Abstract
Solar energy, along with other renewable resources, is going to play a key role in waste treatments and CO2 conversion. Photocatalytic technologies can be considered an effective solution for these purposes. This work summarises the setup of two different reactor configurations for two challenging processes: nitrate/ammonia abatement from water and CO2 photoreduction. The project goal was the setup of proper photoreactors and the preliminary investigation of some critical operating process parameters. Concerning the CO2 photoreduction, large efforts have been devoted to study an innovative high-pressure reactor developed properly to overcome the main limitation of the reaction, i.e. the limited solubility of carbon dioxide in water. On the other hand, nitrate and ammonia conversion was investigated, exploring the reactor setup and the analysis of critical products such as nitrogen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Photochemistry has been proposed as the science of the future, especially considering the progresses obtained in the photochemical conversion of solar energy (Balzani et al. 2008). In addition, photocatalysis is a research field in continuous growth due to the huge impact of the potential applications. During the last decade, photo-assisted reactions on semiconducting metal oxides acquired more and more importance in the photocatalytic field due to the potential simpler industrial applicability. The most relevant environmental application is the removal of pollutants and the decontamination of industrial waste streams. In particular, the strong oxidation ability of photo-excited semiconductors has received growing attention as summarised by Ochiai et al. for TiO2 photocatalysts (Ochiai and Fujishima 2012). However, photochemistry is a relatively young science and sometimes follows different rules than traditional chemistry, e.g. it shows a different dependence of reaction rate on temperature (Balzani et al. 2010).
In this perspective, insufficient stress was dedicated to the photocatalytic reduction reactions (CO2 reduction, nitrate reduction, etc.), combined with a generally non-negligible lack concerning the photocatalytic reactor design, which is usually less addressed with respect to materials design. Indeed, most literature deals with the optimisation of the photocatalytically active material, often neglecting the engineering issues. Some reviews on the topic partially address this point, e.g. (Ola and Maroto-Valer 2015), which however considers photoreactors design in two pages out of 24. More expertise is available from this point of view for water treatment, i.e. the mineralisation of organic pollutants (McCullagh et al. 2011). The scale up and the industrial application of these technologies need a deep effort also on this point and related topics, such as the irradiation efficiency, photoreactor geometry optimisation and the evaluation of transport phenomena. The largest effort in this perspective is present for heterogeneous photocatalytic reactor design in the context of wastewater treatment and air cleaning. For instance, membranes were presented as innovative technology. The aim of this approach is to avoid the catalyst recovering step from the solution at the end of the process, which is a consistent problem for a possible large scale application. The photocatalyst can be immobilised on an inert surface such as glass, quartz, concrete or ceramics. Maira et al. developed a membrane reactor for the photochemical oxidation of organic molecules (Maira et al. 2003). A porous stainless steel plate coated with TiO2 constitutes the membrane and is directly irradiated by the light source. Drawbacks of this technology are the mass transfer limitations of reactants to the surface of the catalyst and the loss of photocatalytic efficiency because of the low area/weight exposed. Moreover, fouling is a major problem, particularly critical in the case of industrial wastes. The related enhancement of transmembrane pressure can be a very big obstacle for the application.
Some pre-commercial photocatalytic applications for water treatment are also considered, mainly dealing with concentrated solar systems (Spasiano et al. 2015).
CO2 photoreduction is an intriguing and challenging reaction, called artificial photosynthesis: CO2 and H2O being the reactants and organic molecules and oxygen the products. Since H2O and CO2 do not absorb sunlight, photosynthetic process must be based on the excitation of a photosensitizer, which is a photocatalyst in the artificial case, whilst in the case of nature is a proper photosynthetic system (Balzani et al. 2015). The interest for the process grew recently due to the possibility to achieve realistic targets capable to meet the 2020 objectives set for EU Member States (Setti and Balzani 2011; Zecchina 2014).
Considering the CO2 photoreduction, the reactor configurations reported in literature are very limited, because generally the processes are performed in batch mode. Usubharatana et al. highlighted the most recent development for photoreactor layouts (Usubharatana et al. 2006). Generally, working in liquid phase, the semiconductor is suspended as slurry in powder form.
