Abstract
Many serious diseases caused by Staphylococcus aureus appear to be associated with biofilms. Therefore, we investigated the biofilm-forming ability of the methicillin-resistant S. aureus (MRSA) isolates collected from hospitalized patients. As many as 96 % strains had the ability to form biofilm in vitro. The majority of S. aureus strains formed biofilm in ica-dependent mechanism. However, 23 % of MRSA isolates formed biofilm in ica-independent mechanism. Half of these strains carried fnbB genes encoding surface proteins fibronectin-binding protein B involved in intercellular accumulation and biofilm development in S. aureus strains. The biofilm structures were examined via confocal laser scanning microscopy (CLSM) and three-dimensional structures were reconstructed. The images obtained in CLSM revealed that the biofilm created by ica-positive strains was different from biofilm formed by ica-negative strains. The MRSA population showed a large genetic diversity and we did not find a single clone that occurred preferentially in hospital environment. Our results demonstrated the variation in genes encoding adhesins for the host matrix proteins (elastin, laminin, collagen, fibronectin, and fibrinogen) and in the gene involved in biofilm formation (icaA) within the majority of S. aureus clones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is a Gram-positive bacterium responsible for many infections ranging from folliculitis to septicemia, pneumonia, osteomyelitis, or endocarditis (Götz et al. 2006). Many infections are mediated by the ability of S. aureus to adhere to catheters and other medical devices and form multicelllular communities embedded in polymeric matrix known as the biofilm. Treatment of Staphylococcus–biofilm-associated infections is extremely difficult because inside the biofilm, S. aureus becomes more resistant to antibiotic treatment and the action of the host immune system. Biofilm formation is a complex process that can be subdivided into relatively distinct phases: primary attachment of cell, cell-to-cell adhesion and proliferation, biofilm maturation, and finally detachment of planktonic cell from the biofilm (Götz 2002; Mack et al. 2006). Several cell surface proteins of S. aureus referred to as MSCRAMMs have been implicated in primary attachment to both native tissues and abiotic surface. Having successfully attached itself, the bacteria start to accumulate in multiple bacterial layers and construct a multilayered architecture. The accumulative phase is linked to the production of polysaccharide intercellular adhesin (PIA) which is composed of linear β-1,6-linked glucosaminylglycans. PIA is synthesized by the products of ica locus, particularly by the enzyme N-acetylglucosaminyltranferase encoded by the icaA gene (Mack et al. 2006). The ica-dependent biofilm formation is the main mechanism described in S. aureus strains (Mack et al. 2006). Recently, O’Neill et al. (2007) have indicated that deletion of the ica locus has no effect on biofilm formation in some methicillin-resistant S. aureus (MRSA) strains. Intercellular adhesion and biofilm development in S. aureus strains are mediated by a 140-kDa cell wall surface protein biofilm-associated protein (Bap) (Cucarella et al. 2001). Additional components such as proteins other than Bap, DNA, or teichoic acid have also been suggested to be important in biofilm formation. After biofilm maturation, the detachment of cell from the biofilm may initiate a new cycle of biofilm formation elsewhere. Therefore, the ability of S. aureus strains to form biofilm is considered an important virulence factor in the establishment of chronic infections.
The purpose of this study was to examine the ability of methicillin-resistant S. aureus strains to produce slime and form biofilm in vitro. The biofilm structures were examined via confocal laser scanning microscopy. Moreover, we estimated the prevalence of genes encoding adhesins for the host matrix proteins (elastin, laminin, collagen, fibronectin, fibrinogen, and bone sialoprotein) and genes involved in biofilm formation (icaA and bap). To assess the epidemiological relation of MRSA strains we used multiple-locus variable-number tandem repeat analysis (MLVA).
