Abstract
This work aims at culturing different Chlorella species, monitoring growth and estimating the nutritional quality for further application of complete cells and lipid extracted biomass in Artemia franciscana feeding. We conducted experiments using C. marina, C. salina, C. capsulata, C. stigmatophora and C. vulgaris that were batch cultured for 14 days. C. salina showed the maximal count on the sixth day while C. marina recorded the maximum growth rate (2 ± 0.177). However, C. capsulata and C. stigmatophora recorded the minimum rate (1.5 ± 0.11). Analyses of algal biomass showed that C. capsulata contains maximal lipids and carbohydrates, but the minimal protein (22.8 ± 1.4 %). However, C. salina contained the highest protein (33.1 ± 1.4 %). After oil extraction, there were no significant losses in the other biochemical constituents of the studied Chlorella species. Considering algae metabolites, saturated fatty acids were the main constituent in the fatty acids methyl esters (FAMEs). Palmitic and stearic acids were dominant. Amino acid pools of the experimental marine Chlorella species were found to contain lysine, methionine and histidine; but were deficient in cysteine. The present investigation showed that lipids, proteins and protein to lipid ratio of A. franciscana napulli enriched with mixed cells of Chlorella species were enhanced by (22 %); 1.96 and 1.33 folds, respectively. Furthermore, the growth and survival of A. franciscana showed significant increases when fed on lipid extracted algae residuals, especially that of a mixed diet; which is considered as an important achievement and confirms that the residual algae biomass can be significantly used for aquaculture feeding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The nutritional quality of microalgae biomass plays a noticeable role in the diet of many living organisms including marine animals. They are required for larval nourishment, either for direct or indirect utilization as food for live prey, such as Artemia nauplii, which are successively used for aquaculture feeding (Muller-Feuga 2000).
The microalgae strains are known as an incredible wellspring of valuable biochemicals that can be used as food and food additives (Rocha et al. 2003) where the proteins and poly unsaturated fatty acids (PUFAs) are of principle significance (Spolaore et al. 2006), while the microalgae lipids are extremely significant at various stages of marine fish larvae of many fish, mussel and oyster species (Ronquillo et al. 2012). Algal lipids have been reported to represent a good source for nutrition in aquaculture industry (Adarme-Vega et al. 2012). The quality and quantity of lipids are of incredible significance in the nutritional value of microalgae in aquaculture as mentioned by El-Sheekh et al. (2015).
The content of polyunsaturated fatty acids (PUFA) is crucial for the use of microalgae in aquaculture (Patil et al. 2005). Omega 3 (ω3) and omega 6 (ω6) are quite compelling. In spite of that, animal lack the required enzymes to synthesize PUFA, it must be obtained from food and, therefore, is often known to be vital (Milledge 2011). Therefore, deficiency in (PUFAs) seems to be the main cause of the low survival rates of larvae (Patil et al. 2005). As a result, microalgae have been used as a dietary source for aquatic organisms, with fatty acid contents being the centric agent in the selection of microalgal species (Huerlimann et al. 2010). The complete utilization of algal biomass may involve the combination of different technologies (Wiley et al. 2011). Algae lipids can be extracted for biofuel production and the leftover solids, which are mostly carbohydrates and proteins are useful for larvae enrichment (El-Sheekh et al. 2015).
Successful algal screening mainly relies upon selecting the right species with apropos properties including biomass and fatty acid productivity. The major economic bottlenecks are algae productivity, followed by labor and then the harvesting costs as stated by (Abomohra et al. 2014).
Different green algal genera are used in aquaculture including Chlorella species (Muller-Feuga 2000) relying on the requirements of seafood production (Pulz and Gross 2004). Algae have also been selected in premise to their mass-culture potential of proper cellular size, digestibility and their overall essential nutritional value (Courtois de Viçose et al. 2012). Brine shrimp nauplii are non-selective particle feeders; simple methods have been emerging to incorporate various sorts and varieties of products into the Artemia prior to feeding to larvae. Artemia enrichment is widely applied in marine fish and crustacean hatcheries for enhancing the nutritional quality of Artemia with essential fatty acids for further aquaculture feeding (Sorgeloos et al. 2001).
Therefore, the objectives of this research were aiming to: (1) Monitor the growth and nutritional quality of five Chlorella species. (2) Study the application of the different Chlorella species for Artemia franciscana feeding. (3) Follow up the growth of A. franciscana in terms of fresh weight and survival % that fed on lipids extracted Chlorella species for 12 days to verify whether there is a long-term positive effect.
Five Chlorella species namely, C. salina, C. marina, C. capsulata, C. stigmatophora and C. vulgaris were grown in a batch culture. Algal growth rates, lipid production capacity, in addition to proteins as well as carbohydrate contents and ashes were determined. The algae metabolites including, fatty acids and amino acids compositions were also estimated. The algal cells were harvested by flocculation to reduce the cost of harvest. Complete algae cells and the residual algal biomass after oil extraction, were used for A. franciscana feeding. As A. franciscana nauplii are used in aquacultures within 48 h of enrichment. The proximate and FAMEs analyses of the enriched A. franciscana nauplii were carried out.
2 Experimental
2.1 Algae strains and growth conditions
Five species of genus Chlorella were chosen for this study. C. marina, C. capsulata and C. stigmatophora were kindly provided by Dr. I. Tzovenis, NKUA, was originally isolated from Crete, Greece. C. salina was obtained from the marine hatchery of the National Institute of Oceanography and Fisheries in Alexandria, Egypt. The fresh water alga C. vulgar was obtained from the algae culture collection, Botany department, Faculty of Science, Alexandria University. The starting inocula were: 0.3 ± 0.03 × 106, 0.2 ± 0.02 × 106, 0.3 ± 0.03 × 106, 0.5 ± 0.04 × 106 and 0.5 ± 0.04 × 106 for C. salina, C. marina, C. capsulata, C. stigmatophora and C. vulgaris, respectively. The marine Chlorella species were grown in axenic modified F medium as described by Guillard and Ryther (1962). On the other hand, C. vulgaris was grown in Amaral’s medium (do Amaral and Freire 2012). All growth media were prepared using analytically grade reagents supplied by sigma (St. Louis, USA). The cultures were grown at 28 °C ± 3 °C with the light intensity of 80 µ mol m−2 s−1 in a controlled culture chamber under a regime of 16:8 light/dark cycle.
