Abstract
The interaction of heat shock proteins (HSP) with cellular membranes has been an enigmatic process, initially observed by morphological studies, inferred during the purification of HSP70s, and confirmed after the detection of these proteins on the surface of cancer cells and their insertion into artificial lipid bilayers. Today, the association of several HSP with lipid membranes is well established. However, the mechanisms for membrane insertion have been elusive. There is conclusive evidence indicating that HSP70s have a great selectivity for negatively charged phospholipids, whereas other HSP have a broader spectrum of lipid specificity. HSP70 also oligomerizes upon membrane insertion, forming ion conductance channels. The functional role of HSP70 lipid interactions appears related to membrane stabilization that may play a role during cell membrane biogenesis. They could also play a role as membrane chaperones as well as during endocytosis, microautophagy, and signal transduction. Moreover, HSP membrane association is a key component in the extracellular export of these proteins. The presence of HSP70 on the surface of cancer cells and its interaction with lysosome membranes have been envisioned as potential therapeutic targets. Thus, the biology and function of HSP membrane association are reaching a new level of excitement. This review is an attempt to preserve the recollection of the pioneering contributions of many investigators that have participated in this endeavor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The heat shock response: a tale of rejection
Science, like many other disciplines, is operated with unwritten rules, some of them transmitted from generation to generation, and others shaped by rejection, flout, and recognition. The most important tenet is that scientific claims need to be supported by solid evidence. In some circumstances, new findings contradict conventional wisdom, and they are rejected or ignored. This aspect was clearly noticed in R. J. Ellis’s words “It is my belief that scientists should resist the natural tendency to ignore unexpected observations that do not fit the existing paradigm, but take the risk of pursuing them in hope that they lead to new ideas and discoveries” (Ellis 1996). Certainly, these circumstances have impacted and shaped the progress of the stress response and heat shock protein biology. The story began in the early 1960s, when a talented Italian investigator, Ferruccio Ritossa, found that Drosophila cells exposed to elevated temperatures responded with a robust chromosomal activity, which was confirmed by subsequent experiments. This observation was rejected because it was labeled as “irrelevant to the scientific community” (Ritossa 1962, 1996; De Maio et al. 2012). Why did Ritossa’s manuscript receive this indifferent response from a high-impact journal? We may never know the details as Ritossa did not elaborate in print on the original review prior to his passing in 2014. However, we can reflect upon the times. The biological models that dominated molecular genetics were E. coli and its phage lambda. The early 1960s was part of the golden age of molecular biology, often stylized by the quotation frequently ascribed to Jacques Monod that “What is true for E. coli is true for the elephant.” The famous PaJaMa experiments had been published in 1959 by Arthur Pardee, Francois Jacob, and Jacques Monod. The PaJaMa experiments strengthened the hypothesis that a specific molecule facilitated the production of proteins from DNA. This was followed in 1961 by Jacob and Monod’s paper titled “Genetic Regulatory Mechanisms of the Synthesis of Proteins,” showing how genes could be activated to make a specific enzyme β-galactosidase. Gene expression seemed so precise and selective with specific inducer molecules inactivating specific repressors on specific genes, and it appeared to extend throughout many if not all species. How could thermal energy, capable of being absorbed by any molecule and therefore the antithesis of specificity, initiate these remarkable processes to activate a specific set of genes in Drosophila cells and ultimately found in eukaryotic and prokaryotic cells in general? This simple question became a stumbling block even for investigators who accepted the premise that thermal energy increases in cells could induce the expression of a specific set of proteins.
Ritossa’s initial finding was forgotten for almost 12 years, and when it was recalled, colleagues spoke of it at best as a curiosity of Drosophila biology and at worst as a laboratory artifact. Then the proteins that were expressed in response to high temperatures were identified by Alfred Tissieres and collaborators (Tissieres et al. 1974). Alfred, during a sabbatical leave with Hershel Mitchell, had not intended to search for the heat shock proteins (HSP), as they became known, but his original project had not worked, and he was running out of time to test a new polyacrylamide gel method, so he decided to do a quick experiment to find them. It is quite possible that the Drosophila heat shock genes would not have been selected as models of eukaryotic gene expression had not been known due to this happenstance that those genes actually encoded proteins.
