Abstract
Chitosan (CS), as a biocompatible and biodegradable nature polymer, has excellent antibacterial property. Poly(ethylene oxide) (PEO), as a biomaterial with thermal stability, biocompatibility and biodegradability, has good spinnability. Anthocyanin, as a natural colorant agent with different molecular structures under different pH values, has good antibacterial and antioxidant properties and can be applied as a pH indicator. Electrospun nanofiber membranes (NFMs) show great application prospects in food packaging due to their unique structural and functional advantages. In this work, anthocyanin/CS/PEO nanofiber membranes (NFMs) for food packaging were batch prepared by the spherical section free surface electrospinning (SSFSE), and their yield reached 5.8 ± 0.4 g/h. The morphology, structure and properties of CS-based NFMs were explored, which showed that they were hydrophilic and had excellent antibacterial activity against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). Among them, anthocyanin/CS/PEO NFMs exhibited better mechanical properties and high antioxidant activity. Furthermore, the color changes of anthocyanin/CS/PEO NFMs at different pH values were investigated, illustrating that they could be used as a visual monitoring food packaging, and their application effects in beef storage were studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanofiber membranes (NFMs) prepared by electrospinning (ES) technology have many advantages in food packaging applications, such as its high specific surface area and high porosity, which are suitable for the release of active agents; ES process does not involve high-temperature conditions, which can ensure the stability of active agents in the production process; the rapid evaporation of solvent during ES greatly reduces the safety problems caused by toxic solvents in electrospun materials; electrospun raw materials for food packaging are widely available. Therefore, the application of ES technology in food packaging has attracted extensive attention and researches [1].
Chitosan (CS) is a cheap, safe, non-toxic natural polymer with excellent antibacterial effect and biodegradability [2, 3]. However, it is a cationic polymer with high density charges, which leads to high repulsion between its ionic groups, poor spinnability of pure CS solution, and poor mechanical properties of NFMs prepared thereby [4, 5]. In order to improve the spinnability of CS and the mechanical properties of NFMs, CS can be blended with other natural or synthetic polymers to prepare NFMs [6]. Electrospun CS-based NFMs have a unique three-dimensional network structure, which can have a good barrier to oxygen and water vapor, and have good air permeability and hydrophilicity. As food packaging materials, they can absorb water released during food storage, thus promoting their freshness preservation. Moreover, they exhibit good antibacterial properties and can be used in antibacterial food packaging [7]. Therefore, electrospun CS-based NFMs are very suitable for food packaging [8, 9].
PEO is a good biomaterial with thermal stability, biocompatibility and biodegradability, which is non-toxic and has good spinnability [7]. It is often used to blend with CS to promote the spinnability of CS. Pakravan et al. [10] used traditional single ES(SNES) to prepare CS/PEO NFMs with different component proportions, and studied the rheological properties of CS/PEO spinning solution and the promotion of PEO on CS spinnability.
In order to provide visual monitoring capability for CS/PEO NFMs used in food packaging and meet the needs of different food packaging applications, anthocyanins, which can serve as pH acid–base indicators [11,12,13], were added to CS/PEO NFMs. Anthocyanin is a safe and non-toxic water-soluble natural food pigment, belonging to polyphenols, with multiple functions and nutritional values, which widely exists in fruits, vegetables and other plants [14]. Because of its antioxidant property, anthocyanin is widely used for scavenging pure radicals [15]. Furthermore, it exhibits different molecular structures under different PH values, resulting in its color changing with pH values [16]. Accordingly, many studies have utilized this characteristic to prepare pH acid–base indicators. Especially, during the spoilage process of meat products, proteins undergo decomposition reactions under the action of microorganisms, generating volatile alkaline nitrogen compounds and other metabolic gases, causing changes in the pH value of the surrounding environment of spoiled meat products. Therefore, pH sensitive anthocyanins can be used as indicators to reflect the metamorphism degree of meat products, making food monitoring visual [11]. In addition, anthocyanins also have antioxidant, anticancer, and anti-inflammatory properties [17], making them very suitable as a food packaging material.
