Abstract
Heterogeneous photocatalysis (HPC) has been widely investigated in recent decades for the removal of a number of contaminants from aqueous matrices, but its application in real wastewater treatment at full scale is still scarce. Indeed, process and technological limitations have made HPC uncompetitive with respect to consolidated processes/technologies so far. In this manuscript, these issues are critically discussed and reviewed with the aim of providing the reader with a realistic picture of the prospective application of HPC in wastewater treatment. Accordingly, consolidated and new photocatalysts (among which the visible active ones are attracting increasing interest among the scientific community), along with preparation methods, are reviewed to understand whether, with increased process efficiency, these methods can be realistically and competitively developed at industrial scale. Precipitation is considered as an attractive method for photocatalyst preparation at the industrial scale; sol–gel and ultrasound may be feasible only if no expensive metal precursor is used, while hydrothermal and solution combustion synthesis are expected to be difficult (expensive) to scale up. The application of HPC in urban and industrial wastewater treatment and possible energy recovery by hydrogen production are discussed in terms of current limitations and future prospects. Despite the fact that HPC has been studied for the removal of pollutants in aqueous matrices for two decades, its use in wastewater treatment is still at a “technological research” stage. In order to accelerate the adoption of HPC at full scale, it is advisable to focus on investigations under real conditions and on developing/improving pilot-scale reactors to better investigate scale-up conditions and the potential to successfully address specific challenges in wastewater treatment through HPC. In realistic terms, the prospective use of HPC is more likely as a tertiary treatment of wastewater, particularly if more stringent regulations come into force, than as pretreatment for industrial wastewater to improve biodegradability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
New challenges in wastewater treatment [e.g., the removal of contaminants of emerging concern (CECs) from municipal wastewater treatment plant effluents], the increasing interest/demand for sustainable wastewater treatment methods, and a circular economic approach (e.g., energy saving and recovery, mass recovery from waste, and water reuse) are leading to the investigation and development of increasingly effective wastewater treatment processes and technologies. The main problem is related to the presence of non-biodegradable substances (organic and inorganic) including metals, pharmaceutical compounds, and personal care products, which persist in water and in the environment, causing serious damage to the ecosystem and to human health. For this reason, the study of advanced oxidation processes (AOPs) has increased significantly in recent years. AOPs are characterized by the presence of highly reactive species able to remove and mineralize refractory organic compounds, water pathogens, and disinfection by-products [1]. Among these processes, heterogeneous photocatalysis (HPC) has been proven to be effective in the degradation of a wide range of refractory organic compounds. However, its application in water and wastewater treatment at full scale is far from being successfully implemented due to the complexity of the various real aqueous matrices as well as limitations in the process (e.g., low photoconversion efficiency) and technological limitations (i.e., catalyst preparation method, slurry vs. supported system, reactor design, energy consumption, etc.). In an effort to improve process efficiency while minimizing energy cost, the new trend in HPC research deals with the formulation of semiconductors active in the presence of visible light. In this review these issues were addressed with regard to the application of HPC in urban and industrial wastewater treatment as well as energy recovery through hydrogen production during treatment, by explaining and critically discussing possible advantages and disadvantages of the process. The scope was to provide the reader with some information and tools to understand the current knowledge gaps as well as to evaluate prospective applications of HPC in the treatment of wastewater.
2 Heterogeneous Photocatalysis: An Overview of Consolidated and New Photocatalysts as well as Preparation Methods
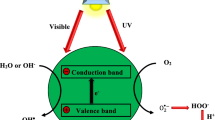
Heterogeneous photocatalysis relies on the interaction between a light source and a solid semiconductor in an aqueous matrix. Depending on the emission spectrum of the light source and the characteristics of the semiconductor (photocatalyst), electrons are promoted from the valence band to the conduction band of the photocatalyst, thus initiating a surface reaction will ultimately result in the formation of highly oxidizing agents, such as hydroxyl radicals (·OH). The efficiency of the HPC process in water/wastewater treatment will depend on the capacity of the system to effectively produce such radicals, which can degrade/oxidize a wide range of contaminants as well as effectively inactivate microorganisms.
The development of photocatalysts should take into account the typical characteristics and requirements for photocatalytic materials. In particular, a photocatalyst must be photoactive, inert, stable, non-toxic, and relatively cheap [2]. One of the most studied photocatalyst for water/wastewater treatment is titanium dioxide (TiO2). TiO2 has been widely investigated because of its very high ultraviolet absorption and high stability. These properties made this photocatalyst ideal for different applications, such as electroceramics, glass, and photocatalytic degradation of chemicals in water and air. It has been investigated in powdered form suspended in aqueous matrices (slurry reactor) or as thin film. TiO2 presents three different crystalline forms: anatase, rutile (more stable), and brookite (uncommon and unstable). Degussa P25 titania is commercially available and consists of 25% rutile and 75% anatase crystalline phases [3]. P25 is used as a benchmark in water and wastewater treatment by TiO2 photocatalysis due to its easy availability, reproducibility, chemical stability, and high photoactivity [4,5,6,7,8]. Beyond TiO2, another interesting photocatalyst with properties similar to titania is ZnO. This photocatalyst has recently attracted increasing attention from the scientific community for its characteristics that include strong oxidation ability, good photocatalytic properties large free-exciton binding energy, and low cost (cheaper than TiO2) [9]. Another category of semiconductors that has found success in photocatalytic applications for water treatment is represented by perovskites, in particular LaFeO3 [10, 11]. LaFeO3 is one of the most common perovskite-type oxides, and it is a promising material with different functionalities, having a general formula ABO3, where position A is the rare earth ion and position B is represented by metal ion. This material is characterized by high stability, non-toxicity, and small bandgap energy (2.07 eV), making perovskite an interesting visible-light-active photocatalyst [12].
2.1 Methods for Improving Photocatalyst Activity
The application of photocatalysts such as TiO2 and ZnO is limited by the fact that ultraviolet (UV) activation is needed (the bandgap energy is about 3.2 eV, and this means that less than 5% of the solar spectrum has sufficient energy to activate the photocatalyst) and by the fast recombination rate of the electron–hole pairs generated [13]. Hence, modification of photocatalysts through metal or non-metal doping or their combination with another semiconductor are common methods used to improve the photocatalytic performance and for activation by visible light irradiation. The particle size and morphology of nanoscale photocatalysts is also a problem in full-scale application for water and wastewater treatment, because the particles should be removed/recovered after treatment. To overcome this drawback, the application of photocatalytic membranes or the use of other photocatalyst-supporting materials has been proposed [14,15,16]. For example, photocatalysts have been supported on activated carbon [14], fibers, [17, 18] membranes, [19, 20] metal [5], and plastics [21]. In this section, the main methods for the synthesis of modified photocatalysts used in wastewater treatment are introduced, along with the methods developed for supporting photocatalysts on suitable materials in order to make them usable on a large scale.
A number of methods can be used to extend the light absorption properties of traditional semiconductors (e.g., ZnO or TiO2) into the visible range, including:
Coupling a primary photocatalyst with different semiconductors with a smaller bandgap or through sensitization with dyes.
Doping the photocatalyst with metals (second-generation photocatalysts).
Doping the photocatalyst with non-metals (third-generation photocatalysts).
2.1.1 Coupling a Primary Photocatalyst with Smaller-Bandgap Semiconductor or Its Sensitization with Dye
As a semiconductor (e.g., TiO2 or ZnO) with a wide bandgap is coupled with other semiconductors having a lower bandgap or is sensitized with specific types of dyes, the absorption properties of the obtained composite are extended into the visible region. This phenomenon is induced either by the light absorption characteristics of the dye or by the other semiconductor coupled with TiO2 or ZnO. When a semiconductor is sensitized with a dye, the visible light is absorbed by the dye molecules bridged to the semiconductor surface, and the electrons pass from the dye’s ground state to an excited state. These excited electrons are then transferred to the conduction band of ZnO or TiO2, which results in a modified photocatalyst with improved photoactivity under visible light. Dyes that have been used for this purpose include ruthenium polypyridyl complexes [22] and different metal-free organic dye molecules such as hemicyanine [23] and indoline [24]. However, it is worth noting that the low stability of the dye used for the sensitization of semiconductors in water/wastewater is the main drawback of this method.
With regard to the coupling of two semiconductors, the aim is to form a heterojunction structure between TiO2 or ZnO and a narrow-bandgap semiconductor such as CdS, MoS2, or In2S3 [25]. The electrons excited by visible light are transferred to TiO2 or ZnO from the narrow-bandgap semiconductor, thus promoting charge carrier separation and, consequently, improving the visible-light photocatalytic activity of the composite [25]. In order to improve ZnO (bandgap of 3.2 eV) activity under visible light, photocatalysts have also been coupled with LaFeO3. This composite was prepared by a process known as solution combustion synthesis, using citric acid as the organic fuel and metal nitrates, thus drastically affecting the bandgap energy, which decreased from 3.2 to 1.94 eV [26]. However, although this method might represent a suitable approach for preparing photocatalysts that work effectively under visible light, the coupling between semiconductors can suffer from the photo-corrosion phenomenon, negatively affecting the photocatalytic activity [27,28,29].
2.1.2 Photocatalyst Doping with Metals
In order to improve the photocatalytic efficiency of primary photocatalysts under visible light, TiO2 and ZnO were also doped with metals (second-generation visible-light-active photocatalysts), by inserting a metal ion in the crystalline structure of the semiconductor. Generally, doping with metal ions is able to generate further energy levels between the valence band and conduction band of the undoped semiconductor, shifting the absorption properties in the visible region due to the decrease in bandgap energy of TiO2 or ZnO. Several metals have been investigated in the synthesis of second-generation visible active photocatalysts, such as Mn, Fe, Co, Ni, Cu for ZnO [5, 30, 31] or Mo, Cr, La, Er, Ce for TiO2 [32]. The main drawback of this type of doping is that metal ions could act as recombination centers of electron–hole pairs, reducing the photocatalytic activity [33, 34].
2.1.3 Photocatalyst Doping with Non-Metals
With together the second-generation visible active photocatalysts are of concern, ZnO or TiO2 doped with non-metals element (third-generation visible active photocatalysts) have been widely investigated. The doping with non-metals can significantly extend the visible light absorption of the doped-photocatalysts and minimize photogenerated charge recombination [35]. Doping process with non-metals (mainly anion of C, N, F, P or S) aims to replace oxygen atoms with these elements in the semiconductor lattice. The high activity under visible light irradiation of the third-generation visible active photocatalysts is due to the decrease of their bandgap by mixing p orbital of the anions with 2p orbital of oxygen [32, 36]. Moreover, the inclusion of these elements in the semiconductor crystalline structure causes the formation of some defects that delay the recombination of the photo-excited species [37, 38].
2.2 Photocatalyst Preparation Methods
One of the main limitations of the application of photocatalytic processes to wastewater treatment at full scale is related to the preparation of the photocatalysts, which significantly affects the cost of the process, particularly compared with homogenous photo-driven AOPs [103, 150]. Unlike HPC technology, which is expensive to produce in terms of photocatalyst preparation and reactor design, UV/H2O2 is easy to implement, and H2O2 is widely available commercially. In order to provide a contribution to fill this gap, the easiest and possibly most cost-effective preparation methods for the synthesis of pure or doped semiconductors are summarized and discussed in the following subsections. A comparison summarizing the main advantages, disadvantages, and prospective applications at large industrial scale is also proposed.
2.2.1 Sol–Gel Method
Sol–gel is the most commonly used method for the preparation of photocatalysts. A sol is made by solid particles homogeneously dispersed in a liquid medium in colloidal form, whereas a gel is an organized three-dimensional continuous solid arrangement having sub-micrometer-sized pores in which the liquid phase is present. During sol–gel synthesis, the sol is produced from the hydrolysis and polymerization reactions of the precursors (usually metal alkoxides). Briefly, the sol–gel method involves four basic steps: hydrolysis, polycondensation, drying, and thermal decomposition of precursors. A schematic of the sol–gel process for the preparation of TiO2-based photocatalysts is showed in Fig. 1 [32].