2.2 Monitoring of algal growth
The growth of the tested organisms was determined by cell count using the haemacytometer slide, where cell numbers were estimated at 24 h intervals. In addition to that, the growth rate was calculated using the formula proposed by Robert (1979):
where: 3.322 = growth constant, t 1 time at the beginning of the experiment, t 2 time at the end of the experiment, N 1 number of cells/ml culture at t 1, N 2 = number of cells/ml culture at t 2.
2.3 Proximate analyses of the studied Chlorella species
2.3.1 Determination of total lipids
For lipid extraction, the algal cells were collected at the end of the logarithmic development stage by centrifugation at 1000g for 5 min. Cells were homogenized with chloroform—methanol (2:1 v/v) and refluxed for a couple of minutes to inactivate the phospholipases. Purification of the extracts was performed according to Bligh and Dyer (1959). The total lipid contents were estimated for different algae cultures.
2.3.2 Estimation of carbohydrate
Total carbohydrate was evaluated using the colorimetric technique by (DuBois et al. 1959). The cells were collected by centrifugation at 5000 rpm for 10 min and measured specimens were blended with 1 mL of 5 % aqueous solution of phenol in a test tube. Therefore, 5 mL of 95 % sulfuric acid was added to the blend. Subsequently, the test tubes were permitted to remain for 10 min. They were then vortexed for a few moments and set for 20 min in a water bath at room temperature for yellow–orange color development. Light absorption was measured at 490 nm using the UV-spectrophotometer.
2.3.3 Determination of total protein
Total protein was extracted from the algal cells, according to the method of Rausch (1981). Protein content, both total and water-soluble, was determined according to Hatree (1972).
2.3.4 Ash determination
Ash contents were estimated according to AOAC (1995). Oxidation of organic matter was done using a muffle at 550 °C overnight.
2.4 Estimation of algal metabolites
2.4.1 Analysis of fatty acids methyl esters (FAMEs)
Investigation of fatty acid methyl esters (FAMEs): Total lipid portions in the distinctive green growth species were subjected to saponification. Afterwards, it was changed into methyl esters taking after the methodology of Radwan (1978). Popularities were measured and recognized utilizing gas chromatography (GC framework Hp, Germany, serial No 6890 D 1530 A serial DE 00000348) furnished with a fire ionization locator; the pressing segment material was SP-2340. The transporter gas was nitrogen and the short speed was 5 mm min−1. Distinguishing proof of FAMEs was done by contrasting their maintenance times and those of the benchmarks’. Measurement depended on the inner defamed strategy.
2.4.2 Analysis of amino acid composition
Different amino acids, except for tryptophane, were extracted and determined by the method described by Spackman et al. (1958) using a Beckman 119 CL amino acid analyzer.
2.5 Enrichment of Artemia franciscana with the studied Chlorella species
Brine Shrimp A. franciscana was created by hatching Artemia cysts through a decapsulation technique. They were hatched as depicted by Lavens and Sorgloos (1996). Produced nauplii were harvested after 24 h and then washed with filtered sea water. After 6 h from hatching time, Artemia nauplii (instar II) were grown on their growth media in the presence of 0.5 g L−1 of dried biomass of the experimental Chlorella species. A trial to use a mixture of the dried experimental Chlorella species was also carried out. After 48 h, the enriched Artemia was harvested by plankton net (100 μm). Then, the proximate analyses were carried out by the previously described methods. Bligh and Dyre method (lipid), DuBois’ method (carbohydrate) and Hatree’s method (protein) were performed. Fatty acid fractions were also measured through Radwan esterification method (1978) which analyzes the fatty acid compositions of each A. franciscana treatment. All these methods were mentioned before in details in the algal analysis.
2.6 Following up the growth and survival of Artemia franciscana fed on lipid extracted Chlorella biomass
Artemia franciscana specimens were cultivated in 500 mL of 15 g L−1 artificial sea water. Approximately, 0.5 g L−1 of dried lipid extracted biomass of the different experimental Chlorella species were added to each culture which was aerated using air pump. Six treatments were decided to run the experiments for 12 days, one experiment for each Chlorella species and another one for a mixed diet of the different Chlorella species. Survival of A. franciscana was recorded by estimating the number of living A. franciscana for each experimental trial. Also, Twenty Artemia were sampled from each experimental trial at the beginning and end of the experiment to estimate their growth by measuring fresh weight (Pacheco-Vega et al. 2015).
2.7 Statistical analysis
All values presented in this study are the means of triplicate trials for each treatment; error bars in the figures depict the standard deviations (SD) of these triplicates.
3 Results
3.1 Monitoring of algae growth
The growths of the studied algae were compared and illustrated graphically (Fig. 1). Chlorella species started their stationary phase of growth on the 6th day of incubation and reached the maximum number on the 10th day. C. salina showed the best performance of cell division (26.5 mL−1 × 106); while C. marina showed the lowest (10 mL−1 × 106). On the other hand, C. marina recorded the maximum growth rate (2.0) after 3 days of incubation, followed by C. vulgaris (1.78). Similarly, the two algae C. capsulata and C. stigmatophora recorded the same growth rate (1.5).