Material and methods
Bacterial strains
Eighty MRSA were collected from treated patients in a large hospital in Poznań for 4 years (Table 1.). The bacteria were identified by conventional methodology and using Vitek 2 system (bioMérieux, France). All isolates were resistant to oxacillin, which was confirmed by the presence of mecA gene (Geha et al. 1994).
Detection of slime production by Congo red agar plate test and biofilm assay
The ability to form slime was tested on Congo red agar (Arciola et al. 2002). Briefly, isolates were incubated on brain heart infusion agar (Oxoid) supplemented with 0.8 μg/mL of Congo red (Sigma) and 36 μg/mL of sucrose. The plates were subsequently incubated for 24 h at 37 °C and additionally overnight at room temperature. Slime-producing strains develop black colonies, whereas slime non-producing isolates form red colonies. Biofilm formation of S. aureus cell on polystyrene was quantified by using the microtiter plate assay (Kim et al. 2008; Fredheim et al. 2009). Isolates were cultivated overnight in 96-well flat-bottomed tissue culture plates at 37 °C with trypticase soy broth (bioMérieux, France) supplemented with 0.25 % glucose as a growth medium. After incubation, the cultures were gently removed, the wells were washed three times with phosphate-buffered saline, and air dried and stained with 0.4 % crystal violet solution for 10 min. The plate was washed, the adherent cells were resuspended in ethanol–acetone mixture (70:30), and finally the absorbance at 490 nm was determined. Isolates were considered biofilm-positive if they had an A 490 > 0.25. Each isolate was tested in triplicate.
Confocal laser scanning microscopy
Overnight cultures of different strains were added to cell culture chamber wells 9Lab-TekII, (Nalge Nunc International) and statically incubated for 24 h as described by Qin et al. (2007). Biofilms and bacteria were stained by using SYTO 9 and propidium iodide (PI) (Live/Dead BacLight Bacterial Viability kits, Invitrogen) for 15 min and observed under the microscope (Carl Zeiss LSM 510-Axioveut 200 M) equipped with detector and filter sets for monitoring SYTO9 and PI. The Carl Zeiss confocal software was used for the analysis of biofilm images, which allowed for collections of stacks three-dimensional reconstruction.
Detection of adhesins and biofilm-encoding genes
Isolation of DNA was performed by using the Genomic DNA Plus kit (A&A Biotechnology, Poland). A multiplex polymerase chain reaction (PCR) was applied for simultaneous amplification of the bbp, can, eno, ebps, fnbB, fnbA, fib, clfA, and clfB genes (Tristan et al. 2003). Another PCR assay was applied to determine the presence of the icaA and bap genes (Cucarella et al. 2001; Peacock et al. 2002). The amplification products were electrophoresed in 1.5 % agarose gel, stained with ethidium bromide, visualized on a UV light transilluminator, and documented with V.99 Bio-Print system (Vilber Lourmat, Torcy, France).
Multiple-locus variable-number tandem repeat analysis
A PCR was applied to simultaneously amplify part of the hypervariable number of tandem repeat (VNTR) regions of the spa, sspsA, clfA, clfB, sdrC, sdrD, and sdrE genes (Sabat et al. 2003). GelCompar II (version 3.5; Applied Maths, Kortrijk, Belgium) software was used to analyze the MLVA banding patterns. The Dice band-based similarity coefficient was calculated with band position tolerance of 1 %. MLVA dendrogram was constructed by unweighted pair-group method (UPGMA) with arithmetic means. We defined clusters as two or more isolates with more than 95 % similarity.