In our previous work [8], CS/PEO NFMs were prepared in batch by the modified free surface ES device (MFSE), and its yield was 3.1 ± 0.1 g/h. In this paper, in order to further improve the yield of NFMs for smart food packaging that can monitor changes in food quality in real-time, a spherical section free surface ES (SSFSE) device was used to prepare anthocyanin/CS/PEO NFMs in batches, and their yield reached 5.8 ± 0.4 g/h, while the yield of CS/PEO NFMs was also increased to 6.0 ± 0.5 g/h. Herein, anthocyanins used were extracted from purple cabbage, and the color changes of the anthocyanin extraction solution and anthocyanin/CS/PEO NFMs at different pH values were discussed. Finally, the application effects of anthocyanin/CS/PEO NFMs as a visual monitoring food packaging in beef storage were studied, indicating that they had great application potential in the field of smart food packaging.
2 Materials and Methods
2.1 Materials
Purple cabbage was purchased from supermarket. Citric acid (ASC, ≥ 95%) was purchased from Aladdin Biochemical Technology Co Ltd. (Shanghai, China). Ethanol (95%), acetic acid (AA, analytical pure), hydrochloric acid (analytical pure) and sodium hydroxide (analytical pure) were purchased from Jiangsu Qiangsheng Functional Chemical Co Ltd. (Suzhou, China). Phosphate buffer saline (without calcium and magnesium) (PBS, pH = 7) was offered from Corning Co Ltd. (Glendale, USA). 1,1-Diphenyl-2-trinitrophenylhydrazine (DPPH) were obtained from Suzhou Shengnokang Biotechnology Co Ltd. Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) were supplied by Soochow University. Nutrient agar and nutrient broth mediums were obtained from SCAS Ecoscience Technology Inc. Na2HPO4 and KH2PO4 were supplied by Sinopharma chemical reagent Co., Ltd. (Shanghai, China).
2.2 Preparation of Anthocyanins and Their Response Tests at Different pH Values
50 g of washed and dried purple cabbages was weighed, cut into pieces, and placed in a beaker. 200 ml of ethanol with 1% hydrochloric acid was added in the beaker, and the cabbage pieces were soaked at room temperature for 24 h. The cabbage was taken out, and the filtered solution was placed into a rotary evaporator for evaporation and concentration until it became viscous, and then it was dried in an oven to obtain a purple red crystalline solid, which was anthocyanins extracted from purple cabbages. The anthocyanin solid was ground into powders and weighed to calculate the extraction rate.
Citrate buffer solution (CBS) with pH = 3 and hydrochloric acid-sodium hydroxide buffer solution (HSBS) with pH = 11 were prepared, respectively. A certain amount of extracted anthocyanin powders were weighed and added to solutions with different pH values. The color changes of these solutions were observed and recorded.
2.3 Preparation of Spinning Solution
According to our previous work [18], a certain amount of extracted anthocyanin powders were added to the optimal CS/PEO mixed solution (60 wt% AA, 4 wt% CS/PEO) prepared, in which the weight ratio of mixed solute CS/PEO to anthocyanin was 3:1. The mixed solution was stirred in a magnetic stirrer for 12 h to obtain a uniformly mixed anthocyanin/CS/PEO spinning solution.
2.4 SSFSE Process and Batch Preparation of NFMs
As shown in Fig. 1, the SSFSE device was used to prepare anthocyanin/CS/PEO NFMs in batch. According to the previous work, the spinning parameters were set as follows: the applied voltage was 60 kV, and the spinning distance was 18 cm. ES experiments were conducted at room temperature (20 °C) and 60% relative humidity. The prepared spinning solution was poured into the solution reservoir, and the high-voltage power supply was slowly turned on. After applying the electric field force, the surface tension of the solution was overcome, multiple jets were excited on the solution surface, and the jets were stretched and refined to form nanofibers on the receiving device.
2.5 Measurements and Characterizations
2.5.1 Property of Spinning Solutions
The viscosity and conductivity of spinning solutions were determined by a viscometer (SNB-1, FangRui Instrument Co., Ltd., Shanghai, China) and a conductivity meter (DDS-307A, Shanghai IE Scientific Instrument Co., Ltd., Shanghai, China), respectively. All measurements were repeated five times at room temperature to obtain the average value.
2.5.2 Yield of NFMs
After spinning for 30 min under a stable condition, the NFMs prepared by SSFSE apparatus were weighed using a precise electronic balance. And the test was repeated five times for obtaining the average value.