3.2 Proximate analyses of the studied Chlorella species
3.2.1 Complete algae cells
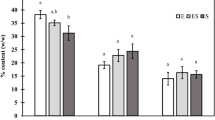
The valuable constituents of Chlorella species are in Fig. 2. Lipid production potential showed that C. capsulata produced the highest lipid content (44.6 ± 3.4 %), followed by C. salina and C. marina (26.8 ± 2.08 and 26.7 ± 2.6 %), respectively. However, C. vulgaris came in last on the list (17.6 ± 2.5 %).
Carbohydrate content ranged from 12.8 ± 0.33 to 22.0 ± 0.57 % in C. salina and C. capsulata, respectively. Algal proteins are represented as the sum of the monomeric soluble and structural amino acids entrained in biomass; data recorded for different Chlorella species showed that proteins ranged from 22.8 ± 1.4 % in C. capsulata to 33.1 ± 1.4 % in C. salina. However, it recorded 30.6 % in C. vulgaris.
Ash contents present in the studied Chlorella species ranged between 7.9 ± 0.2 and 9.2 ± 0.2 % of the algal dry weight in C. salina and C. vulgaris, respectively.
3.2.2 Lipid extracted algae biomass
In lipid extracted algal biomass, the data recorded in Fig. 2 revealed that carbohydrate contents ranged between 15.5 ± 0.40 and 12.0 ± 0.31 % in C. marina and C. salina, respectively. It decreased by about (0.6 %) and (5.4 %) in C. stigmatophora and C. capsulata, respectively, when compared to the complete C. stigmatophora and C. capsulata cells. Protein content of C. vulgaris decreased by only 0.6 %. The two algae, C. stigmatophora and C. capsulata decreased by nearly 1.7 %, 1.2, respectively. However, C. marina and C. salina recorded about a 2.5 % decrease in protein contents in comparison to the complete corresponding algal cells. The ash contents present in the five studied Chlorella species ranged between 7.6 ± 0.2 and 9.1 ± 0.2 % of the algal dry weight in C. salina and C. vulgaris, respectively. Less than a 1 % decrease in ash contents in all the studied Chlorella species has been recorded.
Generally, the biochemical constituents, including proteins and carbohydrates as well as ashes in the lipid extracted Chlorella species were not significantly different from the complete algae cells.
3.3 Fatty acids compositions in the experimental Chlorella species
Fatty acids compositions in the different experimental Chlorella species are shown in Table 1. The highest percentages of SFA were observed for C. salina and C. capsulata followed by C. stigmatophora (94.2; 93.0; 87.0 %), respectively.
Different types of saturated fatty acids were detected in the different experimented Chlorella sp. Palmitic acid (C16:0) was found predominant in the algal lipid. The highest percentages were recorded with C. capsulata and C. stigmatophora. In addition, stearic (C18:0) and undecanoic (C11:0) acids are the most fatty acid composition of the experimented species. All of the 5 experimented Chlorella species contained little quantities of mono unsaturated eicosanoic acid (C20:0).
Fatty acids with different degrees of unsaturation have been recorded. The MUFAs [Oleic acid (18:1) and myristic acid (C14:1)] along with pentadecanoic acid, lauric acid and capric acid were determined. The highest values for the USFA were reported in C. marina and C. vulgaris, each containing 20 %. Also, C. vulgaris didn’t show any contents of mono unsaturated myristoleic acid (C14:1) or saturated capric acid (C10:0). On the other hand, C. marina contained the highest percentage of polyunsaturated α-linolenic acid (C18:2c).
3.4 Amino acid composition of delipidated cells
The individual amino acids of delipidated C. salina, C. marina, C. capsulata, C. stigmatophora and C. vulgaris were recorded in Table 2. The results showed that Krebs cycle family and aliphatic amino acids family surpassed the other groups of amino acids. The total amino acids of Krebs cycle family in the examined species of Chlorella represented nearly 50 % of the total amino acids. The percentages of aliphatic amino acids for C. salina, C. marina, C. capsulata, C. stigmatophora and C. vulgaris represented by therionine, serine, glycine, alanine, valine, leucine and isoleucine were 47.24, 43.12, 85.75, 68.34 and 72.04 %, respectively. That was found to be the opposite of aromatic amino acids (Tyrosine and Phenylalanine), which were detected in traces. In addition to acidic amino acids, there were the asparatic acid and the glutamic acid for delipidated biomass of C. salina, C. marina, C. capsulata, C. stigmatophora and C. vulgaris; they measured at 38.05, 15.82, 3.92, 7.18 and 10.62 %, respectively. Besides that, basic amino acids (lysine and arginine) had results of 6.69, 24.27, 6.32, 2.85 and 11.97 % in the same species, respectively. Finally, sulfur containing amino acids (cysteine and methionine) and secondary amino acid (proline) were found in a few fractions.
3.5 Feeding of Artemia franciscana with the different studied Chlorella species
3.5.1 Biochemical constituents
The study was extended to apply both the complete and the lipid extracted algae cells in A. franciscana feeding. The results in Table 3 indicated that A. franciscana enriched with complete cells of the experimented Chlorella species resulted in improvements of their lipid contents. The increase in the percentage of lipids were 53, 20, 8 % as well as 10 and 37 % for C. salina, C. marina, C. capsulata as well as C. stigmatophora and C. vulgaris, respectively in comparison to the controlled A. franciscana. The lipid contents of A. franciscana increased by 1, 4, 3, 0.00 and 5 % for C. salina, C. marina, C. capsulata as well as C. stigmatophora and C. vulgaris, respectively, when compared with the control. When A. franciscana was enriched with mixed complete algae cells, the lipid contents increased by about 22 %, which was the same discovered result after the enrichment with C. marina complete cells.