Preparation of TiO2-based photocatalysts by sol–gel method [32]
Typically, a solution containing a precursor salt of the doping element (metal or non-metal) is added as the sol is formed. In this way, strong covalent bonds are created between the dopant element and the very reactive monomeric species of the precursor of the semiconductor (e.g., TiO2 or ZnO). Several photocatalysts active under visible light have been prepared by the sol–gel process, including TiO2 doped with nitrogen, boron [39], cerium [40], fluorine [41], iron, zinc [42], molybdenum, and chromium [43]. In addition, ZnO has been doped with different metals and non-metals (e.g., nitrogen, aluminum, silver, copper, cobalt) [44,45,46,47,48].
A Sol–gel method was also recently used for doping ZrO2, a metal oxide with a very large bandgap (about 5 eV), an energy corresponding to a negligible fraction of the solar light at the earth's surface [49]. In this case, sol–gel synthesis was carried out using Ce isopropoxide and Zr propoxide solutions. Sol–gel synthesis has also been coupled with a dip-coating procedure to immobilize visible active photocatalysts on macroscopic and transparent supports in order to formulate structured photocatalysts for use in water/wastewater treatment applications. For example, an N-doped photocatalyst was immobilized on glass spheres and tested for the removal of organic dyes from wastewater under visible light irradiation [50]. In the preparation procedure, triton X-100 (used as binder) was dissolved in isopropyl alcohol, and the pH of the solution was adjusted with nitric acid to about pH 2. Titanium isopropoxide, used as titanium precursor, was then added to the mixture [50]. The N-doped TiO2 coating was achieved by leaving the glass spheres in the solution for 10 min, with subsequent calcination for 30 min at 450 °C. This method was able to obtain N-doped TiO2 nanoparticles which were well dispersed on a glass substrate.
2.2.2 Hydrothermal Synthesis
Hydrothermal synthesis requires the use of high temperature and water pressure. When another solvent is used instead of water, this method is known as “solvothermal” [51]. The synthesis of photocatalysts with this method is typically carried out in steel vessels operating at high pressure (autoclaves) under controlled temperature, and the formation of nano-catalysts takes place in the liquid medium [52].
This method has been found to be very effective for incorporating dopants into the crystalline structure of TiO2 or ZnO. Because of the high photocatalytic activity achieved by the controlled synthesis of hollow TiO2 particles, it has attracted much interest among the scientific community [53]. For example, Zhou et al. prepared flower-like F-doped TiO2 hollow microspheres using a hydrothermal synthesis method by controlling the hydrolysis of TiF4 in a autoclave lined with Teflon at a reaction temperature of 180 °C [54].
2.2.3 Precipitation Method
The preparation of photocatalysts through the precipitation method consists of the chemical transformation of a highly soluble metal precursor into another substance of lower solubility, which precipitates in solution. The conversion into the low-solubility compound (and then into the precipitate) is usually obtained by changing (generally by increasing) the pH of the solution [55]. To avoid a rapid precipitation in solution (that can cause a strong increase in particle size), it is better if the mixing and the generation of the precipitant are carried out separately. At the laboratory scale, this is possible through the use of a base. The precipitation method using a base has been applied for the preparation of various catalysts. Upon increasing the pH of the solution, the precipitation of a hydroxide is induced. The semiconductor typically prepared through this method is ZnO. In particular, the preparation involves the reaction of zinc salts such as Zn(NO3)2, Zn(CH3COO)2, or ZnSO4 with solutions containing NH4OH, NaOH, etc. [56, 57]. For the doping of ZnO with metals in order to shift its absorption to the visible region, the precursor salt of the doping element can be added to the solution of the zinc precursor before inducing precipitation [9, 58, 59]. The obtained precipitate is then transformed into the ZnO-doped photocatalyst through thermal treatment.
2.2.4 Solution Combustion Synthesis
Solution combustion synthesis (SCS) is a well-known synthesis method used for the preparation of inorganic compounds for many catalytic, photocatalytic, and electrocatalytic applications. The method is based on the redox reactions that take place between a fuel and an oxidant in the presence of metal cations. Oxidants are metal precursors such as metal nitrates, while fuel is an organic material such as glycine, urea or citric acid. The final products of this synthesis are characterized by high purity, narrow particle distribution, and good agglomeration [60, 61]. Another advantage is the possibility of using different precursors, both soluble and insoluble. Solution combustion synthesis is characterized by three main steps: (1) the formation of the combustion mixture, (2) the formation of the gel, and (3) the gel combustion [62]. A schematic description of these three steps is shown in Fig. 2 [62]. The metal precursors are mixed in water solution with an organic fuel. The product obtained at the end of the combustion process is a soft powder, typical of combustion synthesis processes whose characteristics depend on the parameters chosen for the synthesis, such as the type of fuel.

(Adapted from [62])
Schematic representation of the three main steps of solution combustion synthesis (SCS)
The SCS process is very fast. Fast kinetics inhibits the sintering of the particles and guarantees a certain degree of porosity in the powder obtained. The microstructure of the material can change in relation to the type and quantity of fuel. It was also observed that the interaction of citric acid (fuel) with metal cations occurs through formation of metal chelates forming a gel network, where metal cations are uniformly distributed [63].
One of the most well-studied and commonly used perovskites for wastewater and water treatment purposes is LaFeO3 [64, 65]. The characteristics of LaFeO3 photocatalysts prepared with SCS using different amounts of citric acid have been reported in the literature [11]. The results in terms of X-ray diffraction (XRD) patterns showed well-indexed diffraction peaks, clearly indicating the formation of an orthorhombic perovskite-type structure. Moreover, it was possible to observe from the XRD that the crystallite size of perovskite decreased as the amount of citric acid used in the synthesis was increased. On the other hand, different amounts of citric acid caused changes in the crystalline structure of perovskites [11]. When LaFeO3 was prepared with the highest amount of citric acid (2.15 g in 100 mL of distilled water), a partially amorphous structure with respect to the LaFeO3 synthesized using the lowest fuel content was observed. It is worth noting that the SCS method was also applied for the preparation of an LaFeO3 layer on corundum or cordierite honeycomb monoliths and studied as a structured catalytic system for the photo-Fenton oxidation of organic pollutants in aqueous solution [66, 67]. The thin walls of the monolithic supports (triangular channel) were treated with aqueous solutions of iron and lanthanum nitrate in the presence of citric acid and ethylene glycol. The samples were dried and calcined in air at 900 °C for 4 h to form a grainy porous LaFeO3-supported layer [68].
2.2.5 Ultrasound (or Sonochemical)-Assisted Synthesis
Sonochemical synthesis is a method that uses the principles of sonochemistry for the synthesis of new molecules or particles by the application of ultrasound. The chemical effects of ultrasound derive from acoustic cavitation. The collapse of the bubbles in the liquid generates a huge amount of energy from the conversion of the kinetic energy of the liquid motion into heating. The high local temperature and pressure, combined with extraordinarily rapid cooling, provide a unique means of driving chemical reactions under extreme conditions [69]. The implosion of the bubbles creates extreme conditions that enable synthesis to be conducted on the benchtop in liquid at room temperature that in other cases would have required high temperature and pressure or long reaction times [70]. During the preparation of nanomaterials with ultrasonic irradiation, the phenomena responsible for sonochemistry can be characterized under “primary sonochemistry”, “secondary sonochemistry”, and “physical modifications” [71]. Xu et al. reported “primary sonochemistry” as the reaction occurring inside the collapsing bubbles and “secondary sonochemistry” as the reaction in solution phase occurring outside the bubbles. These phenomena are responsible for the chemical effect of ultrasound and occur only if the reaction is sonication-sensitive or when the energy released during cavitation collapse participates as reaction intermediate [70]. In particular, sonolysis of water produces highly reactive H· and OH· radicals, which can be utilized for various sonochemical reactions. These generated free radicals can further react with each other to form new molecules and radicals or diffuse into the bulk liquid to serve as oxidants. The reaction that produces free radicals can occur within the collapsing bubble (thermolytic center), at the interface between the bubble and bulk liquid, or in the adjacent liquid. Several studies have reported that the predominant effect in heterogeneous sonochemical reactions used for the production or modification of semiconductors is the physical one [72]. In particular, it is the impact of jets of liquid at high speed on the particle surface that can cause erosion and corrosion phenomena, by modifying the particle surfaces. In this way, surface nanostructures or disaggregated particles can be generated [71].
The mechanisms governing ultrasound applications can be summarized as follows [73].
Homogeneous reactions that proceed via radical mechanisms are affected by sonication, while ionic reactions are not affected by ultrasound.
Heterogeneous reactions involving ionic species are influenced mainly by the physical effects of cavitation (e.g., by the reduction in particle size). In this type of reaction, the chemical effects are not dominant, so it is important to select the appropriate operating parameters.
Heterogeneous reactions involving radicals or combined mechanisms (ionic and radical) are significantly influenced by the ultrasound effect. The radical reactions are intensified by the presence of ultrasound, but the physical effects also greatly affect the mass transfer rate, improving its efficiency.
As regards the ultrasonic application for photocatalyst synthesis, the preparation of anatase and rutile TiO2 was reported by Huang et al. [74]. The authors compared the sol–gel method with the sonochemistry procedure, highlighting that the ultrasound process resulted in a perfectly crystalline TiO2 structure. The procedure used to obtain the titania photocatalyst typically involves the treatment of the precursor deionized water solution by ultrasound. During the process, the precipitates are separated by centrifugation, then washed and vacuum-dried overnight until the final product is obtained. In addition, the application of high-intensity ultrasound irradiation allows a mesoporous TiO2 to be obtained without the addition of surfactant compounds [75]. According to the scientific literature, this method has the capacity to (1) increase crystal growth rate, (2) decrease induction periods and metastable zone width, (3) improve particle size distribution and morphology, and (4) achieve higher control of the nucleation process [76]. Moreover, the synthesis of photocatalysts by ultrasound is considered a “green technology”, as it is a fast, clean, and efficient option that enables the formation of reaction conditions at ambient temperatures in cases that would otherwise require high temperature and pressure to yield the same results. An additional benefit is that it is an extremely versatile synthetic procedure that cab be used for the development of various photocatalysts. The materials synthesized by this method show clear improvements in terms of photocatalytic performance over photocatalysts synthesized using traditional methods.
2.2.6 Comparison of Photocatalyst Preparation Methods
Table 1 summarizes the main advantages, disadvantages, and feasibility of large-scale application of the photocatalyst preparation methods discussed in the previous paragraphs.
Although all of the methods described can generate effective photocatalysts, from a large-scale application point of view, it appears that SCS and hydrothermal synthesis are less strongly recommended methods, as they require high thermal energy and high pressure [83]. On the other hand, sol–gel and precipitation methods may be feasible at a large scale if no expensive metal precursors are required.
3 Wastewater Treatment by HPC: Great Potential but Some Limitations and Drawbacks
The efficiency of photocatalytic processes in water and wastewater treatment can be negatively affected by different factors, including low photoconversion efficiency and the occurrence of reactive oxygen species (ROS)-scavenging substances in the target water/wastewater matrix. Moreover, technological limitations such as the selection of a more suitable photocatalyst and preparation method or the choice between slurry (need to recovery the powder photocatalyst after treatment) and supported photocatalyst systems have thus far discouraged the application of heterogeneous photocatalysis for water and wastewater treatment at full scale.
3.1 Photoconversion Efficiency
Low photoconversion efficiency is a major limiting factor in the application of photocatalytic processes, even when compared with other AOPs. Quantum yield can vary widely depending on the photocatalyst, experimental conditions, and wastewater matrix [86]. Taking UV/H2O2 AOPs as an example, the photolytic decomposition of H2O2 is characterized by a quite high Φ and ·OH production yield [87], making HPC not so competitive in terms of energy efficiency. However, it is worth noting that TiO2 can also be effective under sunlight, and more effective than sunlight/H2O2 [88].