2.5.3 Surface Morphology and Element Compositions of NFMs
The surface morphology and diameter distribution of NFMs were observed by SEM. All samples were dried at 20 °C and then sprayed with gold 90 s to improve their conductivity. 10 SEM images of each sample and 100 nanofibers in each SEM image were randomly selected, and further analyzed by ImageJ to obtain the diameter distribution of NFMs.
2.5.4 Attenuated Total Reflection-Flourier Transform Infrared (ATR-FTIR) Spectra of NFMs
FTIR spectra of nanofiber membranes were obtained by ATR-FTIR (Nicolet5700, Thermo Nicolet Company, Waltham, MA, USA). The test parameters were 32 scans on average, with a spectral resolution of 4 cm−1 and wave numbers ranging from 400 to 4000 cm−1.
2.5.5 X-ray Diffraction (XRD) of NFMs
The crystalline structures of NFMs were measured by an X-ray diffractometer (XRD, Philips X’Pert-Pro MPD, PANalytical, Holland). All samples were measured and placed on the sample plate. The test parameters were a voltage of 40 kV, a current of 40 mA, a radiation source of Cu target, a scan angle range of 5–70° and a scan speed of 2°/min.
2.5.6 Mechanical Property of NFMs
The mechanical properties of NFMs were investigated by a universal electromechanical test machine Instro-3365 (Intro, Norwood, MA, USA). All samples were 40 mm × 20 mm NFMs and were placed at relative humidity of 60% and 20 °C for 24 h to stabilize the structure of NFMs. The measurement parameters were: the pretension was 0.2 cN, the clamping length was 20 mm, and the drawing rate was 100 mm/min. The test was repeated five times for accuracy.
2.5.7 Wettability of NFMs
The water contact angle (WCA) measurement was carried out using a CA tester (Krüss Company, Hamburg, Germany) to indicate the wettability of NFMs. The samples were cut into 30 mm × 30 mm square membranes and placed in a vacuum dryer overnight. The four corners of the sample were flattened by a glass slide to remove the impact of its surface on the test data. The volume of droplet used was 3μL. The WCA measurements for each sample were conducted at five different locations on the same sample.
2.5.8 Antioxidant Test
DPPH (0.00394 g) was diluted with ethanol to 100 mL for obtaining a 39.4 mg/L DPPH solution. The NFMs were placed in a 6-well plate, 3 ml of DPPH solution was added to each well, and the absorbance value of each sample was measured at the maximum wavelength of 517 nm. The measurement was conducted in triplicate. The free-radical scavenging rate was calculated as follows:
where A0 and A1 indicate the absorbance values of blank and sample, respectively.
2.5.9 Antibacterial Property of NFMs
The antibacterial activity of the NFMs against S. aureus and E. coli was characterised by antibacterial test. PBS was prepared by adding 2.84 g Na2HPO4 and 1.36 g KH2PO4 to 1 L of deionized water. To reduce the effect of other irrelevant parameters on the antibacterial test, all the samples were disinfected with ultraviolet (UV) light for 1 h. Individual colonies were incubated in a nutrient medium for 18–24 h to obtain a bacterial solution. A diluted bacterial solution with a colony concentration of 3–5 × 105 CFU/ml was prepared by mixing 1 ml of bacterial solution with 9 ml of PBS solution.
150 mg of the test sample was placed in a conical flask containing 1 ml of diluted bacterial solution and 14 ml of self-made PBS. The flask was shaken well in an incubator shaker and kept at 25 °C for 18 h. Afterwards, 1 ml of the solution was removed from the flask and poured into a nutrient agar plate (20 ml of agar), which were placed in a thermostatic shaker and incubated at 37 °C for 24 h. Finally, the contamination status was observed and bacterial colony counts were obtained. In addition, the antibacterial rate was calculated using the following formula:
where Nc is the bacterial colony count of the standard cotton cloth after 24 h of contact with bacteria, Ns is the bacterial colony count of CS-based NFMs after 24 h of contact with bacteria.
2.5.10 Response Tests of NFMs at Different pH Values
CS/PEO NFMs with and without anthocyanins were separately immersed in three buffer solutions with different pH values, namely CBS with pH = 3, PBS with pH = 7, and HSBS with pH = 11. The color changes of NFMs were observed and recorded.