Results in Table 3 showed that A. franciscana enriched with the complete cells of the experimental Chlorella species resulted in the improvement of its carbohydrate content. The percentage of carbohydrate increased by 11, 9.23, 11.9 % as well as 16.3 and 9.6 % for C. salina, C. marina, C. capsulata as well as C. stigmatophora and C. vulgaris, respectively, when compared to the control. On the other hand, there were fewer increases in the carbohydrate contents of A. franciscana that had been enriched with lipid extracted biomass of the different experimented Chlorella species when compared to the control. The carbohydrate content in A. franciscana increased by 10.4, 9.8, 10.2, 12 and 7.24 % for C. salina, C. marina, C. capsulata as well as C. stigmatophora and C. vulgaris, respectively when compared to the controlled. In contrast to the lipid contents, when A. franciscana enriched with mixed complete experimented algae cells, the carbohydrate contents increased by about only 5.5 %. However, there was a lower increase level in the carbohydrate contents of A. franciscana that had been enriched with a mixture of lipid extracted algae meal (4.5 %) when compared to that of the controlled. Generally, the carbohydrate content in A. franciscana that was enriched with different experimental Chlorella species was more or less the same when compared to those that were enriched with the lipid extracted ones.
By detecting the protein content in tested A. franciscana, whether enriched with complete algal biomass or the lipid extracted biomass; both were found to have protein ranging from 43 to 70 %. This refers to the highest protein percentage of A. franciscana enriched with C. vulgaris and C. stigmatophora as recorded in Table 3. The results also indicated that the enrichment of A. franciscana on the complete cells of the experimented Chlorella species improved their protein contents. The percentages of protein increased to 27.2, 26.1, 22.13 % as well as 36.26 and 39.33 % for C. salina, C. marina, C. capsulata as well as C. stigmatophora and C. vulgaris, respectively, when compared with control. On the other hand, there were fewer increases in the protein contents of A. franciscana that had been enriched with lipid extracted biomass of the different experimented Chlorella species when compared to the control. The A. franciscana protein contents increased by 11.9, 17.4, 16.13, 22 and 28.83 % for C. salina, C. marina, C. capsulata as well as C. stigmatophora and C. vulgaris, respectively, when compared to the control. The protein contents of A. franciscana which had been enriched with mixed complete algae cells, increased by about 34.28 %. On the other hand, lower increases in the protein contents of A. franciscana that were enriched with mixtures of lipid extracted algal residues (29.78 %) were recorded. Upon comparing the protein content of A. franciscana that had been enriched with complete algal cells, and that enriched with the same lipids extracted residues, it appeared obvious that the protein contents of A. franciscana were significantly higher in those enriched with the complete experimental algal cells than that enriched with lipid extracted cells. The protein contents decreased by 15.3, 8.7, 6, 14.26 % as well as 10.5 and 4.5 % for C. salina, C. marina, C. capsulata, C. stigmatophora as well as C. vulgaris and those enriched with the mixed species of algae.
The results presented in Table 3 revealed that protein/lipid ratios of A. franciscana that had been enriched with the different experimental Chlorella species were highly improved when compared to the controlled. The ratio had increased by about 0.16, 0.34, 0.59, 0.11 and 0.28 % for those enriched with C. marina, C. capsulata, C. stigmatophora, C. vulgaris and mixed algae, respectively.
3.5.2 Fatty acid composition
From A. franciscana fatty acid composition shown in Table 4, saturated fatty acids were found to be abundant in A. franciscana that was enriched with complete algal cells of the five experimental Chlorella species. Upon comparing results, it has been reported that palmitic acid represents the most abundant with low concentrations of capric, undecyclic, lauric, tridecyclic, myristic, pentadecenoic, heptadecyclic and octadecanoic acids. Results in Table 4 indicated that the SFA increased to 80 % in A. franciscana that was enriched with C. salina and C. marina. Also, enrichment of A. franciscana with the same two Chlorella species resulted in an improvement of C18:3ω3 (Alpha-Linoleic) by nearly 9.2 and 11.4 folds, respectively. A. franciscana was enriched with a mixture of the five experimental Chlorella species. The results in Table 5 indicated that the SFA increased to 72.6 %. The contents of PUFAs-ω3 had increased by nearly 7.9 folds in A. franciscana that was enriched with the mixture of the studied Chlorella species. This gave them a good chance to be used in A. franciscana feeding.
3.5.3 Growth and survival follow up
Growth and survival of A. franciscana that fed on the lipid-extracted biomass were monitored to verify whether there is a long-term positive effect of exhausted Chlorella biomasses which could be an important achievement. After 12-day feeding trials, the results (Fig. 3) revealed that the survival and growth of A. franciscana that fed on a mixed diet of the residual algae biomass yielded the superior survival and average fresh weight of A. franciscana recording 91.8 ± 4 % and 4.2 ± 0.4 mg, respectively. However, using the residual biomass of the different marine Chlorella species, the survival of A. franciscana recorded moderate values ranging between 68 ± 3.3 and 78 ± 3.2 % for C. capsulata and C. salina, respectively. Furthermore, the lowest survival accompanied with fresh weight of A. franciscana was recorded upon using C. capsulata: 68 % and 2.9 mg, respectively, after the same period of growth.
4 Discussion
In this study, we are interested to know about the five experimental Chlorella species for their growth and the characterization of their biomass contents for further applications in A. franciscana feeding.
Monitoring of the algae growth was determined during the exponential phase that mainly relied upon the inocula sizes, culture conditions and the composition of the growth media as revealed by Jayasankar and Valsala (2008). On the 10th day, the five studied Chlorella species reached their maximum cell number and the alga C. salina was superior in growth, while C. marina was the lowest grown alga. The growth rate of C. marina was recorded for its maximum growth rate (2.0) after 3 days of incubation, followed by C. vulgaris after 4 days. Under this administration, Mandalam and Palsson (1997); and Scarsella et al. (2010) reported that C. vulgaris growth rate was equal to 1.4 cell 104/h, with a biomass productivity of 0.27 g L−1.