Another challenging process is the photoconversion of N-containing compounds, such as inorganic ammonia, nitrites and nitrates, and some organic N-containing molecules (dyes, pesticides, drugs, etc.). They are harmful contaminants for drinking water, inducing acute and/or chronic diseases, especially affecting infants and children. Furthermore, when released in waste waters they contribute to eutrophication, or possibly contaminate ground water. This is particularly relevant in agriculturally intensive zones and in the case of various industrial processes involving, e.g. nitration reactions.
Also in this case, although several progresses were achieved concerning the improvement of semiconductor properties in terms of light absorption and photocatalytic efficiency, proper reactor configuration is still much less explored. For instance, Shaban et al. investigated carbon-modified titanium dioxide nanoparticles under different reaction conditions (Shaban et al. 2016). Although the work reports a very interesting kinetic study identifying a pseudo-first-order reaction kinetics, the reactor configuration was simply constituted by an open glass tank, without considering the identification and quantification of nitrogen formation (as in the majority of the reports). Mohamed and Baeissa explored recently the synthesis and catalytic performance of Pd/NaTaO3 nanoparticles for the photoreduction of nitrate. Also in this case very interesting results concerning the Pd loading were reported, but the reactor configuration used was not even mentioned (Mohamed and Baeissa 2014). By contrast, Luiz et al. reported several metal modified TiO2 photocatalysts with a detailed explanation of the lab scale reactor (Luiz et al. 2012). An immersion lamp was present, able to ensure the best photo-irradiation of the catalyst surface. Although also for more traditional photoxidation treatments this configuration is the best (Usubharatana et al. 2006; Ochiai and Fujishima 2012; Galli et al. 2017), this configuration is not easily applied to solar irradiation. The only way to maintain the same configuration would be the use of optical fibres as well detailed by Nguyen et al., but such technology is in a very preliminary phase (Nguyen and Wu 2008). By contrast, the use of the external solar irradiation can be enhanced using properly designed parabolic collectors able to collect and enhance in a marked way the solar irradiation power directly on the catalyst surface (Ochiai and Fujishima 2012).
In the present work, we proposed new reactor configurations for some photocatalytic processes, using heterogeneous photocatalysts. An innovative high-pressure photoreactor was set up to study the CO2 photoconversion route toward the production of chemicals and fuels (mainly methanol and methane). For the abatement of N-containing compounds, we developed nanostructured photocatalysts and the relative processes, focusing on selectivity towards innocuous N2, to be applied for the treatment of waste waters to meet legislative specifications.
2 Experimental
2.1 Catalysts preparation
Detailed synthesis description of the catalyst used for the CO2 photoreduction can be found elsewhere (Compagnoni et al. 2016a). Briefly NaAuCl4·2H2O (Aldrich, 99.99% purity), NaBH4 (Fluka, purity >96%) and urea (Aldrich, purity >99%) were used in the synthesis. TiO2 P25 from Evonik was used as support. A deposition–precipitation methodology was performed.
On the other hand, the detailed synthesis description of the catalyst used for the N-compounds abatement involved a one-step technique: Flame Spray Pyrolysis. The details of the apparatus can be found in previous works (Chiarello et al. 2005, 2007). This technique allows the continuous and one-step synthesis of oxides, single or mixed. It is based on a specially designed burner fed with oxygen and an organic solution of the precursors, with the solvent acting as fuel for the flame. FP-synthesised samples usually show good phase purity, once the procedure is optimised for each material, along with nanometer-size particles and hence very high surface area (up to 250 m2/g). In addition, the high temperature of the flame in principle should also ensure thermal stability, provided that a solvent with sufficiently high combustion enthalpy is chosen. The sample was synthesised using a solution of Titanium(IV)-isopropylate (Aldrich, pur. 97%) in xylene, with a 0.67 M concentration referred to TiO2. The solutions were fed to the burner nozzle with a flow rate of 2.2 mL/min and a 1.5 bar pressure drop across the nozzle, which was also fed with 5 L/min of O2.
2.2 Apparatus for CO2 photoreduction tests
The innovative photoreactor has been described elsewhere (Rossetti et al. 2014b, 2015; Galli et al. 2017). Briefly, the photoreactor was made of AISI 316 stainless steel. The lamp is introduced vertically in the reactor axis and a magnetic stirrer ensures proper liquid mixing. The internal capacity is ca. 1.3 L, and the reactor is filled with 1.2 L solution. The temperature was kept constant through a double-walled thermostatic system. A medium-pressure mercury vapour lamp (125 W) emitting in the wavelength region from 254 to 364 nm was used during testing. The harmful overheat of the lamp bulb was avoided by continuous heat removal by an air circulation system. The catalyst, ca. 0.5 g, has been dispersed in demineralised and outgassed water (1–1.2 L). The suspension has been saturated with CO2 at different temperature and pressure before starting irradiation.