2.5.11 Application of NFMs in Beef Storage Period
CS/PEO NFMs with and without anthocyanins were separately cut into 5 cm × 5 cm squares. 10 g of beef was weighted and placed on a petri dish. The NFM sample was fixed inside the petri dish cover. The changes of the beef and NFMs were observed and recorded every 24 h, lasting for 5 days at room temperature.
3 Results and Discussion
3.1 Anthocyanin Contents in Purple Cabbage
Table 1 shows that an average of 2.7 g anthocyanin can be extracted from every 50 g of purple cabbage. The purple cabbage used in the experiment was the same, but the time interval between each experiment was 4–5 days. It can be seen that the anthocyanin content slightly decreases over time, which may be due to the oxidation and degradation of anthocyanins by enzymes during the storage of purple cabbage, resulting in poor stability of anthocyanins [19, 20]. The stability of anthocyanins can be effectively promoted by encapsulating anthocyanins in nanofibers through ES technology.
3.2 Response of Anthocyanins at Different pH Values
Table 2 shows the color changes of anthocyanins extracted from purple cabbage in different pH solutions. It can be seen that adding anthocyanins to solutions with different pH values causes color changes in transparent and colorless solutions, appearing purple red in acidic solutions with pH = 3, blue in neutral solutions with pH = 7, and yellow in alkaline solutions with pH = 14. This is because the molecular structure of anthocyanins changes in solutions with different pH values, resulting in color changes of solutions. In acid solutions, the main molecular structures of anthocyanins are flavylium cations (red), and the color of solutions is mainly red. When the pH value of solution is 4.0–7.0, their molecular structures gradually transform into quinoidal bases (blue), and the red color of the solution weakens while its blue color appears. When pH is 8.0–9.0, the molecular structures of anthocyanins are mainly carbinols (colorless) and quinoidal bases, and the solution appears blue-green. When pH > 11, their structures are converted to chalcones (yellowish), and the solution shows yellow [21].
3.3 Solution Viscosity and Conductivity
The solution viscosity and conductivity have significant impacts on the yield and morphology of the electrospun NFMs [22]. The polymeric spinning solution must have appropriate electrical conductivity to supply a repulsive force sufficient to overcome the surface tension of droplets at the spinneret, thus forming a polymer jet [23]. The viscosity and conductivity of solutions are indicated in Table 3. It can be seen that with the addition of anthocyanin, the solution viscosity and conductivity increased accordingly. The increase in solution viscosity is because of the increase of the solution concentration [24, 25], while the increase in solution conductivity is due to the presence of hydrochloric acid in the anthocyanins derived from the extraction process, which forms ions in solution, thus enhancing the solution conductivity [26].
3.4 Yield of NFMs
As shown in Fig. 2, the yield of CS/PEO NFMs prepared using the SSFSE significantly increases compare to the MFSE [4], from 3.1 ± 0.1 g/h to 6.0 ± 0.5 g/h. However, with the addition of anthocyanin, the yield of anthocyanin/CS/PEO NFMs has little change, which is 5.8 ± 0.4 g/h.
3.5 Morphology of NFMs
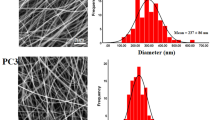
The microscopic morphology of CS/PEO and anthocyanin/CS/PEO NFMs was detected by SEM. As shown in Fig. 3, all the nanofibers had a smooth surface, and anthocyanin/CS/PEO nanofibers have a smaller average diameter and a more uniform distribution (100 ± 10 nm) compare with CS/PEO nanofibers (154 ± 16 nm). This is because when anthocyanins are added, the presence of more charges increase the charge repulsion in the jet due to the higher conductivity of anthocyanin/CS/PEO spinning solution. Therefore, the jet is subjected to a stronger electric field force, which causes it to be more fully stretched, resulting in the formation of finer nanofibers [16].
3.6 ATR-FTIR Analysis of NFMs
Figure 4 shows FTIR spectra of CS/PEO and anthocyanin/CS/PEO NFMs. It could be seen that the CS/PEO NFM have distinct characteristic peaks at 1025 cm−1, 1108 cm−1, 1344 cm−1, 1550 cm−1 and 2887 cm−1. Among them, the characteristic peaks at 1108 cm−1, 1344 cm−1 and 2887 cm−1 belonged to –C–O–C–, –CH2 and –CH– vibrations of PEO, respectively, while the characteristic peaks at 1025 cm−1 and 1550 cm−1 belonged to C–O–C, –NH2– of CS [27]. For the anthocyanin/CS/PEO NFM, all of the above characteristic peaks exist. In addition, the characteristic peak of anthocyanin/CS/PEO NFM shows significant fluctuations near 2887 cm−1 (corresponding to –CH2 and –CH–), due to the abundant hydroxyl groups in purple cabbage anthocyanins, which enhance the vibration of the NFM after the addition of anthocyanins [28, 29].