Results and discussion
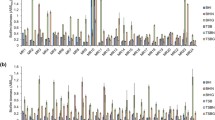
Our results indicated that the vast majority (93 %) of S. aureus isolates had the ability to produce biofilm in vitro. Of the 74 biofilm-positive strains, 56 carried the icaA (76 %) gene and produced slime on Congo red agar (CRA). Arciola et al. (2001) have reported that only 61 % of S. aureus strains from catheter-associated infections carry icaA genes and produce slime on CRA. Data reported by others have indicated that nearly all S. aureus strains carry ica genes (Peacock et al. 2002; Rodhe et al. 2007). Our results indicated that two icaA-positive strains did not have the ability to produce slime and form biofilm. It could be explained by appearance of rare slime-negative mutants, which emerged as a result of passages and prolonged culture on CRA plate (Arciola et al. 2001). It is also well-known that the ica expression is strongly influenced by environmental factors such as glucose, temperature, osmolarity, and growth in anaerobic conditions (Beenken et al. 2004; Kim et al. 2008). None of the MRSA strains were positive for bap genes. We found that 23 % of MRSA strains were biofilm-positive although they did not carry icaA and bap genes. These data provided evidence for ica-independent biofilm formation in MRSA strains. Our results are coincident with the findings reported by O’Neill et al. (2007, 2008) and Izano et al. (2008). It should be noted that 50 % of MRSA strains carry multifunctional surface proteins fibronectin-binding protein B (FNBP). According to O’Neill et al. (2008), FNBP proteins are involved in intercellular accumulation and biofilm development in S. aureus strains. Importantly, 9 of 18 ica-negative and biofilm-positive strains carry fnbB genes, whereas 9 of 18 ica-negative and biofilm-positive strains did not carry fnbB genes. The absence of PIA, BAP, and FNBP among these nine biofilm-positive strains strongly suggests that S. aureus may use also different strategies to form biofilm. It has been indicated that DNA is a structural component of the S. aureus matrix and extracellular DNA fragments of >11 kb can function as intercellular adhesins (Izano et al. 2008). Furthermore, wall teichoic acids have been shown to participate in biofilm cohesion (Gross et al. 2001). We compared the architecture of biofilm created by S. aureus strains by using confocal laser scanning microscopy (CLSM). Six biofilm-positive S. aureus strains were selected for this study: two strains (Bacteriology Collection of Department of Microbiology A. Mickiewicz University, Poznań (MPU S) 8 and 14) positive for ica genes, two (MPU S 6 and 13) positive for fnbB, and two (MPU S 23 and 37) negative for ica genes and fnbB gene. We analyzed the entire thickness of bacterial biofilms as well as the surface of chamber well coverage of biofilms. Figure 1 exemplifies the differences between the ica-positive (Fig. 1a) and ica-negative strains (Fig. 1b, c). Microscopic images showed that the two ica-positive strains created the thickest biofilms with compact architecture. The thickness of biofilm formed by MPU S 8 and 14 strains was about 25 and 23 μm, respectively. In contrast, biofilm created by ica-negative strains was much thinner than that of ica-positive strains. Four S. aureus strains (MPU S 6, 13, 23, and 37) formed relatively thin biofilm structures; the average thickness of biofilm formed by ica-negative strains was 13 μm. Beside the differences in biofilm thicknesses, we also observed the differences in the area covered by biofilm. The CLSM images of MPU S 6, 13, 23, and 37 S. aureus biofilms showed that not all chamber wells were covered by multiple layers of cells. The structures of these biofilms were relatively loose and there were areas on chamber wells with only loosely attached cells or cellular aggregates. It should be emphasized that S. aureus MPU S 8 and 14 formed biofilm structures in which bacterial cells were very closely packed. The common characteristic of biofilm produced by the S. aureus strains was the low number of dead cells observed in mature biofilms (Fig. 1). However, we observed more dead cells in the ica-negative strains.