Na2SO3 (ca. 0.85 g/L) was used as hole scavenger. Its consumption has been evaluated by iodometric titration, showing a conversion ranging from 78 to 94%. Negligible productivity has been observed without its addition. The gas phase over the liquid has been analysed by a gas chromatograph (Agilent 7890) equipped with a TCD detector and proper setup for the quantification of H2, CH4 and polar/non-polar light gases. Every test was typically carried out for 70–100 h. The maximum error on chromatographic analysis is 4 and 5.5% for H2 and CH4, respectively. Repetition of the same test under identical conditions led to an experimental error up to 10%, increasing to a maximum deviation of 19% in case of very low productivity in gas phase for H2. Some repetitions have been carried out on recycled catalysts too, during the setup of the analysis, evidencing a productivity within the given experimental error up to three cycles. However, the tests reported in this work have been carried out on fresh aliquots of catalyst.
Light intensity has been measured using a photoradiometer model Etta-Ohm HD 2102.2.
2.3 Apparatus for nitrate/ammonia photoabatement
Photocatalytic reduction of NO3 − and NH3/NH4 + in water was carried out in a Pyrex reactor. TiO2-P25 and TiO2-FP (prepared by Flame Pyrolysis) were suspended in an aqueous NH4Cl (0.2 M) or NaNO3 solution (0.006 M) without any other compounds; the suspension was stirred using a magnetic stirrer. The reacting suspension was thoroughly degassed and then exposed to He. The light source was a medium-pressure mercury vapour lamp with an irradiation power of 69.5 W/m2 on the top of the liquid phase, emitting in the wavelength region from 254 to 364 nm. The temperature of the reaction vessel was kept at 298 K by a circulating cooling water. The evolved gases were analysed using an online gas chromatograph (Agilent Technology, mod. 5980, He carrier) equipped with two columns connected in series (Molecular Sieve and Poraplot Q) with a thermal conductivity detector (TCD). Ammonium was determined using a Nessler’s reagent (Sigma-Aldrich) and analysed by a spectrophotometer (Perkin Elmer Lambda 35) at a wavelength of 420 nm. The concentrations of NO3 − and NO2 − in the reaction solution were determined using ion chromatographs (Metrohm, 883 Basic IC plus).
3 Results and discussion
3.1 CO2 photoreduction
CO2 photoreduction is an uphill reaction which requires a high amount of energy. At pH 7, the direct acquisition of an electron to form an activated CO ·2 requires −1.9 V (referred to NHE). As a comparison, under the same conditions proton reduction requires −0.41 V. However, the formation of stable species is less energy demanding. For instance, the reactions
are characterised by −0.49, −0.53 and −0.48 V, respectively (Ola and Maroto-Valer 2015).
As mentioned in the introduction, one of the biggest limitations of CO2 photoreduction in liquid phase is its limited solubility in water. For this reason, an innovative high-pressure photoreactor was set up. A screening of the operative conditions was performed using a 0.1 wt% Au/TiO2 material. The choice of titania was based on the wide number of studies performed on this material in literature and in our previous research experience (Barzan et al. 2014; Nichele et al. 2014; Rossetti et al. 2014a).
The main photocatalyst features (metal loading, support crystalline phase, preparation method) were deeply investigated elsewhere (Rossetti et al. 2014b, 2015; Compagnoni et al. 2016a).
After a wide screening up to 20 bar, the effect of pressure was evaluated in the range between 5 and 10 bar to maintain a good productivity both for gas and liquid phase products (Fig. 1). At lower pressure, the productivity was too low due to the limited solubility of CO2 in water. The effect of the pressure can be evaluated in a quantitative way considering the solubility data as a function of pressure and temperature (Rossetti et al. 2015). By contrast, at pressure higher than 10 bar the products evolution in gas phase is hindered for thermodynamic reasons.
An optimal value of 6 bar was chosen as the best condition for obtaining the highest hydrogen and methane productivities. Hydrogen was formed mainly by the photoreforming of the organic products generated in liquid phase during the reduction of CO2, whereas CH4 was directly derived from CO2 photoreduction and evolved in gas phase.