Growth monitoring, in terms of cell numbers or biomass, and the biochemical composition are two essential attributes to evaluate the prospective of a species as promising candidates for different applications as demonstrated by Araújo and Garcia (2005).
In this study, Chlorella species began their stationary phase of growth on the sixth day of incubation and achieved the maximum number on the 10th day, in conjunction with the most elevated lipid content. C. capsulata had accumulated the highest lipid content (44.6 ± 3.4 %) followed by C. salina (26.8 ± 2.08 %) and C. marina (26.7 ± 2.6 %). Comparable results were recorded by several past studies [Huerlimann et al. (2010), Muthukumar et al. (2012) and Moheimani (2013), Sudha et al. (2013)]. Besides, microalgae produced and stored lipids in the form of phospholipids and glycolipids which can be used as feed in aquaculture as indicated by Muthukumar et al. (2012). Ilavarasi et al. (2011) discussed lipid production as a process organized by algae growth. However, Feng et al. (2011) discussed the lipid accumulation on the bases that after day 8, the growth of algae nearly ceased, thus resulting in the increased lipid synthesis.
The total lipid extracted from different experimental Chlorella species, C. salina and C. capsulata followed by C. stigmatophora was found to have accumulated enormous saturated fatty acids (94.2; 93.0; 87.0 %), respectively. However, Shahar (2014) reported that the major fraction of fatty acids belonging to C. salina was PUFAs. The main components of SFAs in the studied Chlorella species were palmitic acid (C16:0) as well as stearic acid (C18:0). In this respect, El-Sheekh and Hamouda (2016) reported that palmitic acid is the major constituent in the crude lipids of the green alga Ankistrodesmus falcatus. Moreover, Bakhtiarvandi et al. (2014) stated that SFAs are utilized as energy substrate and, therefore, SFAs are needed for nutrition.
Several researches concluded the presence of UFAs in algae lipids; they have been considered as wellsprings of PUFAs for aquaculture industry as stated by Patil et al. (2005). Oleic acid was recorded in C. marina, C. stigmatophora and C. vulgaris. These outcomes are additionally in accordance with those reported by (Ötleş and Pire 2001). Furthermore, Gerasimenko et al. (2010) stated that algal lipids could be a source of polyunsaturated fatty acids (PUFAs) of ω-3 and ω-6 series. In our study, PUFAs-ω6 has been detected with nearly low concentrations in all the studied Chlorella species. These results are also in line with findings of Brown et al. (1997) who revealed that the PUFAs contents in marine Chlorella sp. were very low. It is important to draw attention that the GC mass device might miss some essential fatty acids within the algal lipid that may be essential for nutrition because of the coating material of the device.
Carbohydrate production in algae is crucial since it acts as structural components in the cell wall and the compounds stored intracellular (Markou et al. 2012). They added that the output of carbohydrate content rely on the microalgal species, the cultivation parameters and the environmental parameters. Carbohydrate content of the studied Chlorella species ranged from 12.8 ± 0.33 to 22.0 ± 0.57 % in C. salina and C. capsulata, respectively. In this appreciation, it was reported that distinctive strains of Chlorella spp. displayed different behaviors and accumulated compounds, including carbohydrates, in variable quantities (Barsanti and Gualtieri 2006).
Different metabolic studies have confirmed the capacities of microalgae as a novel source of protein. The average quality of most of the studied algae is equal or even superior to that of other traditional high quality plant proteins (Spolaore et al. 2006). Protein contents of the studied Chlorella species ranged from 24.8 ± 1.4 % in C. capsulata to 33.1 ± 1.4 % in C. salina. However, in the lipids extracted from C. marina cells, the protein content was observed to have decreased by about (8 %). In this respect, green algae were reported to contain proteins in addition to various nutrients (El-Sheekh and El-Kassas 2014). Additionally, the recorded data lies within the range reported by Guccione et al. (2014) on their study using Chlorella for protein and biofuels. On the contrary, Grigorova (2006) showed that Chlorella protein content was 55 % of the alga dry weight.
Considering amino acids contents in the experimental Chlorella species, proteins from four trial marine Chlorella species were found to be deficient in cysteine and moderately possessing methionine and histidine; however, they contain enough obvious quantities of lysine. Similarly, Fabregas and Herrero (1998) presumed that lysine concentration in marine micro algae species surpassed that in beef or a whole egg. Therefore, it seems logical to assume that Chlorella’s protein, and possibly cells, may serve as an acceptable source of amino acids in animal enrichment, and if supplemented with cysteine, methionine and histidine, it would be identical to other proteins of high nutritional quality.
The ash contents of the studied Chlorella species was in the extent reported by Grigorova (2006), who showed that ash content of Chlorella was 8.7 %. On the contrary, other studies revealed that green algae including C. salina contained significantly higher contents of ashes [Wong and Chan (1980); (Bi et al. 2013)].
Surprisingly, the results revealed that there weren’t any significant differences between complete and lipid extracted algae residue. This finding reflects a significant economic value of the five studied Chlorella species for aquaculture feeding at the level of a commercial scale. Besides that, the determination of the chemical composition of food plays a key role in larviculture (Pettersen et al. 2010). Therefore, the experimental algal meal of the delipidated Chlorella species, including the recorded nutritional components, may serve as viable feed stuff in aquaculture.
Feeding marine creatures under culture conditions is one of the most important challenges to improve performance and ensure production processes (Cisneros and Vinatea 2009). Artemia (brine shrimp) is probably the most popular live eating regimen in aquaculture (Khairy and El-Sayed 2012). Therefore, this study was extended to use the different, both complete and delipidated experimental Chlorella species for A. franciscana feeding.