CLSM images of 24-h biofilms of S. aureus MPU S 8 (a), MPU S 6 (b), and MPU S 37 (c) stained with SYTO9 (green cells) and PI (red cells). The ica-positive strain MPU S 8 showed compact structure. The ica-negative/FNBP-positive strains (MPU S 6) and ica-negative/FNBP-negative strain (MPU S 37) showed less condensed biofilm structures than MPU S 8 strain. The common characteristic of biofilm produced by the S. aureus strains was the low number of dead cells (red cells) observed in mature biofilms. Bar = 20 μm
The vast majority of the isolates were positive for genes encoding fibrinogen-binding protein (ClfA, ClfB, and Fib) and eno gene encodes laminin-binding protein. The genes encoding fibronectin-binding protein A (fnbA) were absent in all isolates, whereas fnbB genes encoding fibronectin-binding protein B (fnbB) were present in 50 % of the strains. None of the S. aureus strains from our collection carried genes encoding bone sialoprotein-binding protein. The lack of bbp genes in S. aureus isolated from urinary tract infections has been also reported by Baba-Moussa et al. (2008). Rodhe et al. (2007) have observed high prevalence of bbp genes in S. aureus strains isolated from prosthetic hip and knee joint infections. This discrepancy in the results may be related to the site of infection from which the strains were isolated. The gene encoding the elastin-binding protein (ebpS) was present only in 15 % of the MRSA, whereas the gene encoding collagen-binding protein was present in 45 % of S. aureus strains.
In this study, we used MLVA analysis to evaluate the clonal structure of S. aureus strains. It has been found that MLVA method is very useful to determine clonality and the spread of S. aureus strains in hospitals in short-time epidemiological investigation (Łuczak-Kadlubowska et al. 2008). On the basis of MLVA profiles, we identified 12 clusters at 100 % similarity level (Fig. 2). As shown in the dendrogram, the largest cluster comprised four isolates. The remaining clusters consisted of two to three strains. The fact that 12 clusters were identified next to 52 unique genotypes indicates a large genetic diversity among S. aureus strains. We compared the presence of icaA, fib, fnbB, cna, eno, and ebpS within each of the 12 clones. The clfA, clfB, fnbA, bbp, and bap genes were excluded from this comparison, as they were present or absent in almost all isolates. Seven clusters included both ica-positive and ica-negative strains, whereas five clusters included only ica-positive strains. Ten clusters contained isolates that carried different genes (fib, fnbB, cna, eno, and ebpS) mediating adhesion to host matrix proteins. Hence, the distribution of individual genes was not influenced by the clonality of the population. Taken these results together, we did not find a specific clone occurring preferentially in the hospital environment. There was also evidence that most MRSA strains were able to produce slime and form biofilm in ica-dependent mechanisms. On the other hand, we found some clinical strains that produced biofilm in ica-independent mechanisms.
Abbreviations
- Bap:
-
Biofilm-associated protein
- CLSM:
-
Confocal laser scanning microscopy
- CRA:
-
Congo red agar
- FNBP:
-
Fibronectin-binding protein B
- MLVA:
-
Multiple-locus variable-number tandem repeat analysis
- MPU S:
-
Bacteriology Collection of Department of Microbiology A. Mickiewicz University, Poznań
- MRSA:
-
Methicillin-resistant S. aureus
- MSCRAMMs:
-
Microbial surface components recognizing adhesive matrix molecules
- PCR:
-
Polymerase chain reaction
- PI:
-
Propidium iodide
- PIA:
-
Polysaccharide intercellular adhesion
References
Arciola CR, Baldassarri L, Montanaro L (2001) Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol 39:2151–2156
Arciola CR, Campoccia D, Gamberini S, Cervellati M, Donati E, Montanaro L (2002) Detection of slime production by jeans o fan optimised Congo red agar plate test based on a colourimetric scale in Staphylococcus epidermidis clinical isolates genotyped for ica locus. Biomaterials 23:4233–4239