3.2 Interfering Substances
A wastewater matrix can significantly affect HPC efficiency due to the presence of natural organic matter (NOM), carbonate species, and other background constituents that can scavenge ROS and absorb light [89, 90]. Indeed, HPC is susceptible to the differences in the light absorption factors of the wastewater. The UVA or solar radiation supplied to the reactor needs to travel through a column of water (path length). The presence of total suspended solids (TSS) and numerous soluble substances in the wastewater results in a loss of transmittance, via absorption but also scattering and reflection. This results in energy loss from light that does not reach the photocatalyst’s surface. At wavelengths relevant for UVA photocatalysis (365 nm), there is considerable absorption (Fig. 3).
UV–Vis absorption spectrum of secondary treated wastewater. Under CC 4.0 from supplementary information of [5]
The path length that the electromagnetic radiation has to travel is also crucial. According to Beer–Lambert’s law (Eq. 1), a directly proportional relationship between path length and absorption exists, and doubling of the path length results in doubling of the absorption (logarithmic units). The molar absorption coefficient (ε) is a constant specific for each chemical compound and the wavelength at which it is measured, while \( l \) is the path length and c is the concentration of the compound in question. In wastewater, the measured absorbance is the summed absorbance of all compounds in the wastewater
This limits reactor design, especially for reactors with immobilized photocatalysts versus suspended (slurry) photocatalysts, since in the former light is absorbed only after passing through the entire path length, not throughout, as would happen in a suspended photocatalyst reactor.
3.3 Suspended Versus Immobilized HPC
HPC for wastewater treatment in a lab setting is commonly studied as a suspended powder. Maintaining a powder in suspension in a large body of water is very energy-intensive. It is also important that the water is somewhat aerated, since an external source of dissolved oxygen enhances the generation of ROS via electron–hole pairs. Anoxic conditions [i.e., lack of O2(aq.)] prevent the generation of both hydroxyl radicals and superoxide radicals, as the electron promoted to the conduction band does not transfer to dissolved oxygen to form superoxide but recombines with the hole [91]. Precipitation of the suspended photocatalyst to the bottom of the tank would be extremely detrimental to the efficiency of the process, since light adsorption would be at a minimum and contact between targeted compounds/bacteria and the catalyst low. Thus, the cost of circulating the wastewater during treatment in a suspended reactor is essential and should be accounted for. Using the photocatalyst in powder form adds a further processing step to remove the catalyst and recover it for reuse. This can be by gravity precipitation, induced agglomeration and precipitation or filtration, all of which further increase operating costs. In an effort to circumvent these costs, catalyst immobilization has been proposed [5, 92,93,94]. Immobilization of a photocatalyst is the coating of the macrostructure with a layer of photocatalyst that is exposed to the water and that can receive UVA/visible light. Numerous materials (glass, alumina, silica, metal, fibers) have been successfully coated with a photocatalyst [95]. This serves the dual purpose of reducing the energy expenditure for keeping a powder in suspension and eliminating the catalyst recovery step. Utilizing immobilized photocatalysts does have its drawbacks. Reactor design in intrinsically more complex and ultimately is a compromise between maximizing the illuminated catalyst-coated surface area and reactor volume while minimizing the path length of wastewater above the coated surface from the light source. The processes used to immobilize a photocatalysts can also be complex or costly. Despite the limitations, pilot-scale reactors with immobilized catalysts are reported in the literature and have been successfully employed for both CEC removal and bacterial inactivation [96, 97].
4 Application of Heterogeneous Photocatalysis to Wastewater Treatment
4.1 Tertiary Treatment of Urban Wastewater
The three conventional stages of urban wastewater treatment consist of a primary stage of physical separation by sedimentation of solids and skimming off of floating contaminants, a secondary stage for reducing biodegradable organic load by bacterial growth, and a tertiary stage of nutrient removal (typically achieved by biological processes) and/or disinfection and/or removal of micropollutants such as CECs (including pharmaceuticals, pesticides, personal care products, and any other pollutant that is found in micrograms-per-liter or lower quantities in wastewater). The tertiary stage is not considered essential, and only a few countries have regulation that require the use of tertiary treatment to remove one or more of the contaminants explained above. When tertiary treatment is used, the main factors driving the choice of treatment are the efficiency in removing the target contaminants set by the local/national regulation and the cost. The tertiary treatment applied depends on the target parameter(s) that need to be brought within regulatory limits. If such a target is the reduction of bacterial load, then chlorination, peracetic acid, or UVC disinfection is commonly employed [98,99,100]. On the other hand, if CECs should be removed, disinfection processes are no longer effective, and advanced treatment methods are necessary [101]. Indeed, in Switzerland, according to the new national water act (Swiss Federal Council Waters Protection Ordinance) [102], 70% of the urban wastewater treatment plants (UWTPs) must be upgraded with ozonation or adsorption treatment methods to remove 14 selected CECs by 80% of inflow levels. In such a context, homogeneous photo-driven AOPs may be competitive with consolidated technologies in the short term, and HPC may be feasible if some limitations/drawbacks are successfully addressed [103].
4.1.1 HPC for Micropollutant Removal and Bacterial Inactivation
HPC has been intensively studied as a potential technology for the mineralization of organic contaminants and the inactivation of bacteria in the effluents of secondary treated urban wastewater. While full mineralization is not considered feasible for urban wastewater, a wide variety of catalysts have been employed for pollutant degradation and disinfection. These include dyes, [104, 105] endocrine disruptors [106], pharmaceuticals [107] including specifically antibiotics [108, 109], and agricultural chemicals [110, 111], and for bacterial inactivation [112, 113. The most imposing obstacle hindering widespread use of heterogeneous photocatalysis as a tertiary treatment in UWTPs is the high cost associated with photocatalysis, which can be more than an order of magnitude greater than established AOPs [103]. Nevertheless, HPC could find application in the treatment of wastewater intended for reuse, which has stricter water quality requirements than current requirements. The need to achieve higher requirements could outweigh the limitations of HPC when compared with other available treatments, especially when considering degradation of organic chemical pollutants, toxic disinfection by-product formation, and antibiotic resistance concerns in wastewater reuse.
4.1.2 Variability in Wastewater Loads and Its Effect on Treatment
As for challenges of a technical nature, the variability in wastewater in terms of organic contaminant load (COD/DOC) has major effects on the process and the duration required for treatment. ROS generated by photocatalysis, such as hydroxyl radicals, are highly reactive but also highly unselective. This translates to reactions between any form of organic matter, whether harmless humic acids or chemical pollutants that are the target of the treatment. Choi et al. measured the pseudo-first-order rate constants of degradation for acetaminophen and carbamazepine in distilled water and real wastewater from different wastewater treatment plants (WWTPs) having different DOC loads [114]. Relative to distilled water, i.e., where DOC is entirely due to the contaminants of interest, real wastewater showed slower degradation of these two drugs by factors of 3–6. While concentrations of ions such as bicarbonate, chloride, and nitrate in low milligrams-per-liter quantities have a negligible effect on the rate constant, humic acid load drastically reduces the rate of removal (Fig. 4). It should also be noted that slowing of removal kinetics is not linear with DOC load, but rather plateaus after a certain load DOC [114].
Effect of initial concentration of humic acid (SR-HA), bicarbonate (HCO3−), nitrate (NO3−), and chloride (Cl−) on the rate constant for the degradation of carbamazepine. Reproduced with permission from [114]
The rate constants of hydroxyl radicals, the dominant radical in HPC, with most organic compounds approach the theoretical maximum imposed by diffusion-controlled reaction kinetics [115]. This means that while there are differences in reactivity between different organic compounds in water, these differences tend to be mostly of the same order of magnitude (log kOH 9.5 ± 1) [116]. As CECs occur at concentrations many orders of magnitude lower than interfering substances, but both have a rate constant within one order of magnitude, this results in a very small fraction of the ROS probabilistically reacting with CECs as dictated by competitive kinetics between the ROS formed and organic compounds in the water phase. The reduction in the rate of pollutant removal due to high contaminant load and/or the presence of scavengers is a well-established fact [117]. However, such an interfering effect can be overcome to a certain extent in some AOPs, such as ozonation or UVC/H2O2, where the concentration of the oxidant can be increased to achieve a suitable ratio between oxidant and total DOC load. By increasing the oxidant load (ozone, hydrogen peroxide, etc.), the concentration of hydroxyl radicals increases, yield a higher rate. The rate constant (k) is not affected by the concentration of reactants; thus, at the same temperature, increasing the concentration of the reactants increases the overall reaction rate of both CECs and DOC (since they compete for reaction with ·OH as per Eqs. 2 and 3).
Fixing the rate of oxidant to the [DOC] (concentration of DOC) thus aids in stabilizing the reaction rate.
The performance of the catalyst in the application of HPC to water/wastewater treatment peaks at a certain catalyst load. A further increase in catalyst loading would be counterproductive, resulting in reduced efficiency due to the increased opacity of the aqueous matrix. Other factors such as light intensity can be increased, but only up to a certain threshold of saturation. Additionally, commonly used low-pressure mercury lamps are not dimmable, and increasing the intensity would require increasing the number of active bulbs. Moreover, adsorption and electrostatic forces should not be underestimated in heterogeneous photocatalytic process. HPC involves reaction between two materials in different states of matter, and pollutant adsorption on the surface of the catalyst has a drastic effect on removal efficiency. This is because radicals are generated at the semiconductor–water interface, and these species have a very short half-life due to their very high reactivity. Having compounds adsorbed at the site of radical generation increases the probability of reaction of these radicals with the adsorbed species. This is also applicable for HPC for water disinfection, as E. coli and other Gram-negative bacteria are negatively charged due to the ionized phosphoryl and carboxylate components in the cell wall [118, 119], and consequently they are attracted to positively charged semiconductor photocatalysts. These effects also influence the rate of removal/inactivation in other ways; for example, bicarbonate ions are known to adsorb to titania, reducing its activity [120] by preventing electron transfers that generate ROS to take place at the site of adsorption.
4.1.3 Irradiance and Transmittance Through Wastewater
The presence of TSS/turbidity and light-absorbing substances in wastewater results in a loss of transmittance (via absorption but also scattering and reflection) and, consequently, a loss of energy because of the reduced light intensity that will reach the photocatalyst’s surface (as we already saw in Fig. 3). In the day-to-day operation of an HPC tertiary treatment process, the additional variability resulting from this issue can negatively affect process efficiency. Just like ozone content is dosed relative to the DOC load in water, some HPC process parameters can be modified depending on the type of reactor utilized. An online measure of UV–Vis transmittance at appropriate wavelengths has been suggested as a proxy measure for contaminant load [121]. Thus, this can be used to adjust some HPC parameters, e.g., in semi-batch reactors such as raceway reactors [122] the volume of wastewater treated can be increased and decreased accordingly. A lower volume in a fixed reactor results in a shorter water column and thus higher transmittance. Batch reactor residence time can be adjusted as well.
4.1.4 Requirements for Widespread Adoption of Photocatalysis: A Technological Niche for HPC?
Photocatalytic reactors, including solar reactors, have been reviewed in detail in other publications (see [123, 124]), and the obstacles to widespread use are not so much technological, but rather a lack of market demand given the higher costs and levels of treatment obtained by HPC-treated wastewater.
The most critical requirement for widespread adoption of HPC is the establishment of regulations mandating reduced CEC load in discharged effluents and/or water intended for reuse. The case for reducing CECs in water intended for reuse, specifically agricultural irrigation, is especially strong. Paltiel et al. demonstrated that persons consuming crops irrigated with reused wastewater had significantly higher blood levels of carbamazepine compared with those who consumed vegetables irrigated with fresh water [125]. While the levels recorded are orders of magnitudes less than therapeutic levels, there is still cause for concern, both because of chronic exposure to such compounds and due to the cocktail effects from the presence of numerous compounds and their transformation products, many of which are not known. High-level treatment of wastewater intended for food crop irrigation would thus be required if one were applying precautionary measures.