In this study, the enriched A. franciscana has gained significant improvements in its biochemical composition. The carbohydrate contents of A. franciscana increased by values that ranged between 9.23 and 16.3 % when compared to the controlled. Moreover, the protein contents of A. franciscana were doubled in those fed on the complete experimented algal cells. In these contexts, Sun and Wang (2009) revealed that the nutritive quality of microalgae is related to their biochemical composition. Earlier studies by Brown (2002) and Becker (2007) suggested that microalgae have a vital role in aquaculture for providing protein (essential amino acids). Chlorella has high protein contents with a balanced amino acid composition. They can be transferred up through the food chain to improve nourishments of young larvae, ornamental fish, shell fish and bivalves [Muller-Feuga (2000), Cho et al. (2007)]. Furthermore, marine algae can be used as an enrichment media instead of certain commercial media that give the same nutritional level as reported by Senthil et al. (2012). Recently, El-Sheekh et al. (2015) revealed that there were significant increases in the biochemical composition of the A. franciscana (carbohydrate, total protein, total lipid, and omega3 fatty acids), after 24 h of enrichment with the complete Tetraseilimus chuii cells cultured on optimized media (P ≤ 0.001).
The lipids of the studied Chlorella species had been extracted for biodiesel production and the lipid extracted residue of the different experimental Chlorella species was used for A. franciscana feeding. These residues of the different experimented Chlorella species exerted significant improvements in the constituents of A. franciscana, i.e.; that is a possible technique to re-cycle/up-cycle the exhausted biomasses, and would be an important achievement. This is critical in the view of the recent analysis that has shown that to achieve a positive energy steadiness and to produce economically viable biofuels, the residue after extraction must be used for co-products as stated by Wijffels et al. (2010) and Prommuak et al. (2013).
A trial to use a mixed diet of the five experimental algae has been carried out. The contents of PUFAs-ω3 increased by nearly 7.9 folds in A. franciscana that had been fed on the mixture of the different lipid extracted Chlorella species, giving it a decent opportunity to be used in A.franciscana feeding. Correspondingly and in concurrence with our outcomes, Zaki and Saad (2010) concluded that a deliberately chosen mixed diet of microalgae can offer excellent nutrition for larvae, either directly or indirectly (through enrichment of zooplankton). They added that the nutrition sufficiency of live food provided to the newly hatched larvae improves their growth and enhances their survival rate. Furthermore, the use of an algal meal could be a nutritious, economic and environmentally sustainable substitution for animal protein as stated by Dib (2012).
During the experimental work, the protein/lipid ratio of A. franciscana that had been enriched with the complete cells of different experimented Chlorella species was highly improved when compared to the controlled. In this respect, Olsen et al. (2000) reported that imbalances in the lipid composition of the diet, either quantitative or qualitative, resulted in poor larval growth and performance. Large scale research and product development has gone into improving rotifer and brine shrimp nutritional quality by manipulating their diet e.g., by microalgal strain selection or by incorporating dried microalgal biomass into formulated inert diets (Shields and Lupatsch 2012).
The recorded improvements in the A. franciscana survival and fresh weight when feeding on the dried algae residues were similar to that reported by Abomohra et al. (2014) during their study using the dried S. obliques for different Artemia species feeding. The study results were also in accordance with Kim et al. (2002) using dietary supplementation of Chlorella ellipsoidea. Kim et al. (2002) reported higher weight gain and improved feed adequacy and protein efficiency ratios in juvenile Japanese flounders (Paralichthys olivaceus). Furthermore, (Godínez et al. 2004) suggested that the energy content of other studied species of microalgae can affect the growth of Artemia. However, Maldonado-Montiel and Rodríguez-Canché (2005) revealed that biochemical composition of the various algae strains could not be correlated with survival and feed conversion rate values could confirm the best algal feed to Artemia sp. Here in this study, the unexpected improvements in the fresh weight and survival % of A. franciscana that fed on a mixed exhausted algae biomass may be attributed to the nutritional contents of the delipidated dried algae species which complete each other forming an absolute balanced diet that encourage A. franciscana growth and survival.
5 Conclusions
The main goals of this investigation were successfully accomplished. This work examines batch scale cultivation of five Chlorella species as promising microalgae for A. franciscana feeding in simple, efficient and economical methods. The lipids, carbohydrates and protein contents of the experimental Chlorella species were estimated. The alga C. capsulata produced the highest lipid content. FAMEs of total lipids of the experimental Chlorella species showed that C. salina, C. capsulata and C. stigmatophora accumulate enormous saturated fatty acids while unsaturated fatty acids were present in C. marina and C. vulgaris. The study’s results showed that, after lipid extraction, there weren’t any significant losses in the amounts of the algal proteins, carbohydrates and ashes when compared to the values recorded for the complete algae cells. In addition, the amino acid composition of delipidated cell residue was found in agreement with previous studies. The enrichment of A. franciscana with complete cells of the different experimental Chlorella species resulted in an increase of its lipids, carbohydrates and protein contents when compared to the controlled. The enrichment with a mixture of the experimental Chlorella species improved the lipid content by 22 %, protein content as well as Protein/Lipid ratio by about 1.96 and 1.33 folds, respectively. However, the enrichment with the mixture of the delipidated experimental Chlorella species attained the detection of PUFAs-ω3 by 7.9 % in the enriched A. franciscana. Survival and fresh weight of A. franciscana were highly improved when feeding on the mixed dried lipid extracted algae meals. Therefore, the study recommends feeding of A. franciscana with mixed algal residues.