The effect of reaction temperature was also investigated at the same pressure. Increasing temperature influenced the photocatalytic process in a negative way, because it enhanced the electron-hole recombination, thus leading to a lower efficiency. However, from a chemical point of view, the increase in the collision frequency should lead to an enhancement of the reaction rates. The controversial role of temperature is made even more complex for solubility reasons. An increase of temperature decreases the CO2 solubility and consequently its molar fraction in the liquid phase that could come in contact with the surface of the photocatalyst. Other key points are the possible mass transfer limitations and the adsorption rates of the reactant: mass transfer is enhanced by increasing the temperature, whilst the latter is reduced. At 5 bar with 0.1 wt% Au/P25, H2 productivity increased by a factor of ca. 20 when increasing temperature from 50 to 80 °C considering different pH. This can be mainly attributed to the improvement of kinetics with increasing temperature. This is also an important result, because it suggests that a kinetic control of the reaction may be achieved when working under high CO2 pressure, whereas at lower pressure the reaction rate is substantially limited by dissolution equilibria.
The best operative pressure of 6 bar was used for the performance evaluation of different photocataysts, in particular varying the crystalline phase of the titania maintaining the same gold loading. The results for TiO2-P25, TiO2-Rutile and TiO2-Anatase are shown in Fig. 2.
The catalysts were tested for different time, from 3 h up to 72 h. Low reaction time favoured the formation of liquid phase products, whereas prolonged reaction times induced higher formation of gas phase compounds due to the consecutive photoreforming of the organic products produced. The samples reused for 3 times for 72 h did not show significant deactivation or variation of products distribution. The main products found in liquid phase were HCOOH and HCHO in the case of TiO2, whereas for Au-doped samples CH3OH was also observed in significant amount. Also the products distribution in gas phase was different depending on reaction time and catalyst composition. The photoreforming product, H2, was obtained after prolonged irradiation, being the product of a consecutive reaction. Furthermore, its appearance was tightly related to the consumption of the hole scavenger. CO was the main gas phase product of direct CO2 reduction for TiO2, whereas for Au-doped samples CH4 was the main product in gas phase. In general, the productivity in liquid phase was orders of magnitude higher than that of gas phase products.
This aspect is important because many papers are available on the effect of titania polymorphs in the oxidative photocatalytic applications, but only few for the reductive photochemistry. The highest methane formation was obtained for the mixed-phase P25 sample. The direct photoreduction of CO2 to methane is a complex multielectronic pathway and the great activity usually exhibited by the titania mixed phase is ascribed to the electron transfer from one phase to the other, which acts as electron sink, thus inhibiting the electron-hole recombination. Therefore, only by increasing the lifetime of the photogenerated electron the direct CO2 photoreduction can safely proceed to methane. Also the photocatalytic activity towards H2 production was higher in the case of the P25 mixed phase than for the pure anatase and rutile phases, although the difference was less marked than the case of methane. Delavari and co-workers explored the gas phase reaction using immobilised TiO2 nanoparticles on a stainless steel mesh (Delavari and Amin 2014). They obtained an increase of CO2 and CH4 conversions (CO2 and CH4 were co-fed for the production of HCOOH in this reference work) due to the increase of the anatase/rutile ratio in TiO2, directly correlated with the enhancement of surface area for their specific case. A detailed Langmuir–Hinshelwood model was applied to the experimental results, but no considerations about the crystalline form and the direct consequences on CO2 reduction were advanced by the authors. Furthermore, the co-feeding of methane with CO2 did not allow to study the effect on methane formation. It should be also mentioned that kinetics still needs refinement in photocatalysis. Indeed, pseudo-homogeneous models are often found in the literature, instead of specifically derived kinetic models, which can be more appropriate for reactor modelling (Camera-Roda et al. 2016).
Mixed-phase titania nanomaterials were studied by Chen et al. for gas phase CO2 photoreduction, using the DC magneton sputtering as preparation method (Chen et al. 2009). All the mixed-phase sputtered samples displayed greater activity with respect to single phase ones. The result was correlated to the high density of interfacial sites.
