010
Fattom AI, Sarwar J, Ortiz A, Naso RA (1996) Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP specific antibodies protect mice against bacterial challenge. Infect Immun 64:1659–1665
Freeman DJ, Falkiner FR, Keane CT (1989) New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol 42:872–874
Geoghegan JA, Corrigan RM, Gruszka DT, Speziale P, O’Gara JP, Potts JR, Foster TJ (2010) Role of surface protein SasG in biofilm formation by Staphylococcus aureus. J Bact 197:5663–5673
Gordon RJ, Franklin RD (2008) Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:S350–S359
Han HR, Pak SI, Guidry A (2000) Prevalence of capsular polysaccharide (CP) types of Staphylococcus aureus isolated from bovine mastitic milk and protection of S. aureus infection in mice with CP vaccine. J Vet Med Sci 62:1331–1333
Khan F, Shukla I, Rizvi M, Mansoor T, Sharma SC (2011) Detection of biofilm formation in Staphylococcus aureus. Does it have a role in treatment of MRSA infections? Trends Med Res 6:116–123
Kogan G, Sadovskaya I, Chaignon P, Chokr A, Jabbouri S (2006) Biofilms of clinical strains of Staphylococcus that do not contain polysaccharide intercellular adhesin. FEMS Micobiol Lett 255:11–16
Kropec A, Maria-Litran T, Jefferson KK, Grout M, Cramton SE, Götz F, Goldmann DA, Pier GB (2005) Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun 73:6868–6876
Magee AD, Yother J (2001) Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun 69:3755–3761
Mah TFC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Maira-Laitren T, Kropec A, Goldmann D, Pier GB (2004) Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide. Vaccine 22:872–879
Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L (2009) Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822
Melchior MB, Fink-Gremmels J, Gaastra W (2007) Extended antimicrobial susceptibility assay for Staphylococcus aureus isolates from bovine mastitis growing in biofilms. Vet Microbiol 125:141–149
Moore PCI, Lindsay JA (2001) Genetic variation among hospital isolates of methicillin-sensitive Staphylococcus aureus: evidence for horizontal transfer of virulence genes. J Clin Microbiol 39:2760–2767
Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S (1991) Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29(10):2240–2244
Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weisser JN (2007) Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun 75:83–90
Nemeth J, Lee JC (1995) Antibodies to capsular polysaccharides are not protective against experimental Staphylococcus aureus endocarditis. Infect Immun 63:375–380
Oliveira M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, Vilela CL (2006) Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol 118:133–140
Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O’Gara JP (2012) Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8(4):e1002626
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179
Szczuka E, Urbanska K, Pietryka M, Kaznowski A (2012) Biofilm density and detection of biofilm-producing genes in methicillin-resistant Staphylococcus aureus strains. Folia Microbiol (Epub ahead of print]); doi:10.1007/s12223-012-0175-9
Tormo MA, Obeda C, Marti M, Maiques E, Cucarella C, Valle J, Foster TJ, Lasa I, Panades JR (2005) Phase-variable expression of the biofilm-associated protein (Bap) in Staphylococcus aureus. Microbiol 153:1702–1710
Verdier I, Durand G, Bes M, Taylor KL, Lina G, Vandenesch F, Fattom AI, Etienne J (2007) Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J Clin Microbiol 49:725–729
Workineh S, Bayleyegn H, Mekonnen H, Potgieter LND (2002) Prevalence and aetiology of mastitis in cows from two major Ethiopian dairies. Trop Anim Hlth Prod 34:19–25
Acknowledgments
This project is financially supported by an Australia India Strategic Research Fund [BF040038] grants made by the Department of Innovation, Industry, Science and Research, Commonwealth Government of Australia to Dr Trilochan Mukkur (Australia), and Department of Biotechnology, Ministry of Science and Technology, India to Drs Nagendra Hegde and Shrikrishna Isloor (India) respectively. Sincere thanks are expressed for the assistance provided by Mr Alain Delhaize in the execution of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babra, C., Tiwari, J.G., Pier, G. et al. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol 58, 469–474 (2013). https://doi.org/10.1007/s12223-013-0232-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-013-0232-z