References
Abomohra AE, El-Sheekh M, Hanelt D (2014) Pilot cultivation of the chlorophyte microalga Scenedesmus obliquus as a promising feedstock for biofuel. Biomass Bioenergy 64:237–244
Adarme-Vega TC, Lim DK, Timmins M, Vernen F, Li Y et al (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Fact 11(1):96
AOAC (1995) Official methods of analysis of the association of official analytical chemists, 16th ed. Carotenoids and ascorbic acid composition from commercial products of cashew apple (Anacardium occidentale L.). J Food Comp Anal 16:647–657
Araújo S, Garcia VMT (2005) Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 246(1):405–412
Bakhtiarvandi NK, Kenari AA, Nazari RM, Makhdoomi C (2014) Ontogenetic changes in lipids, fatty acid, and body composition during larval stages of Caspian kutum (Rutilus frisii kutum). IJFS 13(2):365–383
Barsanti L, Gualtieri P (2006) Algae—anatomy, biochemistry, and biotechnology. CRC Press, Boca Raton, p 301
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210
Bi Z, He BB (2013) Characterization of microalgae for the purpose of biofuel production. Trans ASABE 56(4):1529–1539
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Brown MR (2002) Nutritional value of microalgae for aquaculture. In: Cruz-Suárez LE, Ricque-Marie D, Tapia-Salazar M, Gaxiola-Cortés MG, Simoes N (eds) Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola.Cancún, Quintana Roo, México
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151(1):315–331
Cho SH, Ji SC, Hur SB, Bae J, Park IS, Song YC (2007) Optimum temperature and salinity conditions for growth of green algae Chlorella ellipsoidea and Nannochloris oculata. Fish Sci 73(5):1050–1056
Cisneros R, Vinatea E (2009) Producción de biomasa de Artemia franciscana Kellogg 1906 utilizando diferentes dietas. Ecología aplicada 8(1–2):9–14
Courtois de Viçose GC, Viera MP, Huchette S, Izquierdo MS (2012) Improving nursery performances of Halioti stuberculata coccinea: nutritional value of four species of benthic diatoms and green macroalgae germlings. Aquaculture 334:124–131
Dib M (2012) Chlorela sp.: Lipid extracted algae utilization of algae biodiesel co-products as an alternative protein feed in animal production (Doctoral dissertation, Colorado State University)
do Amaral P, Freire M (2012) Evaluation of algae concentration in manure based media. PhD thesis, University of Kentucky, p 182
Dubois M, Gilles KA, HamiHon JK, Smith F (1959) Phenol-sulphic method in carbohydrates chemistry. Wistler LR, Wolform RL (eds) Academic Press, New York, pp 388–403
El-Sheekh MM, El-Kassas HY (2014) Biosynthesis, characterization and synergistic effect of phytogenic gold nanoparticles by marine picoeukaryote Picochlorum sp. in combination with antimicrobials. Rend Fis Acc Lincei 25:513–521. doi:10.1007/s12210-014-0341-x
El-Sheekh MM, Hamouda RA (2016) Lipids extraction from the green alga Ankistrodesmus falcatus using different methods. Rend Fis Acc Lincei 27:589–595. doi:10.1007/s12210-016-0528-4
El-Sheekh MM, Gheda SF, Khairy HM, El-Shenody RA (2015) Optimization of medium components using Plackett-Burman design for high production of protein, carbohydrates and lipids in the microalga Tetraselmis chuii. Egypt J Exp Bio (Botany) 11(1):77–88
Fábregas J, Otero A, Morales ED, Arredondo-Vega BO, Patiño M (1998) Modification of the nutritive value of Phaeodactylum tricornutum for Artemia sp. in semicontinuous cultures. Aquaculture 169(3):167–176
Feng Y, Li C, Zhang D (2011) Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour Technol 102(1):101–105
Gerasimenko NI, Busarova NG, Moiseenko OP (2010) Seasonal changes in the content of lipids, fatty acids, and pigments in brown alga Costaria costata. Russ J Plant Physl 57(2):205–211
Godínez DE, Gallo R, Gelabert AH, Gamboa DJ, Landa V, Godínez EM (2004) Crecimientolarvario de Artemia franciscana (Kellog, 1906) alimentada con dos especies de microalgas vivas. Zootec Trop 22:265–275
Grigorova S (2006) Dry biomass of fresh water algae of Chlorella genus in the combined forages for laying hens. JCEA 6(4):625–630
Guccione A, Biondi N, Sampietro G, Rodolfi L et al (2014) Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol Biofuels 7(1):1
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms: i. Cyclotella nana Hustedt, and Detonulacon fervacea (cleve) gran. Can J Microbiol 8(2):229–239
Hartee EF (1972) A modification of Lawry method that gives a linear photometric response. Anal Biochem 41:422–430
Huerlimann R, De Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale up production. Biotechnol Bioeng 107(2):245–257
Ilavarasi A, Mubarakali D, Praveenkumar R, Baldev E, Thajuddin N (2011) Optimization of various growth media to freshwater microalgae for biomass production. Biotechnol 10:540–545
Jayasankar R, Valsala KK (2008) Influence of different concentrations of sodium bicarbonate on growth rate and chlorophyll content of Chlorella salina. JMBAI 50(1):74–78
Khairy HM, El-Sayed HS (2012) Effect of enriched Brachionus plicatilis and Artemia franciscana nauplii by microalga Tetraselmis chuii (Bütcher) grown on four different culture media on the growth and survival of Sparus aurata larvae. Afr J Biotech 11(2):399–415
Kim K-W, Bai SC, Koo J-W, Wang X (2002) Effects of dietary Chlorella ellipsoidea supplementation on growth, blood characteristics, and whole-body composition in juvenile Japanese flounder Paralichthys olivaceus. J World Aquac Soc33(4):425e31
Lavens P, Sorgeloos P (1996) (eds) Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper. No. 361. Rome, FAO, p 295