Clues to the functions of heat shock proteins
A few years after the discovery of the proteins, during the bloom of molecular biology, the genes encoding HSP were cloned (Schedl et al. 1978; Livak et al. 1978; Craig et al. 1979), and the mechanisms of transcription regulation were elucidated (Pelham 1982; Wu 1984; Bahl et al. 1987). There was little interest in attempting to discover the functions of the HSP, and in fact, there were no solid clues to what they might be doing in cells. The fact that virtually all molecules absorb thermal energy and are affected by it, even if only to increase the kinetic energy, meant that no clues were provided by the major known inducer. Promising new clues came from two unlikely fields, animal virology, and neuroscience. Lawrence Hightower, while studying Newcastle Disease Virus-infected avian cell cultures, serendipitously found that different amino acid analogs sharing the common property of incorporating into aberrant proteins altering functions and stabilities caused the induction of HSP at normal temperatures. Independently, Fredric White, while studying rat brain slices as in vitro models for protein synthesis, discovered a small set of proteins, rapidly induced in this tissue, that he ultimately determined to be the mammalian equivalent of the Drosophila HSP. He suggested that these proteins were induced in response to the trauma of tissue slicing and incubation in vitro. They jointly published their observations showing that amino acid analogs and tissue trauma induced the same set of proteins in mammalian cells (Hightower and White 1981). A great step that followed was the discovery that the expression of HSP was not limited to lower organisms, tissue preparations, and cells in culture, but also found in mammalian tissues after in vivo hyperthermia (Currie and White 1983). Suddenly, it became possible to test hypotheses that cells were capable of “sensing” the presence of damaged or unfolded proteins and responding by producing cellular defense proteins to meet the challenge. Essentially any stressor capable of causing cellular or tissue damage that directly or indirectly caused the accumulation of abnormal proteins could be an inducer of the heat shock response (Hightower 1980; Ananthan et al. 1986; Edington et al. 1989). Then, HSP were recognized as composed of many different polypeptides with different molecular masses, some of which were constitutively present under normal physiological conditions, whereas others were induced after a variety of stressors (Lindquist 1986; Lindquist and Craig 1988). Then, the new concept of proteotoxic stress was born (Hightower 1991). A subsequent major breakthrough was related to the finding that HSP participate in protein folding during normal physiological conditions as well as after harmful events, and the concept of molecular chaperones was introduced in this context (Ellis 1996), resembling a prior concept coined by Laskey et al. (1978) regarding a nuclear protein, nucleoplasmin, preventing the aggregation of histones during nucleosome assembly. The folding capacity of HSP was related to an intrinsic ATPase activity. For example, Sadis and Hightower (1992) used the unfolded precursor protein apocytochrome c to show that HSP70 and its constitutively expressed cognate HSC70 can distinguish between unfolded and folded forms of the protein. In this case, the HSP70/HSC70 ATPase activity was only stimulated by the unfolded form. Moreover, the old notion of hyperthermia tolerance observed during approaches to eradicate malignant tumors, initially reported by Crile (1963), was indeed mediated by HSP, a process coined “stress tolerance” (Landry et al. 1982; Subjeck et al. 1982; Li and Werb 1982), which gave a new perspective to the field. During the following years, a great deal of effort was directed at purifying the proteins (Welch and Feramisco 1982; Guidon and Hightower 1986a, b) and developing specific antibodies (Welch and Feramisco 1984; Welch and Suhan 1985; 1986). With these tools on hand, the biology of HSP flourished, resulting in a very exciting period of discovery that continues to the present and it is impulse into the future.
The encounter of heat shock proteins with membranes
Morphological studies for the detection of HSP within cells revealed the presence of these proteins in various subcellular compartments, including in close proximity to membranes (Velazquez et al. 1980; Velazquez and Lindquist 1984). The apparent presence of HSP within membranes was also later observed by others (LaThangue 1984; Welch and Suhan 1985). Although the potential interaction of the proteins with membranes was not further investigated, a surprising observation was encountered during the purification of rat HSP70/HSC70 from cellular extracts. Guidon and Hightower (1986a; b) found that the purified protein was still associated with fatty acids. This observation became the first solid evidence for the interaction of HSP70s with lipids. These pioneering observations were also forgotten for many years, and the attention was directed at the role of HSP70 in protein folding and thermotolerance. It was relatively easy for skeptics to dismiss the association of noncovalently associated fatty acids with HSP as simply a gratuitous presence of a small amount of unesterified and nonspecific fatty acids in purified protein preparations. This was despite the fact that the same fatty acids, palmitic and stearic acids in the same 1:1 ratio, were associated with purified HSC70 and HSP70 from two organs, liver and brain, with very different free fatty acid compositions (Guidon and Hightower 1986a; b). The interest in the association of HSP with membranes was regained by observations regarding the presence of these proteins on the cell surface. The first report on this occurrence was in 1992, in which HSP90 and HSP70 were detected on the surface of several tumor cell lines (Ferrarini et al. 1992). Additionally, HSP70 was detected on the surface of retroocular fibroblasts obtained from patients suffering from Graves’ ophthalmopathy, an autoimmune inflammatory disorder (Heufelder et al. 1992). Moreover, HSP70 was also found in T cell lines infected with leukemia virus I, triggering the production of antibodies against the HSP (Chouchane et al. 1994). These early observations did not receive any major attention, probably because it was unknown whether the protein was inserted into the membrane or just associated with plasma membrane proteins. It was not until Gabriele Multhoff ‘s remarkable work showing in very elegant studies that HSP70 was exclusively present on the surface of tumor cells, embedded into the plasma membrane (Multhoff et al. 1995). This annotation was very controversial at that time, particularly because the majority of available antibodies did not recognize the protein on the cell surface, except for one commercially available, which was rapidly discontinued, probably due to the lack of business. Multhoff ‘s group performed an epitope mapping of HSP70, identifying a motif coined “TKD” (TKDNNLLGRFELSG) that was exposed outside the cell (Botzler et al. 1998). A new antibody for this epitope was raised and distributed, allowing several groups to confirm Multhoff’s initial findings. Today, there are extensive reports demonstrating the presence of several HSP on the surface of various cells (Table 1). Moreover, there are several excellent reviews on the topic (Multhoff and Hightower 2011; De Maio 2011; De Maio and Vazquez 2013; Shevtsov et al. 2020; Elmallah et al. 2020).