3.7 XRD Analysis of NFMs
XRD patterns of CS/PEO and anthocyanin/CS/PEO NFMs are exhibited in Fig. 5. The XRD pattern of CS/PEO NFM shows a broad peak at 22.3° due to the semi-crystalline nature of CS, and also exhibits two small peaks at 19.57° and 23.7°, which are low intensity peaks assigned to the PEO phase [30]. The XRD pattern of anthocyanin/CS/PEO NFM is similar to that of CS/PEO NFM, but its peak intensity is lower than that of CS/PEO NFM. It might be due to the formation of amorphous regions between anthocyanins and CS/PEO through intermolecular interactions, thus reducing the crystalline region content of the sample [31].
3.8 Mechanical Property of NFMs
As shown in Table 4, the breaking strength and elongation at break of anthocyanin/CS/PEO NFM are greater, due to its smaller and more uniform distribution of nanofiber diameter. In addition, the crystalline region of anthocyanin/CS/PEO NFM becomes smaller, which enhances its flexibility and fracture elongation [32], indicating its better mechanical property.
3.9 WAC of NFMs
CS is a hydrophilic biomaterial [33]. As shown in Fig. 6, the WAC of CS/PEO NFM is 56.2 ± 1.5°, and the WCA of anthocyanin/CS/PEO NFM slightly decrease to 54.8 ± 2.3° after adding anthocyanin, implying that both NFMs were hydrophilic. Anthocyanin is a water-soluble pigment, but its addition not affect the change in hydrophilic of the NFM a lot, probably due to the presence of phenolics in the anthocyanin, which formed hydrogen bonds when combined with the polymer [34, 35]. The hydrophilicity of NFMs can facilitate the diffusion of H+ or OH– into the NFMs, allowing these ions to interact with anthocyanins, thereby giving a rapid color response as a function of pH [26].
3.9.1 Antioxidant Activity of NFMs
The antioxidant activity of CS/PEO NFM might be related to the reaction of free radicals with the hydroxyl group at the C-6 position and the amino group at the C-2 position of CS to form stable macromolecule [36]. As shown in Fig. 7, the free radical scavenging rate of CS/PEO NFM is 58.6 ± 1.4%. Anthocyanins belong to phenolic compounds and have high antioxidant activity [17]. The combination of anthocyanin with CS can significantly improve its antioxidant activity [37]. Thus, the scavenging rate of anthocyanin/CS/PEO NFM to free radical removal is as high as 94.1 ± 2.1%, indicating its high antioxidant activity.
3.9.2 Antibacterial Effects
The antibacterial properties of CS/PEO and anthocyanin /CS/PEO NFMs against S. aureus and E. coli were characterized. Blank cotton fabric was used as a comparative sample. As shown in Table 5, these two NFMs all have good antibacterial properties against S. aureus and E. coli, with inhibition rates of 99.9%. It is possible that the positively charged primary amine groups of CS are available to interact with the negatively charged groups presented on the bacterial cell wall [20]. In addition, the hydrophilicity of CS/PEO and anthocyanin/CS/PEO NFMs contributed to their anti-adhesion properties of bacteria [38], resulting in their good antibacterial properties.
3.9.3 Response Analysis of NFMs at Different pH
Table 6 shows the color responses of CE/PEO and anthocyanin/CS/PEO NFMs in different pH solutions, respectively. It can be observed that the CS/PEO NFM appears white in the solution with pH values of 3–11, indicating that the color of CS/PEO NFMs does not change with the pH value of the surrounding environment. However, the anthocyanin/CS/PEO NFM produces color changes with the changes in environmental pH values, in which the NFM appears purple red at pH = 3, light blue at pH = 7, and yellow at pH = 11. This illustrates that the color changes of anthocyanin/CS/PEO NFM in different pH solutions are similar to the previously observed color changes of anthocyanin solutions, demonstrating that the main reason for the color changes of NFM is the structural changes of anthocyanins. However, compared to the colour response of anthocyanin solutions, anthocyanin/CS/PEO NFMs exhibited a slightly lighter color due to the special structure of the NFM.