The synergistic effect between rutile and anatase for photocatalytic applications is not new in the literature, but the deep explanation of the phenomena is still under debate (Ohtani 2010). The more consistent argumentation considers the low electron-hole recombination as consequence of the spatial separation caused by the interconnection between rutile and anatase particles. Li et al. investigated several TiO2 polymorphs as a photocatalyst for CO2 reduction under UV light illumination (Li et al. 2008). They found a very different methane yield varying the crystalline phase of titania. The synergistic effect was apparently demonstrated because higher photocatalytic activity was obtained using a mixed-phase TiO2 nanocomposite, prepared by a low-temperature hydrothermal method (SSA = 8.6 m2 g−1) with respect to commercial P25 (SSA ~ 50 m2 g−1) followed by commercial anatase. On the contrary, Ohtani et al. supported a contrasting explanation, considering the lack of information about the direct evidence of the inter-particle charge migrations and the expected lower level of activity of pure anatase or rutile particles alone (Ohtani 2010). For this reason in another work (Ohtani et al. 2010), they prepared a reconstructed P25 (mixture of pure anatase, rutile and amorphous titania) and demonstrated a less probable synergistic effect for the oxidative decomposition of acetic acid, acetaldehyde and methanol dehydrogenation. However, no reductive photocatalytic processes were considered in their results. Although the explanation is still far from an unmistakable solution, the higher activity of mixed rutile-anatase TiO2 was still reported for CO2 photoreduction in the literature and our results confirmed this trend also for the present unconventional high-pressure conditions.
At last, much lower activity was achieved without the addition of Na2SO3. This compound was tested during different blank tests, showing negligible conversion in the absence of irradiation or without photocatalyst. Therefore, it is not directly responsible of CO2 reduction. On the contrary, it acts as a hole scavenger, allowing to prevent the fast recombination of the photogenerated electrons and holes. A very interesting behaviour was noticed by us when looking to its conversion vs. time. During standard testing for 24 h, it was almost completely converted. However, immediately after its consumption, H2 production started in gas phase, due to the photoreforming of the previously produced organic products. At the same time, the concentration of HCHO, HCOOH and (when present) CH3OH decreased. Therefore, we can conclude that the sulphite preferentially acts as hole scavenger and is substituted by organic compounds when consumed. This allows the use of the sacrificial agent to tune products composition. If organic products in liquid phase are desired, a continuous supply of sulphite should be provided. By contrast, if a fuel mixture is desired in gas phase, a starting batch of sulphite can be added as initiator, leaving the formed organics as hole scavengers after its consumption. In this way, a consecutive process is self-sustained, in which CO2 photoreduction generates organics, which are consequently oxidised back to CO2 with simultaneous formation of H2 (Galli et al. 2017).
3.2 Photoconversion of N-containing compounds
The photo-oxidation of ammonia/ammonium at pH 7 is much less demanding than the photoreduction of CO2 from a thermodynamic point of view. The redox couple N2/NH4 + has a potential −0.276 vs. NHE, whereas the overoxidised species \( {\text{NO}}_{2}^{ - } \) and \( {\text{NO}}_{3}^{ - } \) lead to 0.343 and 0.363 V, respectively (Wang et al. 2014).
A preliminary study using the photoreactor configuration shown in Fig. 3 was carried out for two different but tightly related processes: ammonia photo-oxidation and nitrate photo-reduction. A semi-batch configuration was adopted, using a continuous gas stream of 20% O2 in He bubbled in an aqueous solution of ammonium chloride for the photo-oxidation process, whilst only He was used for the nitrate reduction tests. This setup allowed to estimate the nitrogen formation using simulated air in the case of ammonia photo-oxidation and He during nitrate photoreduction. The external lamp was placed on the top of the photoreactor to simulate a future application directly by sunlight.
One of the challenges of this kind of process is the quantification of molecular nitrogen produced. Indeed usually the selectivity to N2 is evaluated by difference, considering the total N-balance and measuring only N-compounds in liquid phase (nitrate, nitrite and ammonia) (Ren et al. 2015). Typically the reason is related with the ammonia produced and stripped in gas phase. In particular, when you have an online gas-chromatograph for this process, ammonia can damage the columns, o-rings and possibly the TCD filaments. For this reason, a proper adsorber was set up, constituted by a column filled with a zeolite (in acidic form and pre-activated) able to adsorb the gaseous NH3 possibly stripped by the gas stream through the liquid phase inside the reactor.
