Abstract
The impact of probiotic supplementation of canine-derived strain Lactobacillus fermentum AD1-CCM7421 in freeze-dried form on quantitative composition of microbiota and short-chain fatty acid profile in feces of dogs was demonstrated by two independent studies (straightforward repeated-measures model; study I: a dose of 2 g per dog for 2 weeks, 108 CFU/g, n = 12; study II: 1 g per dog for 1 week, 107 CFU/g, n = 11. The results revealed a significant increase of lactic acid bacteria population persisting also after the cessation of probiotic application in both studies. A reduction of clostridia (study I, p sum < 0.01) and tested Gram-negative bacterial genera (coliforms, Aeromonas sp., Pseudomonas sp., study II, p < 0.05) was also detected. The strain AD1-CCM7421 colonized the canine digestive tract in sufficient numbers (105–106 CFU/g) and it persisted in the majority of dogs after cessation of probiotic application. An increase of short-chain fatty acid concentrations (study I: butyric, succinic, valeric, formic acid) especially in the early post-treatment phase (p < 0.05) most likely led to a decrease of fecal pH value (p < 0.05) without negative influence on fecal consistency throughout the studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The importance of balancing or improving intestinal microbiota also in dogs is supported by the current evidence of frequent medical problems connected with the gastrointestinal tract which are reported to be at the 3rd and 4th place in canine health problems (Vet Pet Insurance 2011). The use of probiotic microorganisms could be of great benefit to dogs, also taking into account the low microbial supply by currently preferred commercial food products (expansion–extrusion process above 100 °C).
In this study, the effect of lyophilized form of probiotic, canine-derived Lactobacillus fermentum AD1-CCM 7421 strain was evaluated (our isolate, deposited in the Czech Collection of Microorganisms in Brno, Czech Republic, protected as the Utility model no. 4914 by Industrial Property Office of the Slovak Republic) in relation to microbiology, consistency and short-chain fatty acids (SCFA) detected in fecal samples of healthy dogs.
Materials and methods
Animals and diet
Two independent canine studies (straightforward repeated-measures experimental design) with different time of treatment period and sample collections were carried out. Experiment I: 14-day application (n = 12, rifampicin-marked strain L. fermentum AD1-CCM7421 2 g per dog, 108 CFU/g of milk powder); experiment II: 7-day application (n = 11, 1 g per dog, 107 CFU/g). Dogs were housed individually in a fully environmental but covered facility sized 3.0 × 3.0 m with a 1.5 × 0.8 m box. They had access to fresh water at all times and received commercial extruded dry dog food twice a day (Purina Pro Plan Dog Adult Medium Breed; Nestlé Purina PetCare Company, USA; crude protein 27.0 %, crude fat 17.0 %, crude fiber 2.0 %, ash 7.0 %, 15 MJ (3,580 kcal) metabolizable energy per kg). Experimental procedures were approved by the Ethic Commission of the Institute of Animal Physiology, Slovak Academy of Sciences (Košice, Slovakia).
Sampling procedures
The fresh fecal samples were collected (study I: days 0, 7, 14, 21, 28, 49; study II: days 0, 7, 21) during morning individual walking. The determination of fecal score (visually: 1 = hard, dry feces; 2 = hard, formed; 3 = soft, formed, moist; 4 = soft, unformed; 5 = watery liquid) and pH measurement were performed immediately.
Microbiological analysis
The samples of feces (1 g) were mixed with sterile Ringer buffer (pH 7.0; Merck, Germany) and homogenized using a stomacher (IUL Instruments, Spain). Microbial populations were determined according to the standard microbiological method by serial dilution onto the following selective media: MacConkey agar (Becton Dickinson, USA), Mannitol salt agar (Oxoid, UK), M-Enterococcus agar (Becton Dickinson), MRS agar (Becton Dickinson), MRS agar with rifampicin (100 μg/ml) for L. fermentum AD1-CCM 7421, Pseudomonas agar (Biomark, India), Aeromonas medium base (Oxoid) and Clostridium difficile agar base (Oxoid). Plates were incubated aerobically at 37 °C for 24–48 h except for Aeromonas and Pseudomonas plates incubated at 25 °C for 48–72 h. Clostridia were cultivated at 37 °C for 48 h using anaerobic conditions. The results were expressed as log10 CFU per gram of feces.
SCFA analysis
Feces (1 g) was diluted in 100 ml deionized water, homogenized (stomacher; IUL Instruments) and filtered through a filter paper. A sample of 30 μl was used for analysis of SCFA (formic, lactic, succinic, acetic, propionic, butyric, valeric acids) by capillary isotachophoresis (Isotachophoretic analyser ZKI 01; Radioecological Institute, Košice, Slovakia). A leading electrolyte of the following composition was used in the pre-separatory capillary: 10 mmol/l HCl + 22 mmol/l ε-aminocaproic acid + 0.1 % methyl-hydroxyethylcellulosic acid, pH = 4.3. A solution of 5 mmol/l caproic acid + 20 mol/l histidine was used as a finishing electrolyte. This electrolytic system worked at 250 μA in the pre-separatory and 50 μA in the analytic capillary.