3.9.4 Application of NFM in Beef Storage
Table 7 shows the color changes of CS-based NFM over time after storing beef at room temperature. It can be found that there is no significant change in the color of CS/PEO NFM, while anthocyanin/CS/PEO NFM exhibits significant color changes during beef storage. This is because at the beginning, the beef appears bright red in color, with a high glossiness. However, the beef is prone to spoilage at room temperature. After 24 h, the beef turns dark red, loses its lustre, and smells slightly sour. At this time, the beef is in the early stages of spoilage. Under the action of microorganisms, its proteins undergo decomposition reactions, forming alkaline substances such as amine compounds, which make the pH value of the beef become alkaline, thus causing the color of anthocyanin/CS/PEO NFM to change gradually from white to light yellow. As the storage time increases, the degree of beef spoilage gradually intensifies, and the beef become grey-green in colour with obvious putrefaction odor after 48–96 h of storage. The concentration of alkaline compounds generates by beef putrefaction also increases, resulting in the gradual increase of the pH value in beef, making the NFM gradually change from light yellow to yellow-green.
4 Conclusion
In this paper, anthocyanin/CS/PEO NFMs for smart food packaging was prepared in batches by using SSFSE technology, and their yield reached 5.8 ± 0.4 g/h. 2.7 ± 0.1 g of anthocyanin could be extracted from every 50 g purple cabbages, which was a natural colorant agent with different molecular structures under different pH values, and could change color with pH values. The morphology, structure and properties of CS-based NFMs were explored, which showed that they were hydrophilic and had excellent antibacterial activity against E. coli and S. aureus. With the addition of anthocyanin, anthocyanin/CS/PEO NFMs exhibited better mechanical properties and high antioxidant activity. Furthermore, based on the response of anthocyanin to pH values, the color changes of anthocyanin/CS/PEO NFMs at different pH values were investigated. The results showed that the color response of anthocyanin/CS/PEO NFM to different pH values was similar to the anthocyanin solution, with a color change from purple, blue to yellow as the pH value increased, illustrating that they could be used as a visual monitoring food packaging. When anthocyanin/CS/PEO NFM was used as the beef packaging material, its color changed from white, light yellow to yellow-green as the beef deteriorated during the storage period, realizing visual monitoring of beef quality.
Data Availability
All data included in this study are available upon request by contact with the corresponding author.
References
H. Liu, C.R. Gough, Q. Deng, Z. Gu, F. Wang, X. Hu, Int. J. Mol. Sci. 21, 4019 (2020)
I. Kohsari, Z. Shariatinia, S.M. Pourmortazavi, Int. J. Biol. Macromol. 91, 778 (2016)
M. Arkoun, F. Daigle, R.A. Holley, M.C. Heuzey, A. Ajji, Packag. Technol. Sci. 31, 185 (2018)
A. Ahmed, L. Xu, J. Yin, M. Wang, F. Khan, M. Ali, Fiber. Polym. 21, 1945 (2020)
E. Yildiz, G. Sumnu, L.N. Kahyaoglu, Int. J. Biol. Macromol. 170, 437 (2021)
D. Wang, W. Cheng, Q. Wang, J. Zang, Y. Zhang, G. Han, Compos. Sci. Technol. 182, 107774 (2019)
D. Surendhiran, C. Li, H. Cui, L. Lin, Food. Packag. Shelf. 23, 100439 (2020)