Preliminary results were obtained using bare titania photocatalyst. This semiconductor was chosen considering its well-known photoactivity and photoresistance towards corrosion (Ohtani et al. 2010). Zecchina and co-workers reported in a detailed study the photocatalytic decomposition of sulphur mustard (bis(2-chloroethyl)sulphide) using several metal-doped dispersed titania-silica catalysts. The improved activity using metals also reported in different photochemical processes (Centi and Perathoner 2009; Chen et al. 2015) revealed the future route for further improvement of the photo-efficiency of the process, and underlined the key role of the preparation technique.
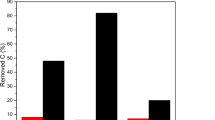
In this perspective, a home-made TiO2 photocatalyst was compared with the P25 (Evonik). The selected preparation technique was the flame spray pyrolysis, a scalable and industrially mature technique based on a special designed burner (Rossetti et al. 2009; Compagnoni et al. 2016b). This technique is alternative and less studied methodology than traditional sol–gel routes, mainly due to the impossibility to synthesize sophisticated materials such as nanovoid-structured TiO2 (Usseglio et al. 2007). By contrast, the technology introduces several advantages: it is a one-step synthesis, includes a flash calcination, and has easier scalability to industrial level; the structure is tunable by choosing proper operative conditions (Strobel and Pratsinis 2007; Teoh et al. 2010; Kemmler et al. 2013). The activity results in terms of conversion are shown in Fig. 4. The titania prepared by flame pyrolysis revealed higher activity, reaching ca. 20% conversion, with respect to much lower performance of the commercial sample. Both catalysts were fully selective to N2, without the formation of nitrites or nitrates. Conversion was significatively improved by adding 0.1 wt Pd. The metal was chosen to improve the charge separation and to increase the oxidation activity. The catalyst remained fully selective to N2, reaching 32% conversion of ammonia.
The semi-batch configuration of the photoreactor has the advantage of a constant oxygen content, together with the removal of the formed N2. Although the conversions are still not impressive, these data are very interesting as preliminary results for the reactor configuration chosen. Indeed this configuration could be easy applicable using sunlight, adopting proper parabolic collectors as aforementioned. At the moment, a newly designed photoreactor is being realised to improve the irradiation efficiency and catalyst dispersion to further improve reactants conversion.
4 Conclusions
Two kinds of unconventional photoreactor configurations were explored. In the case of CO2 photoreduction, the novelty was related with the possibility to achieve challenging and non-trivial operative conditions, able to operate up to 20 bar. This system was employed to improve the solubility of CO2 in water, one of the main limitations for CO2 photoconversion in liquid phase. Interesting productivity for gas phase photoregenerated fuels has been achieved by using 0.1 wt% Au/TiO2 catalysts. Operating at intermediate pressures favours the formation of gas phase products, in the form of a fuel mixture composed of H2 + CH4. The highest productivities for gas phase products were achieved at 6 bar. The screening of catalyst polymorphs revealed better performance of rutile-anatase mixed phase in accordance with the results reported in the literature at atmospheric pressure.
On the other hand, the photocatalytic conversion of NH3/NH4 + and \( {\text{NO}}_{3}^{ - } \) over titania prepared by different methodologies was investigated as a candidate treatment process for water purification. A semi-batch photoreactor configuration was set up. Titania prepared by Flame Pyrolysis showed the best photocatalytic performance compared to the reference P25 (Evonik).