Statistical analysis
The repeated-measures analysis of variance (ANOVA; Dunnett’s post-test) with the level of significance set at p < 0.05 was used to evaluate each experiment.
Results and discussion
The microbial analysis of fecal samples revealed a significant increase of lactic acid bacterial (LAB) population during the treatment phase in both canine experiments differing in the length of L. fermentum CCM 7421 application (p sum < 0.05 and p sum < 0.001, respectively; Table 1). In addition, higher counts of LAB were also noted after cessation of probiotic supplementation. The probiotic strain CCM 7421 was detected in fecal levels up to 105–106 CFU/g during the treatment phase and in the amount of 102–104 CFU/g in the post-treatment phase (days 21, 28 and 49). The significant decrease of clostridial population (monitored only in study I) of up to 2 log cycle on day 14 (p sum < 0.01) may be beneficial to the dog because it can provide prevention against diseases caused by C. difficile or C. perfringens.
A similar decrease of clostridia was detected also by Baillon et al. (2004) after L. acidophilus DSM 13241 application to healthy dogs for 4 weeks. However, the possible mechanisms of this effect are not well understood; animal and cell culture studies demonstrated that some probiotic strains can stimulate immune function, resist C. difficile colonisation, and hydrolyze C. difficile toxin (Castagliuolo et al. 1996; Buts 2009).
The counts of tested Gram-negative bacterial genera tended to decrease especially when the probiotic strain CCM AD1-7421 was supplemented to dogs for 1 week (study II; Table 1). In detail, the fecal populations of Aeromonas sp. (p sum < 0.05), Escherichia coli (p sum < 0.05) and Pseudomonas sp. (p sum < 0.05) were significantly lower after 1 week of application (day 7). A similar decreasing tendency was also indicated in the study I but the changes were not significant. Consistency of feces was not changed during the experiments (according to the fecal score; Table 1).
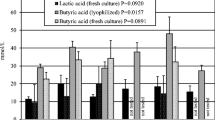
A significant decrease of fecal pH was observed in both experiments on day 21 (p < 0.05; Table 1). The pH value continued to decrease in the post-treatment phase with a maximum decrease 2 weeks after the end of the probiotic application.
This interesting fact supports the results of the fecal concentration of tested SCFA, most of which reached the highest concentration just on day 21 (or on day 28; Table 2). The total concentration of SCFA (lactic, acetic, propionic and butyric acid) was also the highest on day 21. According to Hoshi et al. (1994), lactic and succinic acids, but not SCFA, are major determinants of lumen pH in the large intestine. The concentrations of the following acids increased significantly: formic (p < 0.01, day 21), succinic (p < 0.05, days 21 and 49), butyric (p < 0.05, day 28) and valeric acid (p sum < 0.001). An increase of SCFA in the post-treatment phase may be due to an accumulation ability (slower absorption) of some acids in the intestinal lumen (Umesaki et al. 1979) produced by bacterial fermentation stimulated by the addition of the probiotic. These SCFA, especially propionic, formic, butyric and lactic acids, which have a broad antimicrobial spectrum including also yeasts and fungi, could contribute to the observed antimicrobial effect (Naughton and Jensen 2001).
In summary, L. fermentum CCM 7421 strain brought about a positive shift in intestinal microbial community in favor of lactic acid bacteria at the expense of unfavorable Gram-negative genera together with an increase of SCFA important in the colonic ecology and in various systemic metabolic functions. This effect was more pronounced in the study in which a lower dose and shorter length of probiotic treatment was used.
References
Baillon MLA, Marshall-Jones ZV, Butterwick RF (2004) Effects of probiotic Lactobacillus acidophilus strain DSM13241 in healthy adult dogs. Am J Vet Res 65:338–343
Buts JP (2009) Twenty-five years of research on Saccharomyces boulardii trophic effects: updates and perspectives. Dig Dis Sci 54:15–18
Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C (1996) Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun 64:5225–5232
Hoshi S, Sakata T, Mikuni K, Hashimoto H, Kimura S (1994) Galactosylsucrose and xylosylfructoside alter digestive tract size and concentrations of cecal organic acids in rats fed diets containing cholesterol and cholic acid. J Nutr 124:52–60
Naughton PJ, Jensen BB (2001) A bioreactor system to study survival of Salmonella typhimurium in pig gut content. Berl Münch Tierärztl Wochenschr 114:378–381
Umesaki Y, Yajima T, Yokokura T, Mutai M (1979) Effect of organic acid absorption on bicarbonate transport in rat colon. Pflügers Arch 379:43–47
Veterinary Pet Insurance Co. (2011) Top 10 Pet Medical Conditions of 2010. http://press.petinsurance.com/pressroom/02222011Pet_Conditions_2010.aspx
Acknowledgement
The study was supported by the Slovak Scientific Agency VEGA, project no. 2/0005/09.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strompfová, V., Lauková, A. & Gancarčíková, S. Effectivity of freeze-dried form of Lactobacillus fermentum AD1-CCM7421 in dogs. Folia Microbiol 57, 347–350 (2012). https://doi.org/10.1007/s12223-012-0139-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-012-0139-0