A.E. Mohamed, A. Shetta, J. Kegere, W. Mamdouh, Int. J. Biol. Macromol. 215, 387 (2022)
S.F. Hosseini, Z. Nahvi, M. Zandi, Food. Hydrocolloid. 89, 637 (2019)
M. Pakravan, M. Heuzey, A. Ajji, Polymer 52, 4813 (2011)
C.J. Luo, M. Nangrejo, M. Edirisinghe, Polymer 51, 1654 (2010)
Y.M. Shin, M.M. Hohman, M.P. Brenner, G.C. Rutledge, Polymer 42, 9955 (2001)
M.M. Hohman, M. Shin, G. Rutledge, M.P. Brenner, Phys. Fluids. 13, 2201 (2001)
A. Castaneda-Ovando, M. de Lourdes Pacheco-Hernandez, M.E. Paez-Hernandez, J.A. Rodriguez, C.A. Galan-Vidal, Food. Chem. 113, 13 (2009)
H. Wu, K. Yang, P. Chiang, Molecules 23, 13 (2018)
R. Nayak, R. Padhye, I.L. Kyratzis, Y.B. Truong, L. Arnold, Text. Res. J. 83, 12 (2013)
C. Lee, K. Na, Macromol. Res. 28, 289 (2020)
A. Ahmed, J. Yin, L. Xu, F. Khan, J. Mater. Res. Technol. 9, 9059 (2020)
S. Zhang, P. Li, W. Qian, S. Zhang, F. Li, H. Zhang, X. Wang, R. Sun, Euphytica 212, 83 (2016)
Y. Zhao, X. Qi, Z. Liu, W. Zheng, J. Guan, Z. Liu, J. Ren, H. Feng, Y. Zhang, Foods. 11, 1787 (2022)
H. Almasi, S. Forghani, M. Moradi, Food. Packag. Shelf. 32, 100839 (2022)
C. Henriques, R. Vidinha, D. Botequim, J.P. Borges, J. Silva, J. Nanosci. Nanotechno. 9, 3535 (2009)
S. Wang, M.F. Marcone, S. Barbut, L. Lim, Food. Res. Int. 52, 6 (2013)
S.Q. Li, J. Yin, L. Xu, Fiber. Polym. 23, 1225 (2022)
C. Cametti, S. Sennato, D. Truzzolillo, J. Chem. Phys. 131, 34901 (2009)
L. Prietto, V.Z. Pinto, S.L.M.E. Halal, M.G. de Morais, J.A.V. Costa, L. Lim, A.R.G. Dias, E.D.R. Zavareze, J. Sci. Food. Agr. 98, 7 (2018)
L. Yu, S. Dou, J. Ma, Q. Gong, M. Zhang, X. Zhang, M. Li, W. Zhang, Front. Mater. 8, 650223 (2021)
K. Sakulnarmrat, W. Sittiwong, I. Konczak, Int. J. Food. Sci. Tech. 57, 11 (2022)
Y. Mi, M. Cheng, Q. Yu, Y. Si, Ecotox. Eniron. Safe. 224, 10 (2021)
M.M. Abutalib, A. Rajeh, Polym. Test. 93, 107013 (2021)
S.L. Chen, M. Wu, P. Lu, L. Gao, S. Yan, S.F. Wang, Int. J. Biol. Macromol. 149, 271 (2020)
Q.M. Ma, Y. Zhang, F. Critzer, P.M. Davidson, S. Zivanovic, Q.X. Zhong, Food. Hydrocolloid. 52, 533 (2016)
S.S. Narasagoudr, V.G. Hegde, V.N. Vanjeri, R.B. Chougale, S.P. Masti, Carbohyd. Polym. 236, 116049 (2020)
A. Fernandes, E. BrandAo, F. Raposo, E. Maricato, J. Oliveira, N. Mateus, M.A. Coimbra, V. de Freitas, Carbohyd. Polym. 239, 116240 (2020)
P. Mustafa, M. Niazi, Z. Jahan, S. Rafiq, T. Ahmad, U. Sikander, F. Javaid, Polym. Polym. Compos. 29, 1472 (2021)
Z.G. Jiang, J.L. Wang, D. Xiang, Z.K. Zhang, Foods. 11, 1360 (2022)
J. Liu, H.M. Pu, S. Liu, J. Kan, C.H. Jin, Carbohyd. Polym. 174, 999 (2017)
S. Zare, M. Mohammadpour, Z. Izadi, S. Ghazanfari, S. Nadri, H. Samadian, Biology. 11, 1472 (2022)
Acknowledgements
The work is supported financially by National Natural Science Foundation of China (Grant No. 11672198), Jiangsu Higher Education Institutions of China (Grant No. 20KJA130001), Jiangsu Engineering Research Center of Textile Dyeing and Printing for Energy Conservation (Grant No. ERC-Q811580722), and PAPD (A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, A., Zhang, M., Li, S. et al. Batch Preparation and Characterization of Anthocyanin/CS/PEO Nanofiber Membranes for Food Packages. Fibers Polym 24, 3075–3084 (2023). https://doi.org/10.1007/s12221-023-00283-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00283-9