References
Balzani V, Credi A, Venturi M (2008) Photochemical conversion of solar energy. ChemSusChem 1:26–58. doi:10.1002/cssc.200700087
Balzani V, Marchi E, Semeraro M (2010) From the periodic table to photochemical molecular devices and machines. Rend Fis Acc Lincei 21:91–109. doi:10.1007/s12210-010-0073-5
Balzani V, Bergamini G, Ceroni P (2015) Light: a very peculiar reactant and product. Angew Chemie Int Ed 54:11320–11337. doi:10.1002/anie.201502325
Barzan C, Groppo E, Bordiga S, Zecchina A (2014) Defect sites in H2-reduced TiO2 convert ethylene to high density polyethylene without activator. ACS Catal 4:986–989. doi:10.1021/cs500057s
Camera-Roda G, Augugliaro V, Cardillo AG et al (2016) A reaction engineering approach to kinetic analysis of photocatalytic reactions in slurry systems. Catal Today 259:87–96. doi:10.1016/j.cattod.2015.05.007
Centi G, Perathoner S (2009) Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal Today 148:191–205. doi:10.1016/j.cattod.2009.07.075
Chen L, Graham ME, Li G et al (2009) Photoreduction of CO2 by TiO2 nanocomposites synthesized through reactive direct current magnetron sputter deposition. Thin Solid Films 517:5641–5645. doi:10.1016/j.tsf.2009.02.075
Chen W-T, Chan A, Al-Azri ZHN et al (2015) Effect of TiO2 polymorph and alcohol sacrificial agent on the activity of Au/TiO2 photocatalysts for H2 production in alcohol–water mixtures. J Catal 329:499–513. doi:10.1016/j.jcat.2015.06.014
Chiarello GL, Rossetti I, Forni L (2005) Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J Catal 236:251–261
Chiarello GL, Rossetti I, Forni L et al (2007) Solvent nature effect in preparation of perovskites by flame pyrolysis. 2. Alcohols and alcohols + propionic acid mixtures. Appl Catal B Environ 72:227–232. doi:10.1016/j.apcatb.2006.10.026
Compagnoni M, Kondrat SA, Chan-Thaw CEE, et al (2016a) Spectroscopic Investigation of Titania Supported Gold Nanoparticles Prepared by a Modified DP Method for the Oxidation of CO. ChemCatChem 8:2136–2145. doi:10.1002/cctc.201600072
Compagnoni M, Lasso J, Di Michele A, Rossetti I (2016b) Flame pyrolysis prepared catalysts for the steam reforming of ethanol. Catal Sci Technol. doi:10.1039/C5CY01958C (in press)
Delavari S, Amin NAS (2014) Photocatalytic conversion of CO and CH4 over immobilized titania nanoparticles coated on mesh: Optimization and kinetic study. Appl Energy 162:1171–1185. doi:10.1016/j.apenergy.2015.03.125
Galli F, Compagnoni M, Vitali D et al (2017) CO2 photoreduction at high pressure to both gas and liquid products over titanium dioxide. Appl Catal B Environ 200:386–391. doi:10.1016/j.apcatb.2016.07.038
Kemmler JA, Pokhrel S, Mädler L et al (2013) Flame spray pyrolysis for sensing at the nanoscale. Nanotechnology 24:442001. doi:10.1088/0957-4484/24/44/442001
Li G, Ciston S, Saponjic ZV et al (2008) Synthesizing mixed-phase TiO2 nanocomposites using a hydrothermal method for photo-oxidation and photoreduction applications. J Catal 253:105–110. doi:10.1016/j.jcat.2007.10.014
Luiz DDB, Andersen SLF, Berger C et al (2012) Photocatalytic reduction of nitrate ions in water over metal-modified TiO2. J Photochem Photobiol A Chem 246:36–44. doi:10.1016/j.jphotochem.2012.07.011
Maira AJ, Lau WN, Lee CY et al (2003) Performance of a membrane-catalyst for photocatalytic oxidation of volatile organic compounds. Chem Eng Sci 58:959–962. doi:10.1016/S0009-2509(02)00634-6
McCullagh C, Skillen N, Adams M, Robertson PKJ (2011) Photocatalytic reactors for environmental remediation: a review. J Chem Technol Biotechnol 86:1002–1017. doi:10.1002/jctb.2650
Mohamed RM, Baeissa ES (2014) Environmental remediation of aqueous nitrate solutions by photocatalytic reduction using Pd/NaTaO3 nanoparticles. J Ind Eng Chem 20:1367–1372. doi:10.1016/j.jiec.2013.07.020
Nguyen TV, Wu JCS (2008) Photoreduction of CO2 in an optical-fiber photoreactor: Effects of metals addition and catalyst carrier. Appl Catal A Gen 335:112–120. doi:10.1016/j.apcata.2007.11.022