The controversial finding that HSP70 was inserted into the plasma membrane of cancer cells was again unappreciated for many years. The turning point came in the year 2000 at the annual Cold Spring Harbor Meeting “Molecular chaperones and the heat shock response,” in which two posters changed the course of the field. Asea and Calderwood showed elegant studies demonstrating that exogenous HSP70 was capable of activating macrophages producing a robust inflammatory response. This study was later published in a prestigious journal (Asea et al. 2000). This observation opened an extensive line of investigation regarding the role of extracellular HSP in cell signaling and as biomarkers that is still very active today (Calderwood et al. 2007a; De Maio 2011, 2014; Pockley et al. 2014). The second poster by Arispe and De Maio showed that HSC70 (HSPA8) could get inserted into planar lipid bilayers, forming a very stable ion channel with a conductance regulated by nucleotides. The poster was greeted by a very seasoned investigator who shouted at one of the presenters during the initial lunch, “Are you saying that HSP70 is opening pores? Are you crazy?” This observation was later published in the Journal of Biological Chemistry after being rejected by a prominent journal because it did not have any biological importance (Arispe and De Maio 2000). The Arispe and De Maio poster did not cause any major impact at that time, perhaps because there was no other electrophysiologist at the meeting. However, two people were very excited about the observation. The first one was Michael Tytell, who, many years back, showed that a heat shock-like protein was released from the squid giant axon and transferred to the glia (Tytell et al. 1986). The second was Larry Hightower, who previously showed, as indicated above, that the protein was associated with fatty acids (Guidon and Hightower 1986a, b). Thus, the association of HSC70 with membranes could nicely explain their original findings.
The interaction of HSP70s with lipids and membranes
Following the pioneering work of Guidon and Hightower, two publications appeared. Alder et al. (1990) reported that the addition of HSP70 to liposomes produced a leakage of the vesicle contents, probably due to the formation of pores. Moreover, Negulyaev et al. 1996 found that the addition of exogenous HSP70 to patch-clamped membranes activated potassium currents. These observations were under the radar for many years. It was not until 2000 that Arispe and De Maio observed that HSC70 (HSPA8) could form very stable and uniform ion conductance channels upon incorporation into artificial lipid bilayers. The ion conductance pathway displayed a multi-conductance activity by frequently switching between different open levels. The channel was selective for cations, and it was not voltage-dependent. Moreover, the channel conductivity was opened by ATP and closed by ADP (Arispe and De Maio 2000). The HSPA8 channel activity was later confirmed by Macazo and White (2014), and a similar channel activity was also reported for HSPA1 (Vega et al. 2008).
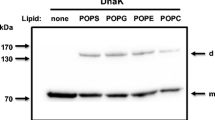
An interesting feature of the interaction of HSP70 with lipid membranes was their high selectivity for negatively charged phospholipids, particularly phosphatidylserine (PS) (Arispe et al. 2004; Schilling et al. 2009; Armijo et al. 2014; Lopez et al. 2016; McCallister et al. 2016). Indeed, the interaction of HSP70 (HSPA1) with membranes was diminished by exchanging portions of PS with phosphatidylcholine (PC) within liposomes (Arispe et al. 2004; Armijo et al. 2014). Additionally, HSPA8 (HSC70) was found associated with PS on the cytosolic side of endosomes during microautophagy (Sahu et al. 2011). Other negatively charged phospholipids also mediated the interaction of HSP70 with membranes, including palmitoyl-oleoyl phosphatidylglycerol (POPG) (Armijo et al. 2014; McCallister et al. 2016) and bis(monoacylglycero)phosphate (BMP), the latter being a major phospholipid of lysosome membranes (Kirkegaard et al. 2010; Mahalka et al. 2014). In addition, HSP70 associates with cardiolipin that is present in mitochondrial membranes (Mahalka et al. 2014). Indeed, mitochondria HSP70 (mtHSP70), also known as mortalin (HSPA9), interacts with membranes containing cardiolipin, particularly resembling the inner mitochondrial membranes (Dores-Silva et al. 2020a). Other studies detected the interaction of HSP70 with the glycosphingolipid Gb3 (Gehrmann et al. 2008) and sulfogalactosyl ceramide (Mamelak et al. 2001), which are also negatively charged. HSC70 (HSPA8) showed also high selectivity for PS in addition to low affinity for PC (Dores-Silva et al. 2021). In contrast with the preceding observations, bacterial HSP70, DnaK, interacted with lipid membranes without any phospholipid specificity (Lopez et al. 2016), suggesting that the ability of HSP70s to associate with membranes may be an ancient characteristic of these proteins, and phospholipid specificity was gained during evolution.