Maldonado-Montiel TD, Rodríguez-Canché LG (2005) Biomass production and nutritional value of Artemiasp. (Anostraca: Artemiidae) in Campeche, México. Rev Biol Trop 53(3–4):447–454
Mandalam RK, Palsson BO (1997) Cell cycle of Chlorella vulgaris can deviate from the synchronous binary division model. Biotechnol Lett 19(6):587–591
Markou G, Chatzipavlidis I, Georgakakis D (2012) Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: improvements through phosphorus limitation process. BioEnergy research 5(4):915–925
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol 10(1):31–41
Moheimani NR (2013) Long-term outdoor growth and lipid productivity of Tetraselmis suecica, Dunaliella tertiolecta and Chlorella sp. (Chlorophyta) in bag photobioreactors. J Appl Phycol 25(1):167–176
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12(3–5):527–534
Muthukumar A, Elayaraja S, Ajithkumar TT, Kumaresan S, Balasubramanian T (2012) Biodiesel production from marine microalgae Chlorella marina and Nannochloropsis salina. J Petrol Technol Altern Fuels 3:58–62
Olsen AI, Olsen Y, Attramadal Y, Christie K, Birkbeck TH et al (2000) Effects of short term feeding of microalgae on the bacterial flora associated with juvenile Artemia franciscana. Aquaculture 190(1):11–25
Ötleş S, Pire R (2001) Fatty acid composition of Chlorella and Spirulina microalgae species. J AOAC Int 84(6):1708–1714
Pacheco-Vega JM, Cadena-Roa MA, Ascencio F, Rangel-Dávalos C, Rojas-Contreras M (2015) Assessment of endemic microalgae as potential food for Artemia franciscana culture. Lat Am J Aquat Res 43(1):23–32
Patil V, Reitan KI, Knutsen G, Mortensen LM, Källqvist T et al (2005) Microalgae as source of polyunsaturated fatty acids for aquaculture. Plant Biol 6:57–65
Pettersen AK, Turchini GM, Jahangard S, Ingram BA, Sherman CD (2010) Effects of different dietary microalgae on survival, growth, settlement and fatty acid composition of blue mussel (Mytilus galloprovincialis) larvae. Aquaculture 309(1):115–124
Prommuak C, Pavasant P, Quitain AT, Goto M, Shotipruk A (2013) Simultaneous production of biodiesel and free lutein from Chlorella vulgaris. Chem EngTechnol 36:733–739
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65(6):635–648
Radwan SS (1978) Coupling of two-dimensional thin-layer chromatography with gas chromatography for the quantitative analysis of lipid classes and their constituent fatty acids. J Chromatogr Sci 16(11):538–542
Rausch T (1981) The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia 78:237–251
Robert RLG (1979) Growth measurements. Division rate. In: RJ Stein (ed) Physiological methods. Culture methods and growth measurements. Cambridge University. Press, Cambridge, pp 29–311
Rocha JM, Gracia JE, Henriques MH (2003) Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Eng 20(4–6):237–242
Ronquillo JD, Fraser J, McConkey AJ (2012) Effect of mixed microalgal diets on growth and polyunsaturated fatty acid profile of European oyster (Ostrea edulis) juveniles. Aquaculture 360:64–68
Scarsella M, Belotti G, De Filippis P, Bravi M (2010) Study on the optimal growing conditions of Chlorella vulgaris in bubble column photobioreactors. Chem Eng 20:85–90
Senthil SL, MaruthuPandi T, Kumar TA, Devi KN, Balasubramanian T (2012) Exigent of microalgae for the enrichment of Artemia salina. J Aquacult Feed Sci Nutr 4(2)
Shahar S (2014) Biochemical composition and antioxidant capacity of marine microalgae Chlorella salina Butcher and Isochrysis maritima Billard and Gayral isolated from Penang coastal waters (Doctoral dissertation, UniversitiSains Malaysia)
Shields RJ, Lupatsch I (2012) Algae for aquaculture and animal feeds. J Anim Sci 21:23–37
Sorgeloos P, Dhert P, Candreva P (2001) Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture 200:147–159
Spackman DH, Stein WH, Moore S (1958) Automatic recording apparatus for use in chromatography of amino acids. Anal Chem 30(7):1190–1206
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96
Sudha KSS, Shalma SM, Naveena BE, Prakash S (2013) Effect of nitrogen concentration on growth and lipid content of Chlorella marina and Dunellialla salina for biodiesel production (IJIIT) 2(3):28–32
Sun Y, Wang C (2009) The optimal growth conditions for the biomass production of and the effects that phosphorus, Zn, CO, and light intensity have on the biochemical composition of and the activity of extracellular CA. B B E2(14):225–231
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science (Washington) 329(5993):796–799
Wiley PE, Campbell JE, McKuin B (2011) Production of biodiesel and biogas from algae: a review of process train options. WER 83(4):326–338
Wong PK, Chan KY (1980) Algal single cell protein production from sewage effluent with high salinity. Experientia 36(9):1065–1066
Zaki MI, Saad H (2010) Comparative study on growth and survival of larval and juvenile Dicentrarchus labrax rearing on rotifer and Artemia enriched with four different microalgae species. Afr J Biotech 9(24):3676–3688
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Research involving human participants and/or animals
All procedures followed were in accordance with the ethical and there are no Human Participants or Animals.
Informed consent
Written Informed consent was obtained from all participants.
Rights and permissions
About this article
Cite this article
El-Kassas, H.Y., Mohammady, N.GE., El-Sayed, H.S. et al. Growth and biochemical variability of complete and lipid extracted Chlorella species (application for Artemia franciscana feeding). Rend. Fis. Acc. Lincei 27, 761–774 (2016). https://doi.org/10.1007/s12210-016-0569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-016-0569-8