Nichele V, Signoretto M, Menegazzo F et al (2014) Hydrogen production by ethanol steam reforming: effect of the synthesis parameters on the activity of Ni/TiO2 catalysts. Int J Hydrogen Energy 39:4252–4258. doi:10.1016/j.ijhydene.2013.12.178
Ochiai T, Fujishima A (2012) Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J Photochem Photobiol C Photochem Rev 13:247–262. doi:10.1016/j.jphotochemrev.2012.07.001
Ohtani B (2010) Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J Photochem Photobiol C Photochem Rev 11:157–178. doi:10.1016/j.jphotochemrev.2011.02.001
Ohtani B, Prieto-Mahaney OO, Li D, Abe R (2010) What is Degussa (Evonic) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J Photochem Photobiol A Chem 216:179–182. doi:10.1016/j.jphotochem.2010.07.024
Ola O, Maroto-Valer MM (2015) Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J Photochem Photobiol C Photochem Rev 24:16–42. doi:10.1016/j.jphotochemrev.2015.06.001
Ren HT, Jia SY, Zou JJ et al (2015) A facile preparation of Ag2O/P25 photocatalyst for selective reduction of nitrate. Appl Catal B Environ 176–177:53–61. doi:10.1016/j.apcatb.2015.03.038
Rossetti I, Fabbrini L, Ballarini N et al (2009) V–Al–O catalysts prepared by flame pyrolysis for the oxidative dehydrogenation of propane to propylene. Catal Today 141:271–281. doi:10.1016/j.cattod.2008.05.020
Rossetti I, Lasso J, Finocchio E et al (2014a) TiO2-supported catalysts for the steam reforming of ethanol. Appl Catal A Gen 477:42–53. doi:10.1016/j.apcata.2014.03.004
Rossetti I, Villa A, Pirola C et al (2014b) A novel high-pressure photoreactor for CO2 photoconversion to fuels. RSC Adv 4:28883–28885. doi:10.1039/C4RA03751K
Rossetti I, Villa A, Compagnoni M et al (2015) CO2 photoconversion to fuels under high pressure: effect of TiO2 phase and of unconventional reaction conditions. Catal Sci Technol 5:4481–4487. doi:10.1039/C5CY00756A
Setti L, Balzani V (2011) Road Map towards an integrated energy management system in Italy. Rend Fis Acc Lincei 22:55–64. doi:10.1007/s12210-010-0110-4
Shaban YA, El Maradny AA, Al Farawati RK (2016) Photocatalytic reduction of nitrate in seawater using C/TiO2 nanoparticles. J Photochem Photobiol A Chem 328:114–121. doi:10.1016/j.jphotochem.2016.05.018
Spasiano D, Marotta R, Malato S et al (2015) Solar photocatalysis: materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl Catal B Environ 170–171:90–123. doi:10.1016/j.apcatb.2014.12.050
Strobel R, Pratsinis SE (2007) Flame aerosol synthesis of smart nanostructured materials. J Mater Chem 17:4743–4756. doi:10.1039/b711652g
Teoh WY, Amal R, Mädler L (2010) Flame spray pyrolysis: an enabling technology for nanoparticles design and fabrication. Nanoscale 2:1324–1347. doi:10.1039/c0nr00017e
Usseglio S, Damin A, Scarano D et al (2007) (I2) n encapsulation inside TiO2: a way to tune photoactivity in the visible region. J Am Chem Soc 2007:2822–2828. doi:10.1021/ja066083m
Usubharatana P, McMartin D, Veawab A et al (2006) Photocatalytic process for CO2 emission reduction from industrial flue gas streams. Ind Eng Chem Res 45:2558–2568. doi:10.1021/ie0505763
Wang H, Su Y, Zhao H et al (2014) Photocatalytic oxidation of aqueous ammonia using atomic single layer graphitic-C3N4. Environ Sci Technol 48:11984–11990. doi:10.1021/es503073z
Zecchina A (2014) Energy sources and carbon dioxide waste. Rend Fis Acc Lincei 25:113–117. doi:10.1007/s12210-013-0253-1
Acknowledgements
The financial support of Fondazione Cariplo through the measure “Ricerca sull’inquinamento dell’acqua e per una corretta gestione idrica”, Grant no. 2015-0186, is gratefully acknowledged. Prof. Laura Prati and Dr. Alberto Villa are gratefully acknowledged for the preparation of Au/TiO2 catalysts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Compagnoni, M., Ramis, G., Freyria, F.S. et al. Innovative photoreactors for unconventional photocatalytic processes: the photoreduction of CO2 and the photo-oxidation of ammonia. Rend. Fis. Acc. Lincei 28 (Suppl 1), 151–158 (2017). https://doi.org/10.1007/s12210-017-0617-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-017-0617-z