Another potential issue that might make HPC a necessity is the environmental dimension of antibiotic resistance. Conventional tertiary treatment processes and ozonation are both associated with an increased prevalence of antibiotic-resistant bacteria (ARB) and antibiotic resistant genes (ARGs) [126]. Such an increase in prevalence results a process of natural selection in bacterial mortality, that is, when non-resistant bacteria are more susceptible to treatment than resistant bacteria. Therefore, while the overall bacterial loads decrease, the percentage of resistant bacteria within the total bacterial load increases [127,128,129]. This shift in population dynamics introduces risks in the downstream environment such that the more prevalent resistance is now more likely to thrive in the environment. Chlorination and UVC treatments are known to cause resistance selection [127, 129]. Ozonation, which is also an AOP with high operating costs, is associated with increased ARB and ARGs as well [126]. Since HPC application for AR resistance mitigation is largely in its early stages, most studies on the matter have dealt with its ability to reduce the absolute number of ARB and ARGs, not specifically on reducing relative abundance [94, 112, 130]. Other studies have targeted abundance [5, 131], but because of the lack of full-scale application in WWTPs, it has not been fully established whether HPC also increases prevalence. If antibiotic resistance associated with wastewater reuse is deemed a high risk in need of regulation by the authorities, and HPC is found to be an effective technology for AR mitigation, the high cost of HPC could be justified by its efficacy and by the lack of suitable alternative treatment methods.
Without fulfilling such a need, photocatalysis needs to become at least one order of magnitude more efficient [103], an obstacle that as yet has not been overcome despite the intense level of research on the matter. The preferential use of modified catalysts over commercially available ones can play a pivotal role. These modifications must result in both higher efficiency in the generation of radicals (i.e., quantum yields for hydroxyl radical generation) and the ability to harness photonic energy beyond the UV range and into the visible range, thus enabling solar treatment. Energy production in a power plant and the transport and powering of UVA lamps involve substantial energy losses at each stage, which can be avoided by using solar reactors as a cost-effective strategy. However, solar-driven treatments have problems as well. Even if future engineering modifications result in a sufficiently efficient visible-light-active photocatalyst that makes HPC competitive with other AOPs, solar treatments are still limited by the actual duration of daylight and by the fact that solar reactors need a large footprint in terms of land area. Thus, they are more feasible in smaller, less densely populated cities, where the value of land is not as high as it is close to major cities. Additionally, solar treatments are limited seasonally, with lower treatment potential in winter, as well as geographically, with latitudes farther from the equator having lower insolation and hence treatment potential. While this limits solar-based technologies for the treatment of all types of wastewater, it is much less an issue for solar-based treatment of water specifically for agricultural reuse. Firstly, areas with intense agriculture activity are located far from major cities, and thus the value of land is low allowing for larger footprints. Additionally, the need for irrigation is stronger in latitudes and seasons where insolation is higher. A wide variety of solar reactors have been proposed, including parabolic-based reactors and raceway pond reactors [124, 132]. Parabolic reactors are more suitable in low-insolation conditions but are more expensive than raceway pond reactors. The latter have been used mostly for homogenous AOP processes [133], since the low flow rate of the water inside the reactors would require a catalyst to be immobilized to avoid powdered catalyst sedimentation.
In summary, there are tangible benefits in risk reduction with tertiary treating to a high standard, especially for the case of wastewater for agricultural reuse. The ripest area for development is the combination of cheap raceway pond reactors with immobilized solar-active photocatalysts being employed as the need arises to alleviate temporal water scarcity bouts.
Even at equivalent outcomes, a consolidated system is always favorable to a newer system. Thus, for HPC to be competitive and turn the tide on other AOPs, it does not need to simply match the benefits of currently employed AOPs, but to exceed them. A possible strong point for immobilized solar HPC is that it would not require additional material input, and would require low energy input only for mixing, as opposed to the more consolidated ozonation or Fenton processes which have these requirements.
Another pathway that can lead to increased acceptance of photocatalysis as a treatment is by combining it with other treatments. There are two main ways to combine treatments: simultaneously and in cascade/sequentially. The most common simultaneous use of HPC is its coupling with ozonation. This type of treatment was recently reviewed [134]. The premise of such a combination is that the combined process is more efficient than either of the separate processes, by means of additional pathways that lead to ROS formation through the interaction of the photocatalyst and dissolved ozone as well as direct reactions with molecular ozone [134]. Sequential applications of HPC and other water treatments are not at all well studied. Examples exist of other AOPs that are applied in combination with or after another treatment such as chlorination exist [135, 136], but no suitable examples for HPC have been noted. The benefit of using HPC in a cascade with other treatments includes the fact that the intensity of treatments can be lower than what would be used individually; for example, if chlorination is to be used prior to a photocatalytic process, it is possible to reduce the quantity of chlorine applied and thus reduce the concentration of chlorinated by-products, while meeting the same targets that would not be possible with only one of the sequential treatments. An additional benefit, due to the different oxidation mechanisms, is that one process may be more active on a certain type of contaminant, while the other process is more active on another type; thus when used in cascade, the overall efficiency is higher.
A different approach for improving photocatalytic performance is to reduce the recombination of electron–hole pairs. A particularly interesting solution, in contrast to doping with metals, is represented by the modification (or combination) of the semiconductor with graphitic materials [137]. These materials are interesting because they have excellent electrical conductivity and are considered promising materials for photocatalysis [138]. In recent years, graphene-based photocatalytic processes have also been implemented through the combination of photocatalysis with other techniques. In particular, several studies report interesting results regarding the application of photoelectrocatalysis as an effective process used to suppress the recombination of charges [137]. In an photoelectrocatalytic process, an external bias potential is used. The electrons generated are accumulated on the external cathode and the holes on the photocatalyst. The electrodes used in photoelectrocatalysis are generally composed of photocatalyst and co-catalyst deposited on conductive glass. Different photocatalytic materials have been used in photoelectrocatalysis, among which TiO2 is one of the most widely studied semiconductors and is used as photoanode. Other materials active in the presence of visible light have also been used for their good performance as photoanodes [139].
However, if the photoanode consists of only one component, it is characterized by the problem of recombination of the charges. For this reason, making changes to the composition of the photoanode can be useful. In particular, an interesting approach is the use of co-catalysts, and among these, graphitic materials are very promising. Graphitic materials with two-dimensional structures are characterized by excellent electrical conductivity and can be used as electron transporters in order to improve the separation of photogenerated charge carriers and to enhance photoelectrocatalytic activity [137]. Various studies have investigated the ability of graphene to attract and transport electrons, improve the adsorption of reagents, and confer the ability to absorb light in the visible field to semiconductors [140,141,142,143,144].
4.2 Gray- and Stormwater Treatment
Gray water includes wastewater generated by households from sources such as sinks, showers, baths, laundry washing machines, and dishwashers, but does not include fecal-contaminated sources. Stormwater originates from precipitation events, including snow and ice melt. Stormwater can be absorbed in soil, stored in water bodies as surface water (e.g., in ponds and lakes), evaporate, or be transported via streams and rivers. HPC has seen some research applications in gray-water and harvested stormwater treatment. Wang et al. [145] used TiO2-graphene oxide composites at various percentages in artificial stormwater for the disinfection of spiked E. coli under solar light. While the catalyst showed good reusability, even after ten cycles, in the optimal case only up to 1 log removal was reported from an initial bacterial load of 104 CFU/mL. The catalyst, due to its surface charge, was very efficient in reducing TSS by co-sedimentation of any waterborne particles with the photocatalyst. The authors suggest that such a treatment could take place with photocatalysis during daytime, coupled with a long period of sedimentation for catalyst recovery and TSS reduction overnight. However, the application of HPC for gray-water or stormwater is not ideal—HPC, as a process that is reactive to virtually all organic compounds, would benefit from an initial biological process in order to remove biodegradable organic compounds. Such a setup was demonstrated by Garcia et al. following the removal of TOC in rainwater using a combined ozonation-photocatalytic system, but only after treatment in a bioreactor for the removal of biodegradable compounds [146]. Other examples of gray-water treatment by HPC have also been reported [147, 148]. While research on HPC treatment of gray/stormwater has been published, it is a very small part of water treatment. Both in terms of microbial contamination and potentially toxic compounds, gray/stormwater is of negligible importance relative to wastewater.
4.3 Industrial Wastewater Treatment and Energy Recovery from Hydrogen Production
Industrial processes utilize water that can be contaminated by any organic or inorganic compounds used in the process. Thus, the characteristics of urban wastewater are completely different from those of industrial wastewater. The role of AOPs in industrial wastewater treatment is often to improve its biodegradability and/or reduce toxicity, before applying a biological process. AOPs can also be used after biological treatment of industrial wastewater to further reduce residual contaminants to meet the standards for effluent disposal or reuse. In the following subsections, the application of HPC to various types of industrial wastewater (food, textile, tanning, and pharmaceutical/pesticide) are critically reviewed by discussing its potential, limitations, and prospects.
4.3.1 Pharmaceutical and Pesticide Industry Wastewater
Pharmaceutical compounds are typically detected in urban wastewater at concentrations in the range of nanograms to micrograms per liter [149], with TOC levels in the low milligrams-per-liter range [150]. Although pharmaceuticals can be resistant to treatment and have deleterious effects downstream, at the levels they are found in urban wastewater they do not cause operational problems to the UWTPs. However, wastewater from pharmaceutical industries presents a distinctive case. These wastewater effluents contain high levels of TOC, with concentrations even reaching grams per liter [151], and high levels of compounds that are specifically designed to be bioactive and hence have a high potential for toxicity. Because the volumes of water are much lower than in urban wastewater, Fenton processes are more commonly used to treat pharmaceutical wastewater, as the high TOC load and lower water volumes make it feasible to alter the pH to meet the requirements for Fenton processes. However, photocatalysis has also been studied at the lab scale for treatment of pharmaceutical industrial wastewater. Ahmadi et al. used a TiO2 coupled with carbon nanotubes to treat pharmaceutical wastewater; 0.2 g/L of catalyst was able to reduce the TOC from 1295 to 228 mg/L in 240 min of irradiation. Deng et al. utilized a silver-based photocatalyst to reduce the TOC of real pharmaceutical wastewater from 25 to 10 g/L in 500 min [152], while Verma et al. used a commercial TiO2 preparation to reduce the COD by approximately 90% after 7 h from an initial load of 2.5 g/L.
Industrial wastewater from the pesticide industry is in many ways identical to that from the pharmaceutical industry. Pesticides, like pharmaceuticals, are bioactive compounds, are designed to be stable in order to perform their designed function, and can interfere with biological treatments. HPC has also been studied for the treatment of industrial wastewater from the pesticide industry. Alalm et al. [153] compared the efficiency and cost of using HPC (commercial TiO2) versus a solar photo-Fenton process for treating real agrochemical/pesticide wastewater with a high COD load of 7 g/L. The wastewater was treated using parabolic solar collectors and showed very high efficacy for both processes, especially when taking into account the COD load present. Under the optimal conditions studied, solar HPC was able to reduce the COD load by 80%, while the photo-Fenton process reached 91%. As already mentioned for pharmaceutical wastewater, Fenton processes are generally preferred for industrial wastewater of this type, since the lower water volumes make pH alteration economically feasible. The authors reached the same conclusion, since they estimated that the best-performing HPC treatment was 1.5 times as expensive as solar photo-Fenton per unit volume of wastewater treated. Other research has been carried out on in situ treatment of wastewater from the agro-industry using innovative solar photocatalytic reactors [154]. Wastewater generated from the washing of equipment used for agricultural pesticide application was treated using commercial titania. While the COD loads of the treated wastewater were not high, in the range of 0.1 g/L, the treatment was able to reduce it by more than 75% of the initial value, with contained costs of operation. Kushniarou et al. also applied a photocatalytic treatment for agro-industry wastewater generated from washing equipment that had been in direct contact with pesticides. With the use of commercial TiO2-P25 and 150 mg/L of persulfate, the grouped concentration of 12 selected pesticides was reduced by > 99% in 1 h [155].