The distribution of phospholipids within the plasma membrane is asymmetric, with PC head exposed outside the cells and PS and phosphatidylethanolamine (PE) located within the cytosolic side of the membrane. This asymmetric distribution is maintained by a complex and energy-consuming mechanism directed at correcting the potential spontaneous flipping of lipids across the bilayer (Leventis and Grinstein 2010). Therefore, cytosolic HSP70 could interact with the negatively charged phospholipids within the inner side of the plasma membrane, a process that could be followed by lipid bilayer insertion. Such an event may allow the exposure of some protein regions on the cell surface. Indeed, studies by Multhoff showed that only partial regions of Hsp70 are displayed on the cell surface (Botzler et al. 1998). The amount of HSP70 inserted into the plasma membrane of tumor cells has been reported to be less than 15% of the total cellular concentration of this protein (Gehrmann et al. 2008). Thus, the question that emerges is why only a fraction of the very abundant HSP70 is associated with the plasma membrane. We have proposed that only substrate-free HSP70 is capable of translocating into the lipid bilayer (De Maio 2011). This assumption is based on the observation that HSP70 did not appear on the plasma membrane immediately after heat shock but rather after several hours of post heat stress recovery (Vega et al. 2008), perhaps because HSP70 is in excess with respect to heat-induced unfolded proteins at late times after the insult (Fig. 1). The same argument could be used to explain how the constitutive HSPA8, which is also very abundant in normal physiological conditions, is not ordinarily present on the cell surface, even though this protein has the capacity to interact with lipids (Arispe and De Maio 2000; Macazo and White 2014). Indeed, HSPA8 is likely primary associated with substrates, particularly nascent polypeptides, perhaps preventing membrane insertion. The exception is the binding of HSPA8 to PS within endosomes as part of the process of microautophagy (Sahu et al. 2011). Another argument is that HSP70 is present, almost exclusively, on the membranes of cancer cells because these transformed cells have a great excess of HSP70 with respect to non-cancer cells (Calderwood et al. 2006), which are likely in larger abundance with respect to potential cellular substrates.
Proposed mechanism for the translocation of HSP70 from the cytosol into the plasma membrane. Proteins are properly folding during normal physiological conditions that become unfolded upon heat shock (1) and the expression of HSP70. These newly expressed HSP70s bind to unfolded polypeptides (2), resulting in the refolding of denatured proteins (3) and an excess of polypeptide-free HSP70 (4), that is now capable of getting inserted into the plasma membrane (5) via the interaction with PS on the inner part of the bilayer
Other HSP70s, such as HSPA5 (BIP, Grp78), have also been found associated with lipid membranes. HSPA5 was detected inserted into the plasma membrane of cancer cells (Suzuki et al. 1991; Delpino and Castelli 2002; Zhang et al. 2010, 2013). In addition, the protein was released outside cells (Delpino and Castelli 2002; Zhang et al. 2013). The plasma membrane insertion and extracellular export of HSPA5 were not very surprising since this protein is a resident of the ER. However, HSPA5 needs to overcome the ER retention signal (KDEL) to reach the cell surface/extracellular environment, which could be a consequence of ER stress (Zhang et al. 2013) or any additional factors. HSPA5 is unlikely to interact with the internal ER membrane because the phospholipid composition of this region does not support membrane insertion (Dores-Silva et al., 2020b). Several domains of HSPA5 have been proposed to be inserted into the plasma membrane, particularly the C-terminus end (Tsai et al. 2015; Tseng et al. 2019). The interaction of HSPA5 with artificial lipid bilayers (liposomes) has confirmed membrane insertion, displaying a high affinity for negatively charged phospholipids (Dores-Silva et al. 2020b). Both HSPA5 N-terminal and C-terminal domains could independently interact with phospholipid membranes, but not at the same levels as the full-length protein, suggesting that the two regions may be involved in membrane insertion (Dores-Silva et al. 2020b).
Another HSP70, HSPA9 (mtHsp70, mortalin), that is mainly located in the mitochondrial matrix was also found to associate with negatively charged membranes, in particular cardiolipin, that constitutes approximately 18% of the inner membrane and less than 1% of the outer membrane (Zinser et al. 1991). Studies using liposomes resembling the composition of both inner and outer mitochondrial membranes showed that, indeed, HSPA9 has selectivity for the inner membrane (Dores-Silva et al., 2020a). A very important observation was the interaction of HSP70 with lysosome membranes specifically mediated by binding to the negatively charged phospholipid BMP that is a major component of this compartment (Kirkegaard et al. 2010; Mahalka et al. 2014). The association of HSP70 with lysosome membranes confers stability to this compartment preventing the leakage of lytic enzymes (Nylandsted et al. 2004). Moreover, the interaction of HSP70 and lysosomes appears particularly important in conditions of lysosome storage disorders, and it has been envisioned as a potential therapeutic target (Kirkegaard et al. 2010; 2016; Balogi et al. 2019).