Baba-Moussa L, Anani L, Scheftel JM, Couturier M, Riegel P, Haїkou N, Hounsou F, Monteil H, Sanni A, Prévost G (2008) Virulence factors produced by strains of Staphylococcus aureus isolated from urinary tract infections. J Hosp Infect 68:32–38
Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS (2004) Global gene expression in Staphylococcus aureus biofilms. J Bacteriol 186:4665–4684
Cucarella C, Solano C, Valle J, Amorena B, Lasa Í, Penadés JR (2001) Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183:2888–2896
Fredheim EGA, Klingenberg C, Rodhe H, Frankenberger S, Gaustad P, Fllaegstad T, Sollid JE (2009) Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol 47:1172–1180
Geha DJ, Uhl JR, Gustaferro CA, Persing DH (1994) Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol 32:1768–1772
Götz F (2002) Staphylococcus and biofilms. Mol Microbiol 43:1367–1378
Götz F, Bannerman T, Schleifer K-H (2006) The genera Staphylococcus and Macrococcus. The prokaryotes, 3rd edn. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt (eds) A handbook on the biology of bacteria. Bacteria: Firmicutes, Cyanobacteria, vol. 4 (ed). Springer Science+ Business Media LLC, New York, 5–75
Gross M, Cramton SE, Götz F, Peschel F (2001) Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun 69:3423–3426
Izano EA, Amarante MA, Kher WB, Kaplan JB (2008) Differential roles of poly-N acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol 74:470–476
Kim J, Kim C, Hacker J, Ziebuhr W, Lee BK, Cho S (2008) Molecular characterization of regulatory genes associated with biofilm variation in a Staphylococcus aureus strain. J Microbiol Biotechnol 18:28–34
Łuczak-Kadlubowska A, Sabat A, Tambic-Andrasevic A, Payerl-Pal M, Krzyszton-Russjan J, Hryniewicz W (2008) Usefulness of multiple-locus VNTR fingerprinting in detection of clonality of community- and hospital-acquired Staphylococcus aureus isolates. Antonie Van Leeuwenhoek 94:543–553
Mack D, Rohde H, Harris LG, Davies AP, Horstkotte MA, Knobloch JK-M (2006) Biofilm formation in medical device-related infection. Int J Artif Organs 29:343–359
O’Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O’Gara P (2007) Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolated from device-related infections. J Clin Microbiol 45:1379–1388
O’Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, Loughman A, Foster T, O’Gara P (2008) A novel Staphylococcus aureus biofilm phenotype mediated by the fibtonectin-binding proteins, FnBPA and FnBPB. J Clin Microbiol 190:3835–3850
Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, O’Neill G, Day NPJ (2002) Virulent combinations of adhesion and toxin genes in natural populations of Staphylococcus aureus. Infect Immun 70:4987–4996
Qin Z, Yang X, Yang L, Jiang J, Qu Y, Molin S, Qu D (2007) Formation and properties of in vitro biofilms of ica-negative Staphylococcus epidermidis clinical isolates. J Med Microbiol 56:83–93
Rodhe H, Burandt EC, Siemssen N, Frommelt L, Burdelski Ch, Wurster S, Scherpe S, Davies A, Harris LG, Horstkotte MA, Knobloch JK-M, Ragunath Ch, Kaplan JB, Mack D (2007) Polysaccharide intercellular adhesion or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and joint infections. Biomaterials 28:1711–1720
Sabat A, Krzyszton-Russjan J, Strzalka W, Filipek R, Kosowska K, Hryniewicz W, Travis J, Potempa J (2003) New method for typing Staphylococcus aureus strains: multiple-locus variable-number tandem repeat analysis of polymorphism and genetic relationships of clinical isolates. J Clin Microbiol 41:1801–1804
Tristan A, Ying L, Bes M, Etienne J, Vandenesch F, Lina G (2003) Use of multiplex PCR to identify Staphylococcus aureus adhesions involved in human hematogenous infections. J Clin Microbiol 41:4465–4467
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szczuka, E., Urbańska, K., Pietryka, M. et al. Biofilm density and detection of biofilm-producing genes in methicillin-resistant Staphylococcus aureus strains. Folia Microbiol 58, 47–52 (2013). https://doi.org/10.1007/s12223-012-0175-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-012-0175-9