In all cases, commercial unmodified titania was used as the photocatalyst under solar or solar-simulated conditions. This catalyst, with a bandgap of about 3.2 eV, is mostly active under UV radiation, which is a minor fraction of the solar spectrum. This presents an opportunity to further enhance the photocatalytic process in the removal of pesticides from agro-industry wastewater, since the use of a doped photocatalyst with a lower bandgap would result in the utilization of a higher fraction of the solar spectrum and higher expected rates of removals. This improvement might be enough to reduce the cost while also having a major advantage over solar photo-Fenton processes in ease of automation, as it does not require the addition of an oxidant such as hydrogen peroxide.
4.3.2 Food Industry Wastewater Treatment and Energy Recovery
Food industry wastewater, in addition to biodegradable organic substances, can also include organic substances that are not easily biodegradable, such as food dyes.
Synthetic dyes are the largest group of additives used in the food industry, and their by-products, such as phenolic compounds and aromatic amines, are toxic to the aquatic environment because of their carcinogenic and mutagenic nature [156]. These dyes generally contain recalcitrant organic and inorganic groups, and their release into the aquatic environment results in a reduction in light transmittance, thus reducing the penetration of solar radiation through the receiving body of water. Dyes have high thermal and photo-stability, and this makes them resistant to biodegradation [157]. For this reason, they are persistent molecules which remain in the environment for long periods. The main consequence of the presence of dyes in aquatic environments is on plants, since the light absorption by the dye present in the water reduces photosynthesis activity and influences the food chain [157]. Furthermore, some dyes can cause carcinogenic and genotoxic effects on humans [67, 158], and AOPs appear to be an attractive option for removing such pollutants from wastewater.
Although homogeneous Fenton reaction is effective in the oxidation of several recalcitrant pollutants and is used in industrial wastewater treatment as a pre-oxidation step before a biological process, it has some drawbacks. One of the main limitations is that the process relies on acidic operating conditions (optimal at pH < 3) to avoid catalyst precipitation. Thus conventional homogeneous photo-Fenton can improve process efficiency but does not solve pH-related problems (pH pre-acidification, pH post-neutralization, and sludge production, treatment, and disposal). This restriction makes this option attractive only for the treatment of acidic wastewater, because application to neutral or alkaline wastewater would increase operating costs. The improvement of photo-Fenton performance under neutral conditions has been a subject of high interest among the scientific community in recent years. In particular, photo-Fenton has been investigated for the removal of CECs from urban wastewater and inactivation of bacteria, and its operation under mild conditions (pH 5–6) resulted in good efficiency [159, 160]. Other possible approaches include the use of heterogeneous and homogeneous (photo)-Fenton-like processes. In homogeneous processes, Fe2+ is replaced by other metals or possibly combined with organic or inorganic ligands to form complexes and/or to stabilize the metals over a wide pH range [161]. Different ligands including nitriloacetic acid, ethylenediaminetetraacetic acid, oxalic acid, tartaric acid [162], and ethylenediamine-N-N0-disuccinic acid [163], as well as metal–organic complexes, have been investigated so far. Another option for overcoming homogeneous photo-Fenton drawbacks includes heterogeneous catalytic systems based on macroscopic supports (such as corundum, cordierite) [164]. Heterogeneous photo-Fenton methods have been investigated for the removal of dyes from aqueous matrices [165] through the immobilization of Fe ions on clays, bentonite, and laponite [166], or by using iron oxides such as goethite or hematite under non-controlled pH conditions [167]. To overcome the drawback of powder catalyst removal after treatment, macroscopic supports appear to be an attractive alternative. Structured catalysts can be purposefully designed to optimize fluid dynamics. The application of such a catalyst (i.e., LaFeO3 loaded on corundum honeycomb monolithic support) for the removal of food azo dyes from aqueous solutions resulted in complete discoloration and mineralization (evaluated in terms of TOC removal) of two food dyes [Allura Red (RED) and tartrazine (TRZ)] (Fig. 5); notably, the process proved to be extremely efficient at spontaneous pH values (equal to 6) [67].
Effects of LaFeO3 structured photocatalyst for photo-Fenton reaction on TOC removal over the run time for a RED and b TRZ dyes [67]
Among food industrial wastewater, dairy wastewater is a category not easily managed through conventional biological process alone [168]. While it does not contain particularly toxic substances, dairy wastewater is characterized by high organic content including fats and proteins, which can decompose. Their treatment is generally managed through biological processes, but several problems have been noted related to operating pH, variations in the organic loading, and high sludge volume produced [169]. Some attempts to treat such wastewater by HPC have been carried out but were not so successful.
HPC was combined with a flocculation process to remove COD and inactivate bacteria from dairy wastewater [170]. Although a TiO2 P25 Evonik photocatalyst resulted in total inactivation of E. Coli after 5 h of treatment (120 W/m2 of UV–Vis light intensity), an increase in COD and TOC was also observed, which the authors attributed to the formation of organic oxidation intermediaries.
An HPC technique using ZnO thin film as photocatalyst immobilized on a metal plate (800 × 250 mm) was investigated as possible pretreatment (before biological processing) of dairy wastewater [171]. The experiments were carried out using a solar reactor with a fixed dairy wastewater volume (3 L) and a liquid flow rate of 13 L/min. According to the Taguchi L8 orthogonal array used in the experimental design, an effective TOC percentage degradation as low as 14.23% for all conditions at an optimal level was recorded (max degradation percentage of 31.42%). Although the authors recommend the process as a possible pre-oxidation step to improve dairy wastewater biodegradability, taking into account wastewater characteristics (turbidity 2786 NTU, COD 6032 mg/L, total solids 10,720 mg/L, oil and grease 2002 mg/L) as well as initial biodegradability (as BOD5/COD = 0.368), it appears to be more appropriate as a post-treatment method after biological processes, assuming that the reactor can be scaled up.
The possibility of energy recovery from agrifood wastewater has attracted increasing interest among the scientific community due to the potential for organic pollutants to be transformed into useful fuels such as methane and hydrogen. The purification of wastewater with simultaneous energy conversion is an attractive strategy in the context of a circular economy. The use of photocatalysis for hydrogen production is one of the most widely investigated and interesting approaches for simultaneously treating and deriving value (through renewable energy production) from wastewater [172]. For example, hydrogen recovery through photocatalytic treatment of wastewater containing high concentrations of sugars (particularly glucose) has been increasingly investigated [11, 173,174,175]. In particular, for the photocatalytic production of hydrogen, the use of suspended and dispersed photocatalysts has unique advantages such as efficient utilization of light energy and fast transport of pollutants between the powder surface and the aqueous medium. An interesting solution for overcoming photocatalyst recovery problems, while at the same time preserving the characteristics and advantages of a slurry reactor configuration with the photocatalyst dispersed in solution, is the use of magnetic particles as catalyst support materials [176]. For example, a Ru-doped LaFeO3 photocatalyst coupled with magnetic Fe2O3 particles was proposed for the photocatalytic production of hydrogen (5460 μmol/L) from glucose degradation (complete removal after 4 h treatment) under visible light irradiation [176]. Notably, the photocatalyst recovered from the photoreactor using an external magnetic force showed high stability, and its activity did not change even after seven cycles of use (Fig. 6).
Photocatalytic degradation (a) and hydrogen production (b) for Ru-LaFeO3/Fe2O3 photocatalysts for different cycles. Initial glucose concentration: 1000 mg/L; catalyst dosage: 1.5 g/L [176]
The hydrogen production observed was competitive relative to the results of other studies in the literature (e.g., 1580 μmol/L under UV irradiation in the presence of ethanol as sacrificial agent and using a perovskite-based catalyst) [177]. It is not possible to make a comparison in the case of glucose as sacrificial agent, since this last compound has thus far been investigated only in the presence of noble metal-based catalysts [178, 179]. Moreover, the magnetic photocatalyst was also tested on real agrifood industrial wastewater (cherry washing process), yielding significant hydrogen production (12,344 μmol/L).
4.3.3 Tannery Wastewater
The tannery industry is one of the most productive sectors for the economies of some countries, but also one of the most environmentally impacting processes due to the huge consumption of water resources and chemicals and the high-polluting wastewater [180]. Tannery wastewater is characterized by a dark brown color, high COD and BOD5, and the presence of chromium(III) and phenols [181]. The application of HPC with different semiconductors has been intensively studied because of its ability to degrade the pollutants into nontoxic molecules [182, 183]. ZnO supported on glass spheres, through dip-coating technique without using complexing chemicals, have been effective in the discoloration and mineralization of non-biodegradable tannery dyes under UV light irradiation, reaching discoloration and mineralization values higher than 70% [184]. Moreover, the developed structured photocatalyst was also effective in the treatment of real wastewater characterized as having a high COD value (11 g/L) (Fig. 7). COD removal as high as 70% was achieved after 180 min of UV irradiation [184].
a Schematic representation of synthesis and photocatalytic tests using ZnO on glass spheres. b COD behavior as a function of run time during the treatment of tannery real wastewater using ZnO on glass spheres [184]
Another reported application of ZnO-based photocatalysts (doped with rare earth praseodymium) is in the purification of industrial wastewater from the dyeing and finishing processes of the leather industry [185]. In particular, a Pr-doped ZnO photocatalyst was able to obtain a discoloration degree higher than 50% after 240 min of UV irradiation, with a TOC removal rate of about 40% (TOC initial values in the range 540–1200 mg/L) after 180 min of UV irradiation.
4.4 What are HPC Prospective Uses for Wastewater Treatment?
According to the results available in the scientific literature and discussed in the sections above, the application of HPC to wastewater treatment is basically at a “technological research” level, which is a technology readiness level (TRL) between 2 and 6 (Fig. 8). A number of disadvantages remain, including (1) technological limitations, (2) lack of regulations limiting the release of specific contaminants into the environment (namely CECs from urban WWTPs), and (3) still low efficiency relative to other consolidated technologies in treating some refractory (industrial) wastewater (Table 2).
Technology readiness level scale [185]
Although HPC was found to be effective for the inactivation of pathogens and the degradation of CECs in secondary treated urban wastewater, it is not competitive with consolidated technologies (namely ozonation) due to technological limitations [103, 150], which rank it at TRL 4. Even if such technological limitations are successfully addressed in the future, the absence of a specific regulation on the release of CECs into the environment (except in Switzerland) or in wastewater treated for reused discourages urban WWTP managers from replacing conventional tertiary treatments (mainly disinfection by chlorine, peracetic acid or UV radiation) with more expensive processes such as AOP, and particularly HPC. The treatment of industrial wastewater is a more complex matter, and process efficiency strongly depends on the specific industrial process. In such a case, wastewater characteristics can change substantially in terms of organic loading (from tens of milligrams to tens of grams per liter of COD) and in quantity of toxic/refractory contaminants. As high organic loads in wastewater are of concern, HPC is less effective than more consolidated processes (namely ozonation and Fenton) as a pre-oxidation step to improve wastewater biodegradability before a less expensive biological treatment step. Nevertheless, HPC could become competitive provided that certain technological limitations are resolved (e.g., reactor design, synthesis, and development of effective supported photocatalysts), as an advanced treatment of industrial wastewater (after biological treatment) to remove specific contaminants not effectively removed by biological processes (e.g., phenols in olive oil wastewater treatment).
5 Conclusions
Although HPC has been widely investigated in recent decades for the removal of several contaminants from aqueous matrices, due to the several issues outlined herein, its application in real wastewater treatment at full scale is still far from becoming a consolidated technology. However, (1) continuing technological developments in terms of increasingly effective photocatalysts (even under visible light) and easily scalable synthesis methods, along with the design of new reactors and the identification of specific applications in wastewater treatment where HPC can be competitive with consolidated technologies, may promote its application at full scale in the mid- to long term. A contribution in that direction can come from an increasing number of investigations under real conditions, developing more reactors at a pilot scale to better investigate their feasibility, and possible scale-up conditions to successfully address specific challenges in wastewater treatment.