Although all HSP70s displayed the same affinity for negatively charged phospholipids, their insertion into membranes is not identical. The interaction of HSPA1 and HSPA8 with lipids was different in a liposome aggregation assay, including differences in insertion kinetics and the effect of calcium and nucleotides (Arispe et al. 2002). Another example is the interaction of HSPA9 with POPS liposomes displaying a saturation profile that was not observed for HSPA1. Thus, the packing of the protein within the lipid bilayer or perhaps translocation into the lumen of the liposome appears to be different among these two HSP70 members (Dores-Silva et al., 2020a). Thermodynamic parameters measured during the insertion into artificial membranes indicated that the process is spontaneous but slightly different for HSPA1, HSPA5, HSPA8, and HSPA9, involving intramolecular interactions, Van der Waals forces, hydrophobic interactions, water displacements, and conformational changes (Dores-Silva et al., 2020a, b, 2021).
Other heat shock proteins also interact with membranes
Small HSP, which play a plethora of biological functions (Carra et al. 2017), have not escaped from the interaction with lipids. The small HSP of bacteria, Hsp17, was initially found sedimenting with membranes (Miyake et al. 1993), and it was later found to localize with the outer microbe membrane (Laskowska et al. 2004). Other studies have shown that alpha-crystallin (HSPB5) interacted with lipid membranes (Borchman and Tang 1996; Ifeanyi and Takemoto 1991). Moreover, this protein oligomerizes at higher temperatures driving the insertion into the membranes of vertebrate lenses (Tjondro et al. 2016). Interestingly, HSPB5 membrane association has been correlated with the development of cataracts (Boyle and Takemoto 1996; Cenedella and Fleschner 1992; Cobb and Petrash 2002). HSPB5 and Hsp17 have been reported to stabilize artificial membranes mediated by interaction with the polar head group and affecting the hydrophobic region of the lipid bilayer (Tsvetkova et al. 2002). Recently, HSPB1 and HSPB5 were found to get inserted into liposomes in which the alpha-crystallin domain characteristic of these proteins is embedded into the lipid bilayer. These two small HSP did not associate with the liposomes identically; neither did they display any phospholipid specificity (De Maio et al. 2019). These observations are similar to prior observations indicating that the interaction of HSPB5 with lipids was not specific for the type of phospholipids (Cobb and Petrash 2002) and was reduced by the presence of cholesterol within the membrane (Tang et al. 1998). HSPB5 has been found associated with a variety of membranes, including lenses (Boyle and Takemoto 1996; Cenedella and Fleschner 1992; Cobb and Petrash 2002; Friedrich and Truscott 2010), mitochondria (Whittaker et al. 2009), and Golgi (Gangalum et al. 2004; Gangalum and Bhat 2009). In addition, HSPH5 was observed participating in exosome assembly and release (Gangalum et al. 2016; Kore and Abraham 2016). Other HSP, such as Hsp90 (Hsp90B1), interacted with a mixture of phospholipids stabilizing the membrane (Li et al. 2018). Moreover, Hsp90 family proteins penetrated phospholipid membranes with high affinity losing part of their alpha-helix conformation (Li et al. 2019). In addition, Hsp90 (Hsp90A1) interacts with phospholipid membranes with higher affinity for unsaturated and negatively charged phospholipids, and the affinity increases in the presence of cholesterol (Zhang et al. 2018). GroESL oligomers also interacted with lipid membranes increasing their stability during heat shock conditions (Torok et al. 1997).
Mechanisms of heat shock proteins membrane insertion
The mechanisms for HSP membrane insertion are complex, poorly understood, and enigmatic, particularly because these proteins do not contain major hydrophobic domains that could explain their incorporation into lipid membranes. Biological membranes have a heterogeneous nature in which a hydrophobic center core is made by the assembly of fatty acid tails that are surrounded by a less hydrophobic environment constituted by the polar lipid heads, containing a fair amount of water that may create a niche for the initial insertion of proteins into membranes (Wiener, and White 1992). Thus, the interaction of proteins with the phospholipid head is likely the initiating event for membrane insertion that may be followed by a conformational change that facilitates the incorporation into the most hydrophobic region of the membrane, which may be part of or secondary to an oligomerization process (Wimley et al. 1998). Based on these assumptions, it is not surprising that HSP70 displays phospholipid head specificity and oligomerizes upon membrane insertion. Recently, the interaction of HSP70 with lipid membranes has been shown to result in a rearrangement of the hydration layer associated with the bilayer (Dhanasekaran et al. 2020). Moreover, the insertion of HSP70s into the lipid membrane is a thermodynamically spontaneous process (Dores-Silva et al. 2020a; b; 2021). HSPA8 was reported binding to PS on the endosome membrane (Sahu et al. 2011), an interaction that was mediated by a cluster of lysine residues on the N-terminus end of the proteins, which was confirmed by site-directed mutagenesis (Morozova et al. 2016). This observation is consistent with increased interaction with PS liposomes at pH 5.0 and lower at pH 9.0 (Dores-Silva et al. 2021).