References
Esplugas S, Gimenez J Contreras S, Pascual E, Rodrı́guez M (2002) Comparison of different advanced oxidation processes for phenol degradation. Water Res 36:1034–1042
Bhatkhande DS, Pangarkar VG, Beenackers AACM (2002) Photocatalytic degradation for environmental applications–a review. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 77:102–116
Ohno T, Sarukawa K, Tokieda K, Matsumura M (2001) Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J Catal 203:82–86
Bekbölet M, Boyacioglu Z, Özkaraova B (1998) The influence of solution matrix on the photocatalytic removal of color from natural waters. Water Sci Technol 38:155–162
Zammit I, Vaiano V, Ribeiro AR, Silva AM, Manaia CM, Rizzo L (2019) Photocatalyst for the degradation of antibiotics and the inactivation of antibiotic-resistant bacteria. Catalysts 9:222
Zhang H, Lv X, Li Y, Wang Y, Li J (2009) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380–386
Yang L, Liu B, Liu T, Ma X, Li H, Yin S, Sato T, Wang Y (2017) A P25/(NH4) x WO3 hybrid photocatalyst with broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Sci Rep 7:45715
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269–8285
Vaiano V, Iervolino G, Rizzo L (2018) Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl Catal B 238:471–479
Su H, Jing L, Shi K, Yao C, Fu H (2010) Synthesis of large surface area LaFeO3 nanoparticles by SBA-16 template method as high active visible photocatalysts. J Nanopart Res 12:967–974
Iervolino G, Vaiano V, Sannino D, Rizzo L, Ciambelli P (2016) Production of hydrogen from glucose by LaFeO3 based photocatalytic process during water treatment. Int J Hydrogen Energy 41:959–966
Li S, Jing L, Fu W, Yang L, Xin B, Fu H (2007) Photoinduced charge property of nanosized perovskite-type LaFeO3 and its relationships with photocatalytic activity under visible irradiation. Mater Res Bull 42:203–212
Huang L, Fu W, Fu X, Zong B, Liu H, Bala H et al (2017) Facile and large-scale preparation of N doped TiO2 photocatalyst with high visible light photocatalytic activity. Mater Lett 209:585–588
Lee D-K, Kim S-C, Cho I-C, Kim S-J, Kim S-W (2004) Photocatalytic oxidation of microcystin-LR in a fluidized bed reactor having TiO2-coated activated carbon. Sep Purif Technol 34:59–66
Chong MN, Vimonses V, Lei S, Jin B, Chow C, Saint C (2009) Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater 117:233–242
Argurio P, Fontananova E, Molinari R, Drioli E (2018) Photocatalytic membranes in photocatalytic membrane reactors. Processes 6:162–189
Zhu H, Gao X, Lan Y, Song D, Xi Y, Zhao J (2004) Hydrogen titanate nanofibers covered with anatase nanocrystals: a delicate structure achieved by the wet chemistry reaction of the titanate nanofibers. J Am Chem Soc 126:8380–8381
Hatat-Fraile M, Liang R, Arlos MJ, He RX, Peng P, Servos MR et al (2017) Concurrent photocatalytic and filtration processes using doped TiO2 coated quartz fiber membranes in a photocatalytic membrane reactor. Chem Eng J 330:531–540
Kwak S-Y, Kim SH, Kim SS (2001) Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ Sci Technol 35:2388–2394
Dzinun H, Othman MHD, Ismail AF, Puteh MH, Rahman MA, Jaafar J (2015) Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem Eng J 269:255–261
Cantarella M, Sanz R, Buccheri MA, Ruffino F, Rappazzo G, Scalese S et al (2016) Immobilization of nanomaterials in PMMA composites for photocatalytic removal of dyes, phenols and bacteria from water. J Photochem Photobiol A 321:1–11
Chen C-Y, Wang M, Li J-Y, Pootrakulchote N, Alibabaei L, Ngoc-le C-h et al (2009) Highly efficient light-harvesting ruthenium sensitizer for thin-film dyesensitized solar cells. ACS nano 3:3103–3109
Chen Z, Li F, Huang C (2007) Organic D-π-A dyes for dye-sensitized solar cell. Curr Org Chem 11:1241–1258
Ito S, Miura H, Uchida S, Takata M, Sumioka K, Liska P et al (2008) High-conversion-efficiency organic dye-sensitized solar cells with a novel indoline dye. Chem Commun 44(41):5194–5196
Zhang Z, Wang W, Wang L, Sun S (2012) Enhancement of visible-light photocatalysis by coupling with narrow-band-gap semiconductor: a case study on Bi2S3/Bi2WO6. ACS Appl Mater Interfaces 4:593–597
Vaiano V, Iervolino G, Sannino D (2016) Photocatalytic Removal of Tartrazine Dye from Aqueous Samples on LaFeO3/ZnO Photocatalysts. Chem Eng Trans 52:847–852
Abe R, Sayama K, Arakawa H (2002) Efficient hydrogen evolution from aqueous mixture of I− and acetonitrile using a merocyanine dye-sensitized Pt/TiO2 photocatalyst under visible light irradiation. Chem Phys Lett 362:441–444
Boschloo G, Hagfeldt A (2005) Activation energy of electron transport in dye-sensitized TiO2 solar cells. J Phys Chem B 109:12093–12098
Tae EL, Lee SH, Lee JK, Yoo SS, Kang EJ, Yoon KB (2005) A strategy to increase the efficiency of the dye-sensitized TiO2 solar cells operated by photoexcitation of dye-to-TiO2 charge-transfer bands. J Phys Chem B 109:22513–22522
Pearton SJ, Norton DP, Ivill MP, Hebard AF, Zavada JM, Chen WM et al (2007) ZnO Doped With Transition Metal Ions. IEEE Trans Electron Devices 54:1040–1048
Iervolino G, Vaiano V, Rizzo L (2018) Visible light active Fe-doped TiO2 for the oxidation of arsenite to arsenate in drinking water. Chem Eng Trans 70:1573–1578
Akpan UG, Hameed BH (2010) The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl Catal A 375:1–11
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Anpo M, Takeuchi M (2003) The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J Catal 216:505–516
Alshammari AS, Chi L, Chen X, Bagabas A, Kramer D, Alromaeh A et al (2015) Visible-light photocatalysis on C-doped ZnO derived from polymer-assisted pyrolysis. RSC Advances. 5:27690–27698
Vaiano V, Sacco O, Sannino D, Ciambelli P (2016) N-doped ZnO nanoparticles supported on ZnS based blue phosphors in the photocatalytic removal of eriochrome black-T dye. Chem Eng Trans 47:187–192
Thompson TL, Yates JT (2006) Surface science studies of the photoactivation of TiO2 new photochemical processes. Chem Rev 106:4428–4453
Di Valentin C, Pacchioni G, Selloni A, Livraghi S, Giamello E (2005) Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J Phys Chem B 109:11414–11419
Gombac V, De Rogatis L, Gasparotto A, Vicario G, Montini T, Barreca D et al (2007) TiO2 nanopowders doped with boron and nitrogen for photocatalytic applications. Chem Phys 339:111–123
Zhang X, Liu Q (2008) Preparation and characterization of titania photocatalyst co-doped with boron, nickel, and cerium. Mater Lett 62:2589–2592
Huang D-G, Liao S-J, Liu J-M, Dang Z, Petrik L (2006) Preparation of visible-light responsive N-F-codoped TiO2 photocatalyst by a sol–gel-solvothermal method. J Photochem Photobiol A 184:282–288
Srinivasan SS, Wade J, Stefanakos EK, Goswami Y (2006) Synergistic effects of sulfation and co-doping on the visible light photocatalysis of TiO2. J Alloy Compd 424:322–326
Wilke K, Breuer H (1999) The influence of transition metal doping on the physical and photocatalytic properties of titania. J Photochem Photobiol A 121:49–53
Macías-Sánchez J, Hinojosa-Reyes L, Caballero-Quintero Ad, De La Cruz W, Ruiz-Ruiz E, Hernández-Ramírez A et al (2015) Synthesis of nitrogen-doped ZnO by sol–gel method: characterization and its application on visible photocatalytic degradation of 2, 4-D and picloram herbicides. Photochem Photobiol Sci 14:536–542
Pal M, Bera S, Sarkar S, Jana S (2014) Influence of Al doping on microstructural, optical and photocatalytic properties of sol–gel based nanostructured zinc oxide films on glass. Rsc Advances 4:11552–11563
Thongsuriwong K, Amornpitoksuk P, Suwanboon S (2012) Photocatalytic and antibacterial activities of Ag-doped ZnO thin films prepared by a sol–gel dip-coating method. J Sol-Gel Sci Technol 62:304–312
Fu M, Li Y, Lu P, Liu J, Dong F (2011) Sol–gel preparation and enhanced photocatalytic performance of Cu-doped ZnO nanoparticles. Appl Surf Sci 258:1587–1591
Lima MK, Fernandes DM, Silva MF, Baesso ML, Neto AM, de Morais GR et al (2014) Co-doped ZnO nanoparticles synthesized by an adapted sol–gel method: effects on the structural, optical, photocatalytic and antibacterial properties. J Sol-Gel Sci Technol 72:301–309
Gionco C, Paganini MC, Giamello E, Burgess R, Di Valentin C, Pacchioni G (2014) Cerium-doped zirconium dioxide, a visible-light-sensitive photoactive material of third generation. J Phys Chem Lett 5:447–451
Vaiano V, Sacco O, Sannino D, Ciambelli P (2015) Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl Catal B 170–171:153–161
Sōmiya S, Roy R (2000) Hydrothermal synthesis of fine oxide powders. Bull Mater Sci 23:453–460
Einarsrud M-A, Grande T (2014) 1D oxide nanostructures from chemical solutions. Chem Soc Rev 43:2187–2199
Anpo M, Kamat PV (2010) Environmentally benign photocatalysts: applications of titanium oxide-based materials, Chap 1. Springer Science & Business Media
Zhou JK, Lv L, Yu J, Li HL, Guo P-Z, Sun H et al (2008) Synthesis of self-organized polycrystalline F-doped TiO2 hollow microspheres and their photocatalytic activity under visible light. J Phys Chem C 112:5316–5321
Regalbuto J (2016) Catalyst preparation: science and engineering, Chap 8. CRC press
Nejati K, Rezvani Z, Pakizevand R (2011) Synthesis of ZnO nanoparticles and investigation of the ionic template effect on their size and shape. Int Nano Lett 1:75–81
Vaiano V, Sacco O, Sannino D, Ciambelli P (2015) Process intensification in the removal of organic pollutants from wastewater using innovative photocatalysts obtained coupling Zinc Sulfide based phosphors with nitrogen doped semiconductors. J Clean Prod 100:208–211
Mittal M, Sharma M, Pandey O (2014) UV–Visible light induced photocatalytic studies of Cu doped ZnO nanoparticles prepared by co-precipitation method. Sol Energy 110:386–397
Amornpitoksuk P, Suwanboon S, Sangkanu S, Sukhoom A, Muensit N, Baltrusaitis J (2012) Synthesis, characterization, photocatalytic and antibacterial activities of Ag-doped ZnO powders modified with a diblock copolymer. Powder Technol 219:158–164
Zhang Y, Stangle GC (1994) Preparation of fine multicomponent oxide ceramic powder by a combustion synthesis process. J Mater Res 9:1997–2004
Manoharan SS, Kumar N, Patil K (1990) Preparation of fine particle chromites: a combustion approach. Mater Res Bull 25:731–738
Deganello F, Tyagi AK (2018) Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog Cryst Growth Charact Mater 64:23–61
Danks AE, Hall SR, Schnepp Z (2016) The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater Horiz 3:91–112
Peng K, Fu L, Yang H, Ouyang J (2016) Perovskite LaFeO3/montmorillonite nanocomposites: synthesis, interface characteristics and enhanced photocatalytic activity. Sci Rep 6:19723–19733
Parida K, Reddy K, Martha S, Das D, Biswal N (2010) Fabrication of nanocrystalline LaFeO3: an efficient sol–gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int J Hydrogen Energy 35:12161–12168
Sannino D, Vaiano V, Ciambelli P, Isupova LA (2011) Structured catalysts for photo-Fenton oxidation of acetic acid. Catal Today 161:255–259
Vaiano V, Iervolino G, Sannino D, Rizzo L, Sarno G, Ciambelli P et al (2015) Food Azo-Dyes removal from water by heterogeneous photo-fenton with LaFeO3 supported on honeycomb corundum monoliths. J Environ Eng 141(12):04015038
Sannino D, Vaiano V, Ciambelli P, Isupova L (2013) Mathematical modelling of the heterogeneous photo-Fenton oxidation of acetic acid on structured catalysts. Chem Eng J 224:53–58
Suslick KS, Price GJ (1999) Applications of ultrasound to materials chemistry. Annu Rev Mater Sci 29:295–326
Xu H, Zeiger BW, Suslick KS (2013) Sonochemical synthesis of nanomaterials. Chem Soc Rev 42:2555–2567
Teh CY, Wu TY, Juan JC (2017) An application of ultrasound technology in synthesis of titania-based photocatalyst for degrading pollutant. Chem Eng J 317:586–612
Moholkar VS, Sivasankar T, Nalajala VS (2012) 20 Mechanistic aspects of ultrasound-enhanced physical and chemical processes. In: Handbook on applications of, pp 501–531
Sancheti SV, Gogate PR (2017) A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason Sonochem 36:527–543
Huang W, Tang X, Wang Y, Koltypin Y, Gedanken A (2000) Selective synthesis of anatase and rutile via ultrasound irradiation. Chem Commun 15(15):1415–1416
Jimmy CY, Zhang L, Yu J (2002) Rapid synthesis of mesoporous TiO2 with high photocatalytic activity by ultrasound-induced agglomeration. New J Chem 26:416–420
Boels L, Wagterveld R, Mayer M, Witkamp G (2010) Seeded calcite sonocrystallization. J Cryst Growth 312:961–966
Tian C, Zhang Q, Wu A, Jiang M, Liang Z, Jiang B et al (2012) Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem Commun 48:2858–2860
Thiagarajan S, Sanmugam A, Vikraman D (2017) Facile methodology of Sol-Gel synthesis for metal oxide nanostructures. In: Recent applications in sol–gel synthesis, Chap 1, IntechOpen
Macwan D, Dave PN, Chaturvedi S (2011) A review on nano-TiO2 sol–gel type syntheses and its applications. J Mater Sci 46:3669–3686
Lin K, Wu C, Chang J (2014) Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater 10:4071–4102
O’Donoghue M (1983) A guide to man-made gemstones. Van Nostrand Reinhold Company
Khalil I, Julkapli N, Yehye W, Basirun W, Bhargava S (2016) Graphene–gold nanoparticles hybrid—synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 9:406–444
Varma A, Mukasyan AS, Rogachev AS, Manukyan KV (2016) Solution combustion synthesis of nanoscale materials. Chem Rev 116:14493–14586
Aghayan M, Rodríguez M (2012) Influence of fuels and combustion aids on solution combustion synthesis of bi-phasic calcium phosphates (BCP). Mater Sci Eng C 32:2464–2468
Bang JH, Suslick KS (2010) Applications of ultrasound to the synthesis of nanostructured materials. Adv Mater 22:1039–1059
Loeb SK, Alvarez PJ, Brame JA, Cates EL, Choi W, Crittenden J et al (2019) The technology horizon for photocatalytic water treatment: sunrise or sunset? : ACS Publications. Environ Sci Technol 53:2937–2947
Liao C-H, Gurol MD (1995) Chemical oxidation by photolytic decomposition of hydrogen peroxide. Environ Sci Technol 29:3007–3014
Fiorentino A, Ferro G, Alferez MC, Polo-López MI, Fernández-Ibañez P, Rizzo L (2015) Inactivation and regrowth of multidrug resistant bacteria in urban wastewater after disinfection by solar-driven and chlorination processes. J Photochem Photobiol B 148:43–50
Rizzo L, Lofrano G, Grassi M, Belgiorno V (2008) Pre-treatment of olive mill wastewater by chitosan coagulation and advanced oxidation processes. Sep Purif Technol 63:648–653
Haroune L, Salaun M, Ménard A, Legault CY, Bellenger J-P (2014) Photocatalytic degradation of carbamazepine and three derivatives using TiO2 and ZnO: Effect of pH, ionic strength, and natural organic matter. Sci Total Environ 475:16–22
Qin G, Sun Z, Wu Q, Lin L, Liang M, Xue S (2011) Dye-sensitized TiO2 film with bifunctionalized zones for photocatalytic degradation of 4-cholophenol. J Hazard Mater 192:599–604
Adán C, Magnet A, Fenoy S, Pablos C, Del Águila C, Marugán J (2018) Concomitant inactivation of Acanthamoeba spp. and Escherichia coli using suspended and immobilized TiO2. Water Res 144:512–521
Lalhriatpuia C, Tiwari D, Tiwari A, Lee SM (2015) Immobilized Nanopillars-TiO2 in the efficient removal of micro-pollutants from aqueous solutions: physicochemical studies. Chem Eng J 281:782–792
Xiong P, Hu J (2013) Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res 47:4547–4555
Coronado JM, Fresno F, Hernández-Alonso MD, Portela R (2013) Design of advanced photocatalytic materials for energy and environmental applications, Springer, pp 123–156
Yu H, Song L, Hao Y, Lu N, Quan X, Chen S et al (2016) Fabrication of pilot-scale photocatalytic disinfection device by installing TiO2 coated helical support into UV annular reactor for strengthening sterilization. Chem Eng J 283:1506–1513
Borges M, Sierra M, Esparza P (2017) Solar photocatalysis at semi-pilot scale: wastewater decontamination in a packed-bed photocatalytic reactor system with a visible-solar-light-driven photocatalyst. Clean Technol Environ Policy 19:1239–1245
Antonelli M, Turolla A, Mezzanotte V, Nurizzo C (2013) Peracetic acid for secondary effluent disinfection: a comprehensive performance assessment. Water Sci Technol 68:2638–2644
Da Costa JB, Rodgher S, Daniel LA, Espíndola ELG (2014) Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 23:1803–1813
Di Cesare A, Eckert EM, D’Urso S, Bertoni R, Gillan DC, Wattiez R et al (2016) Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res 94:208–214
McArdell C (2015) The first full-scale advanced ozonation plant in the Dübendorf WWTP running; the new Swiss water protection act approved. Norman Bulletin 4:36–37
Rizzo L, Malato S, Antakyali D, Beretsou VG, Đolić MB, Gernjak W et al (2019) Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci Total Environ 655:986–1008
Vaiano V, Matarangolo M, Sacco O, Sannino D (2017) Photocatalytic treatment of aqueous solutions at high dye concentration using praseodymium-doped ZnO catalysts. Appl Catal B 209:621–630
.Konstantinou I, Albanis T (2004) TiO2-Assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review 49:1-14
Chiang L-F, Doong R-A (2014) Cu-TiO2 nanorods with enhanced ultraviolet- and visible-light photoactivity for bisphenol A degradation. J Hazard Mater 277:84–92
Calza P, Sakkas VA, Medana C, Baiocchi C, Dimou A, Pelizzetti E et al (2006) Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl Catal B 67:197–205
Reyes C, Fernández J, Freer J, Mondaca MA, Zaror C, Malato S et al (2006) Degradation and inactivation of tetracycline by TiO2 photocatalysis. J Photochem Photobiol, A 184:141–146
Elmolla ES, Chaudhuri M (2010) Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J Hazard Mater 173:445–449
Mijin D, Savić M, Snežana P, Smiljanić A, Glavaški O, Jovanović M et al (2009) A study of the photocatalytic degradation of metamitron in ZnO water suspensions. Desalination 249:286–292
Ahmed S, Rasul MG, Brown R, Hashib MA (2011) Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J Environ Manage 92:311–330
Rizzo L, Della Sala A, Fiorentino A, Li Puma G (2014) Disinfection of urban wastewater by solar driven and UV lamp – TiO2 photocatalysis: effect on a multi drug resistant Escherichia coli strain. Water Res 53:145–152
Wang W, Huang G, Yu JC, Wong PK (2015) Advances in photocatalytic disinfection of bacteria: development of photocatalysts and mechanisms. J Environ Sci 34:232–247
Choi J, Lee H, Choi Y, Kim S, Lee S, Lee S et al (2014) Heterogeneous photocatalytic treatment of pharmaceutical micropollutants: effects of wastewater effluent matrix and catalyst modifications. Appl Catal B 147:8–16
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution. J Phys Chem Ref Data 17:513–886
Luo X, Yang X, Qiao X, Wang Y, Chen J, Wei X et al (2017) Development of a QSAR model for predicting aqueous reaction rate constants of organic chemicals with hydroxyl radicals. Environmental Science: Processes & Impacts. 19:350–356
Jallouli N, Pastrana Martinez L, Ribeiro A, Moreira N, L Faria J, Hentati O, et al (2018) Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. 334:976–984
Wilson WW, Wade MM, Holman SC, Champlin FR (2001) Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods 43:153–164
Gottenbos B, Grijpma DW, van der Mei HC, Feijen J, Busscher HJ (2001) Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 48:7–13
Coleman HM, Marquis CP, Scott JA, Chin SS, Amal R (2005) Bactericidal effects of titanium dioxide-based photocatalysts. Chem Eng J 113:55–63
Thomas O, Causse J, Thomas M-F (2017) Aggregate organic constituents. Chap 4
Fiorentino A, Esteban B, Garrido-Cardenas JA, Kowalska K, Rizzo L, Aguera A et al (2019) Effect of solar photo-Fenton process in raceway pond reactors at neutral pH on antibiotic resistance determinants in secondary treated urban wastewater. J Hazard Mater 378:120737–120746
McCullagh C, Skillen N, Adams M, Robertson PKJ (2011) Photocatalytic reactors for environmental remediation: a review. J Chem Technol Biotechnol 86:1002–1017
Braham RJ, Harris AT (2009) Review of major design and scale-up considerations for solar photocatalytic reactors. Ind Eng Chem Res 48:8890–8905
Paltiel O, Fedorova G, Tadmor G, Kleinstern G, Maor Y, Chefetz B (2016) Human exposure to wastewater-derived pharmaceuticals in fresh produce: a randomized controlled trial focusing on carbamazepine. Environ Sci Technol 50:4476–4482
Alexander J, Knopp G, Dötsch A, Wieland A, Schwartz T (2016) Ozone treatment of conditioned wastewater selects antibiotic resistance genes, opportunistic bacteria, and induce strong population shifts. Sci Total Environ 559:103–112
Liu S-S, Qu H-M, Yang D, Hu H, Liu W-L, Qiu Z-G et al (2018) Chlorine disinfection increases both intracellular and extracellular antibiotic resistance genes in a full-scale wastewater treatment plant. Water Res 136:131–136
Murray GE, Tobin RS, Junkins B, Kushner DJ (1984) Effect of chlorination on antibiotic resistance profiles of sewage-related bacteria. Appl Environ Microbiol 48:73–77
Zheng J, Su C, Zhou J, Xu L, Qian Y, Chen H (2017) Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem Eng J 317:309–316
Karaolia P, Michael-Kordatou I, Hapeshi E, Drosou C, Bertakis Y, Christofilos D et al (2018) Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl Catal B 224:810–824
Moreira NFF, Sousa JM, Macedo G, Ribeiro AR, Barreiros L, Pedrosa M et al (2016) Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res 94:10–22
Amiri H, Nabizadeh R, Silva Martinez S, Jamaleddin Shahtaheri S, Yaghmaeian K, Badiei A et al (2018) Response surface methodology modeling to improve degradation of Chlorpyrifos in agriculture runoff using TiO2 solar photocatalytic in a raceway pond reactor. Ecotoxicol Environ Saf 147:919–925
Carra I, Santos-Juanes L, Acién Fernández FG, Malato S, Sánchez Pérez JA. New approach to solar photo-Fenton operation. Raceway ponds as tertiary treatment technology. Journal of Hazardous Materials. 2014;279:322-9.