Prior studies have shown that HSP70 could form dimers and oligomers in solution (Guidon and Hightower 1986a; b; Benaroudj et al. 1996; Gao et al. 1996; Aprile et al. 2013), a process modulated by nucleotides (Kim et al. 1992; Benaroudj et al. 1996) or temperature (Angelidis et al. 1999; Kiraly et al. 2020). HSPA1 and HSPA9 in solution were observed as homogeneous round complexes of high molecular mass visualized by electron microscopy (Kiraly et al. 2020). Several models have been proposed for the oligomeric complexes, such as binding to the linker between the peptide and nucleotide-binding domains (Chang et al. 2008) and an antiparallel conformation (Morgner et al. 2015). Although no changes in HSP70 secondary conformation have been observed upon membrane insertion, oligomers of this protein have been detected upon incorporation into liposomes (Armijo et al. 2014; Dores-Silva et al. 2020a; 2021). Moreover, studies using atomic force microscopy showed the presence of HSP70 clusters on artificial lipid bilayers (Lamprecht et al. 2018). The best evidence of the oligomerization of HSP70s upon membrane insertion is its ability to form ion conductance channels (Arispe and De Maio 2000; Vega et al. 2008; Macazo and White 2014) which are assembled by various polypeptide subunits or multiple transmembrane domains. The oligomerization process upon membrane insertion may be enhanced by the fluidity of the bilayer as observed using phospholipids with different degrees of fatty acid saturation (Armijo et al. 2014; Lamprecht et al. 2018). Other saturated lipids such as sphingolipids have been reported to be recognized by HSP70 (Gehrmann et al. 2008; Mamelak et al. 2001). Interestingly, cancer cells display elevated levels of the glycosphingolipid Gb3 on the plasma membrane that could explain the presence of HSP70 on the surface of transformed cells (Gehrmann et al. 2008). In this regard, HSP70 have localized within lipid rafts that are rich in sphingolipids and cholesterol (Vega et al. 2008; Nimmervoll et al. 2015; Lamprecht et al. 2018).
As indicated above, HSPA1, HSPA5, and HSPA8 were observed to form oligomers after incorporation into lipid bilayers (Armijo et al. 2014; Dores-Silva et al. 2020a, b, 2021). These oligomeric complexes were stabilized via intermolecular disulfide bonds (Dores-Silva et al. 2020a). HSPA5 contains two cysteine groups, one at the beginning of the N-terminus end and the second at the C-terminus end. In contrast, HSPA1 presents five cysteine groups, with three at the N-terminus end and two at the C-terminus end. HSPA8 has four cysteine groups, two in the nucleotide-binding domain and two in the substrate-binding domain. One of the cysteine groups at the C-terminus end is the only common among all HSP70s. There is no evidence that these cysteine groups form intramolecular bridges in solution nor within the reducing cytosolic environment. Thus, it is possible that the lipid bilayer may provide an oxidative environment allowing the formation of disulfide bridges. Independent membrane insertion of the N-terminus end domain of HSPA1 and HSPA5 could form dimers but not high molecular mass oligomers that were only observed with the full-length protein, whereas membrane insertion of the C-terminus end did not form dimers or oligomers (Dores-Silva et al. 2020a). Based on these observations, we assume that a cysteine within the N-terminus end of the proteins may be within the right conformation to form dimers but not more complex forms. In contrast, we speculate that high-mass oligomers observed upon membrane insertion are the product of intermolecular disulfide bonds between the N-terminus end and the C-terminus domains of adjacent polypeptides assembling in an antiparallel conformation between tandem repeats (Fig. 2).
Why are heat shock proteins inserted into membranes?
The question that emerges is what is the function of HSP membrane association? There is extensive evidence from Vigh’s group showing that HSP stabilize biological membranes (Horvath et al. 2008; Torok et al. 2014; Balogi et al. 2019), which they proposed as a major sensor for thermal stress due to disturbances in membrane fluidity (Csoboz et al. 2013; Balogh et al. 2013). Other studies have indicated lipid membrane stabilization by HSP90 (Li et al.2018, 2019) and HSP70 (Nylandsted et al. 2004). The presence of HSP70 on the surface of cancer cells may confer protection to these cells as well as provide an interface with the immune system (Botzler et al. 1996; Multhoff et al. 2020). Indeed, GRP78/HSPA5 has been implicated in tumor survival, proliferation, and resistance (Pfaffenbach and Lee 2011). In contrast to these observations, the insertion of HSP into membranes could be detrimental. Arispe et al. (2004) showed that exogenous addition of HSP70 could trigger cell death. This observation echoes prior studies showing that an intracellular excess of HSP70 was detrimental in the long term, even though that an early response was protective (Feder et al. 1992). These observations suggest that the potential cytotoxic effect of HSP70 requires that its expression is tightly regulated. Indeed, HSP70 half-life after stress is very short (Mizzen and Welch 1988). Moreover, HSP70 has been reported as a negative regulator of HSF-1, which is the master transcriptional factor for HSP expression (Gomez-Pastor et al. 2018). In addition, the translation of Hsp70 mRNA is reduced in cells that contain large amounts of HSP70 (Theodorakis et al. 1999). Additionally, Hsp70 mRNA has a very short half-life (approximately 1 h) after thermal stress (Theodorakis and Morimoto 1987), which was substantially reduced in cells already containing large amounts of HSP70 (Theodorakis et al. 1999). Also, changes in Hsp70 mRNA stability have been reported in various cell types (DiDomenico et al. 1982; Simcox et al. 1985; Petersen and Lindquist 1989; Ramos and Pastore 2001). In echoes of these observations, HSP70 was found bound to its own message (Balakrishnan and De Maio 2006), a situation that may be part of a mechanism for the self-limiting expression of this protein, as previously proposed (DiDomenico et al. 1982; De Maio 1999). Therefore, it will not be surprising to learn that interaction with membranes may be part of a regulatory mechanism.
HSC70/HSPA8 has been detected on endosome membranes participating in the microautophagy process (Sahu et al. 2011; Morozova et al. 2016). Expression of HSP70 upon heat shock and other stresses was found to increase the endocytosis of transferrin and its receptor (Vega et al. 2010). Moreover, HSP70 accelerates the phagocytotic process in macrophages (Vega and De Maio 2005). The interaction of HSP70s with subcellular vesicles may be necessary for the stabilization of these compartments as proposed for the interaction with lysosome membranes (Kirkegaard et al. 2010; Nylandsted et al. 2004). Moreover, the association of HSP70 with membranes and their intrinsic chaperone activity may raise the possibility that they could be membrane chaperones involved in the insertion of other proteins into membranes. Thus, HSP90, which does not display a significant binding to PS liposomes, was driven into these vesicles after co-incubation with HSPA8 (Dores-Silva et al. 2021). Therefore, HSPA8 may associate with HSP90 in solution prior to membrane association. However, whether HSP90 is inserted into the lipid bilayer or if it is peripherally bound to membrane HSPA8 is unknown. Interestingly, HSPA1 is also capable of bringing HSPA90 into membranes, but this ability is not shared by HSPA5 or HSPA9.
Another possible function for the presence of HSP70s on the plasma membrane may be related to a signal-transducing activity for receptors or co-receptors as proposed for GRP78/HSPA5 (Zhang et al. 2010). Interestingly, HSPA5 has been identified as a receptor for various viruses, including Borna disease (Honda et al. 2009), Coxsackie, dengue virus serotype 2, and Japanese encephalitis (Kottom et al. 2018). Recently, HSPA5 was proposed as an alternative site for the invasion of SARS-CoV-1 (Chu et al. 2018) and SARS-CoV-2 (Ibrahim et al. 2020), the latter being responsible for the COVID-19 pandemic. Latest evidence has shown that HSPA5 forms a complex with the angiotensin-converting enzyme 2 (ACE2) and SARS-CoV-2 spike protein (Carlos et al. 2021). Moreover, reducing surface HSPA5 diminished the membrane presence of ACE2, blocking viral entry. Moreover, HSPA5 displayed higher affinity for the spike protein of the new UK variant of SARS-CoV-2 (VUI202012/01) with respect to the original viral protein as indicated by in silico analysis (Elfiky and Ibrahim 2021).
HSP protein-membrane insertion could also be part of the extracellular export mechanism. With the exception of HSPA5 that is located within the ER, other HSP are present within the cytosol lacking the consensus signal for the classical secretory pathway. Indeed, Hightower and Guidon (1989) showed that the release of HSP70 from cells could not be blocked by classical secretory pathway inhibitors. This early observation was revisited by Hunter-Lavin et al. (2004), showing that indeed HSP70 was released from cells by a mechanism independent of cell death. However, a cloud was raised by Basu et al. (2000), indicating that cell lysis after necrosis was the source of circulating HSP70. Like many things in science, both reports were valid. De Maio and Vazquez (2013) described that HSP70 could be released by cell lysis as well as by the non-classical secretory pathway. The preceding has been described for the export of several cytosolic proteins (Nickel and Seedorf 2008; De Maio 2011). The presence of HSP70 on the plasma membrane could allow this protein to be released via extracellular vesicles or exosomes. Indeed, HSP70s have been reported as a traditional component of exosomes (Lo Cicero et al. 2015). Moreover, HSP70 was found inserted into the exosome membrane (Gastpar et al. 2005; Vega et al. 2008; Gobbo et al. 2016; Chanteloup et al. 2020). In this regard, the formation of bleeds from artificial lipid membranes containing HSP70 was observed upon addition of cholesterol to the bilayer (Lamprecht et al. 2018). Cell surface HSP70 is localized with detergent-resistant membrane microdomains or lipid rafts (Vega et al. 2008; Gehrmann et al. 2008), which could be the precursor for the formation of exosomes (De Maio 2011). Another study has proposed that HSP70 is released associated with secretory-like granules (Evdonin et al. 2006). The insertion of HSP70 into the lysosome-endosome membrane could be an alternative mechanism for extracellular secretion (Nylandsted et al. 2004; Mambula and Calderwood 2006; Juhasz et al. 2013). Similarly, HSPB1 has also been proposed to be secreted via the endo-lysosome pathway (Rayner et al. 2008; 2009). Extracellular HSPs are capable of activating a variety of cellular responses that may be mediated by interaction with surface receptors. Indeed, several extracellular HSP binding proteins have been reported, including LRP/CD91, CD40, CD14, TLRs, c-type lectins, and Scavenger receptors, suggesting that there is not “a receptor” but a variety of binding partners (Calderwood et al. 2007b; De Maio 2014). Interestingly, Shevtsov et al. (2014) showed that exogenous HSP70 were captured by cells triggering the membrane translocation and subsequent export of endogenous HSP70. This observation supports the idea that an excess of subcellular HSP70 drives the appearance of this protein on the cell surface as described above (Fig. 1).