Mehrjouei M, Müller S, Möller D (2015) A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem Eng J 263:209–219
Cho M, Gandhi V, Hwang T-M, Lee S, Kim J-H (2011) Investigating synergism during sequential inactivation of MS-2 phage and Bacillus subtilis spores with UV/H2O2 followed by free chlorine. Water Res 45:1063–1070
Bandala ER, González L, Sanchez-Salas JL, Castillo JH (2011) Inactivation of Ascaris eggs in water using sequential solar driven photo-Fenton and free chlorine. J Water Health 10:20–30
Meng X, Zhang Z (2018) Two dimensional graphitic materials for photoelectrocatalysis: a short review. Catal Today 315:2–8
Li X, Yu J, Wageh S, Al-Ghamdi AA, Xie J (2016) Graphene in photocatalysis: a review. Small 12:6640–6696
Georgieva J, Valova E, Armyanov S, Philippidis N, Poulios I, Sotiropoulos S (2012) Bi-component semiconductor oxide photoanodes for the photoelectrocatalytic oxidation of organic solutes and vapours: a short review with emphasis to TiO2–WO3 photoanodes. J Hazard Mater 211:30–46
Yang M-Q, Zhang N, Pagliaro M, Xu Y-J (2014) Artificial photosynthesis over graphene–semiconductor composites. Are we getting better? Chem Soc Rev 43:8240–8254
Kakaei K (2013) One-pot electrochemical synthesis of graphene by the exfoliation of graphite powder in sodium dodecyl sulfate and its decoration with platinum nanoparticles for methanol oxidation. Carbon 51:195–201
Liu C, Zhang L, Liu R, Gao Z, Yang X, Tu Z et al (2016) Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J Alloy Compd 656:24–32
Jin Z, Zhang Q, Yuan S, Ohno T (2015) Synthesis high specific surface area nanotube gC3N4 with two-step condensation treatment of melamine to enhance photocatalysis properties. RSC Adv 5:4026–4029
Ozer LY, Garlisi C, Oladipo H, Pagliaro M, Sharief SA, Yusuf A et al (2017) Inorganic semiconductors-graphene composites in photo (electro) catalysis: synthetic strategies, interaction mechanisms and applications. J Photochem Photobiol C 33:132–164
Wang G, Feng W, Zeng X, Wang Z, Feng C, McCarthy DT et al (2016) Highly recoverable TiO2–GO nanocomposites for stormwater disinfection. Water Res 94:363–370
Garcia DT, Ozer LY, Parrino F, Ahmed M, Brudecki GP, Hasan SW et al (2018) Photocatalytic ozonation under visible light for the remediation of water effluents and its integration with an electro-membrane bioreactor. Chemosphere 209:534–541
Sanchez M, Rivero M, Ortiz I (2010) Photocatalytic oxidation of grey water over titanium dioxide suspensions. Desalination 262:141–146
Armanious A, Özkan A, Sohmen U, Gulyas H (2011) Inorganic greywater matrix impact on photocatalytic oxidation: does flocculation of TiO2 nanoparticles impair process efficiency? Water Sci Technol 63:2808–2813
Michael I, Rizzo L, McArdell CS, Manaia CM, Merlin C, Schwartz T et al (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995
Rizzo L, Meric S, Guida M, Kassinos D, Belgiorno V (2009) Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res 43:4070–4078
Mascolo G, Balest L, Cassano D, Laera G, Lopez A, Pollice A et al (2010) Biodegradability of pharmaceutical industrial wastewater and formation of recalcitrant organic compounds during aerobic biological treatment. Biores Technol 101:2585–2591
Deng F, Zhao L, Luo X, Luo S, Dionysiou DD (2018) Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem Eng J 333:423–433
Alalm MG, Tawfik A, Ookawara S (2015) Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: operational conditions, kinetics, and costs. J Water Process Eng 8:55–63
Fenoll J, Garrido I, Flores P, Hellín P, Vela N, Navarro G et al (2019) Implementation of a new modular facility to detoxify agro-wastewater polluted with neonicotinoid insecticides in farms by solar photocatalysis. Energy 175:722–729
Kushniarou A, Garrido I, Fenoll J, Vela N, Flores P, Navarro G et al (2019) Solar photocatalytic reclamation of agro-waste water polluted with twelve pesticides for agricultural reuse. Chemosphere 214:839–845
Singh P, Lakhan Singh R (2017) Bio-removal of azo dyes: a review. Int J Appl Sci Biotechnol 5(2):108–126
Gita S, Hussan A, Choudhury T (2017) Impact of textile dyes waste on aquatic environments and its treatment. Environ Ecol. 35:2349–2353
Zhou Z, Lin S, Yue T, Lee T-C (2014) Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J Food Eng 126:133–141
Klamerth N, Rizzo L, Malato S, Maldonado MI, Agüera A, Fernández-Alba AR (2010) Degradation of fifteen emerging contaminants at μgL−1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res 44:545–554
Rodríguez-Chueca J, Polo-López MI, Mosteo R, Ormad MP, Fernández-Ibáñez P (2014) Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl Catal B 150–151:619–629
Wang N, Zheng T, Zhang G, Wang P (2016) A review on Fenton-like processes for organic wastewater treatment. J Environ Chem Eng 4:762–787
De Luca A, Dantas RF, Esplugas S (2014) Assessment of iron chelates efficiency for photo-Fenton at neutral pH. Water Res 61:232–242
Papoutsakis S, Miralles-Cuevas S, Oller I, Garcia Sanchez JL, Pulgarin C, Malato S (2015) Microcontaminant degradation in municipal wastewater treatment plant secondary effluent by EDDS assisted photo-Fenton at near-neutral pH: an experimental design approach. Catal Today 252:61–69
Iervolino G, Vaiano V, Sannino D, Rizzo L, Sarno G, Ciambelli P et al (2015) Influence of operating conditions in the photo-fenton removal of tartrazine on structured catalysts. Chem Eng Trans 43:979–984
Sohrabi MR, Khavaran A, Shariati S, Shariati S (2017) Removal of carmoisine edible dye by fenton and photo fenton processes using taguchi orthogonal array design. Arab J Chem 10:S3523–S3531
Feng J, Hu X, Yue PL (2005) Discoloration and mineralization of Orange II by using a bentonite clay-based Fe nanocomposite film as a heterogeneous photo-Fenton catalyst. Water Res 39:89–96
He J, Tao X, Ma W, Zhao J (2002) Heterogeneous photo-Fenton degradation of an azo dye in aqueous H2O2/Iron oxide dispersions at neutral pHs. Chem Lett 31:86–87
Vourch M, Balannec B, Chaufer B, Dorange G (2008) Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination 219:190–202
Janczukowicz W, Zieliński M, Dębowski M (2008) Biodegradability evaluation of dairy effluents originated in selected sections of dairy production. Biores Technol 99:4199–4205
Murcia JJ, Hernández-Laverde M, Rojas H, Muñoz E, Navío JA, Hidalgo MC (2018) Study of the effectiveness of the flocculation-photocatalysis in the treatment of wastewater coming from dairy industries. J Photochem Photobiol A 358:256–264
Lamas Samanamud GR, Loures CCA, Souza AL, Salazar RFS, Oliveira IS, Silva MB et al (2012) Heterogeneous photocatalytic degradation of dairy wastewater using immobilized ZnO. ISRN Chemical Engineering. 2012:1–8
Lucchetti R, Siciliano A, Clarizia L, Russo D, Di Somma I, Natale F et al (2017) Sacrificial photocatalysis: removal of nitrate and hydrogen production by nano-copper-loaded P25 titania. A kinetic and ecotoxicological assessment. Environ Sci Pollut Res 24:5898–5907
Li Y, Wang L, Cai T, Zhang S, Liu Y, Song Y et al (2017) Glucose-assisted synthesize 1D/2D nearly vertical CdS/MoS2 heterostructures for efficient photocatalytic hydrogen evolution. Chem Eng J 321:366–374
Wang X, Dong H, Hu Z, Qi Z, Li L (2017) Fabrication of a Cu2O/Au/TiO2 composite film for efficient photocatalytic hydrogen production from aqueous solution of methanol and glucose. Mater Sci Eng, B 219:10–19
Speltini A, Scalabrini A, Maraschi F, Sturini M, Pisanu A, Malavasi L et al (2018) Improved photocatalytic H2 production assisted by aqueous glucose biomass by oxidized g-C3N4. Int J Hydrogen Energy 43:14925–14933
Iervolino G, Vaiano V, Sannino D, Rizzo L, Galluzzi A, Polichetti M, et al (2018) Hydrogen production from glucose degradation in water and wastewater treated by Ru-LaFeO3 /Fe2O3 magnetic particles photocatalysis and heterogeneous photo-Fenton 43:2184–2196
Tijare SN, Bakardjieva S, Subrt J, Joshi MV, Rayalu SS, Hishita S et al (2014) Synthesis and visible light photocatalytic activity of nanocrystalline PrFeO3perovskite for hydrogen generation in ethanol–water system. J Chem Sci 126:517–525
Caravaca A, Jones W, Hardacre C, Bowker M (2016) H(2) production by the photocatalytic reforming of cellulose and raw biomass using Ni, Pd, Pt and Au on titania. Proc Math Phys Eng Sci 472:20160054–20160075
Vaiano V, Iervolino G, Sarno G, Sannino D, Rizzo L, Murcia Mesa JJ et al (2015) Simultaneous production of CH4 and H2 from photocatalytic reforming of glucose aqueous solution on sulfated Pd-TiO2 catalysts. Oil Gas Sci Technol 70:891–902
Saxena G, Chandra R, Bharagava RN (2017) Environmental Pollution, Toxicity Profile and Treatment Approaches for Tannery Wastewater and Its Chemical Pollutants. Rev Environ Contam Toxicol 240:31–69
Dixit S, Yadav A, Dwivedi PD, Das M (2015) Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod 87:39–49
Antonopoulou M, Chondrodimou I, Bairamis F, Giannakas A, Konstantinou I (2017) Photocatalytic reduction of Cr(VI) by char/TiO2 composite photocatalyst: optimization and modeling using the response surface methodology (RSM). Environ Sci Pollut Res 24:1063–1072
Shet A, Shetty KV (2016) Photocatalytic degradation of phenol using Ag core-TiO2 shell (Ag@TiO2) nanoparticles under UV light irradiation. Environ Sci Pollut Res 23:20055–20064
Vaiano V, Iervolino G (2018) Facile method to immobilize ZnO particles on glass spheres for the photocatalytic treatment of tannery wastewater. J Colloid Interface Sci 518:192–199
Caracciolo D, Vaiano V, Matarangolo M, Sacco O, Sannino D, Gambicorti T et al (2017) Treatment of dyeing and finishing waters using innovative photocatalysts. Chem Eng Trans 60:187–192
High-level expert group on key enabling technologies—final report”. 2011. Retrieved October 2017:1–56
Acknowledgements
This work is part of a project that has received funding from the European Union’s Horizon 2020 under the Innovative Training Networks (ITN-ETN) programme Marie Skłodowska-Curie Grant (ANtibioticS and mobile resistance elements in WastEwater Reuse applications: risks and innovative solutions) agreement no. 675530.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection “Heterogeneous Photocatalysis”; edited by Mario J. Muñoz-Batista, Alexander Navarrete Muñoz and Rafael Luque.
Rights and permissions
About this article
Cite this article
Iervolino, G., Zammit, I., Vaiano, V. et al. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top Curr Chem (Z) 378, 7 (2020). https://doi.org/10.1007/s41061-019-0272-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-019-0272-1