The proteotoxic and metabolic stress responses work against one another. Dai and colleagues proposed that this antagonism creates a third mechanism to balance cellular homeostasis (Dai, et al. 2015). Tezgin and coworkers have postulated that this new mechanism is actually the caloristasis network, in which HSF1 acts as a master proximal integrator (Tezgin et al. 2020). The term caloristasis was coined to pair with proteostasis, and like the latter, it emphasizes the integrative regulatory interactions by molecules like HSF1, which is necessary to understand cellular energy homeostasis in normal and stressed cells. Where to search for additional regulators is the question. One possibility is that the selective membrane association described above for HSP70 interactions with cardiolipin and the association of mortalin (HSPA9) with inner mitochondrial membranes could position these proteins toward the regulation of oxidative phosphorylation. There have suspicions about a connection between proteotoxic stress responses and downregulation of oxidative phosphorylation almost from the initial discovery of mortalin. Wadhwa and coworkers discussed that the yeast mitochondrial reduced form of nicotinamide adenine dinucleotide dehydrogenase (the initial electron acceptor complex of the mitochondrial electron transport chain leading to oxidative phosphorylation) was identified as a binding partner of mortalin (Wadhwa et al. 2002). Another connection comes through the NF-kB transcription family member RelA, also a mitochondrial binding partner of mortalin (Johnson et al. 2011). These same authors have suggested that tumor cells have become dependent on RelA for rapid growth and survival by virtue of its ability to change cells from oxidative phosphorylation to aerobic glycolysis. It is frequently said that tumor cells are “addicted” to HSP and that they have highjacked a normal defensive maneuver of stressed cells, the acquisition of cytoprotection. This defensive response involves conversion of energy transduction from oxidative phosphorylation to glycolysis to drive biosynthesis for the repair and replacement of damaged molecules, similar to why tumor cells are thought to switch to aerobic glycolysis to drive biosynthesis to support rapid proliferation, known as the Warburg Effect (Tezgin et al. 2020). Thus, mortalin could fulfill its role as a multifunctional integrator of caloristasis and proteostasis through its functions as a regulator of oxidative phosphorylation, as a central component of the mitochondrial protein import machinery, and as part of a damaged protein disaggregating complex (Iosefson et al. 2012). These observations echo early Ritossa’s observations regarding other inducers of the stress response pointing toward mitochondrial energy production, particularly the electron transport chain.
The ability of HSP to interact with phospholipids and their capacity to stabilize membranes could have played a role during the evolution of cellular membranes from protocells to modern cells. Prebiotic fatty acids were likely to form small vesicles due to their amphiphilic nature in aqueous solutions that could encapsulate chemicals, forcing them to react, forming new compounds (Black and Blosser 2016; Damer and Deamer 2015). Thus, these vesicles containing a lipid bilayer are likely the precursor of protocells (Black and Blosser 2016; Segre et al. 2001). A key element for the size expansion from the protocell to more complex structures was the ability to stabilize the lipid bilayer. Elegant studies by Cornell et al. (2019) indicated that primitive membranes could be stabilized by the insertion of amino acids. Thus, the evolution of the protocell to advanced cells was likely mediated by the substitution of fatty acids with glycerophospholipids and amino acids with short peptides. These short peptides involved in membrane stabilization were likely to give rise to longer polypeptides retaining the membrane penetrating capacity. Therefore, proteins with membrane insertion and stabilizing abilities such as HSP may have played an important role in the evolutionary progression of cells (De Maio and Hightower 2020). The capacity of ancestral HSP precursors for the interaction with lipid membranes was likely preserved during the evolution to modern chaperones. In other words, the ability of HSP to get incorporated into membranes was not discarded during the process of gaining new functions such as promoting protein folding. According to this hypothesis, ancient HSP were primary membrane-stabilizing proteins before they became chaperones.
Concluding remarks
The progress from the early initial observations of the association of HSP with fatty acids toward their detection on the cell surface, their insertion into artificial lipid bilayers, and our current understanding of the interaction of these proteins with membranes has been a remarkable journey, marked by a lot of controversies, but full of excitement. The mechanisms of membrane insertion and oligomerization have begun to be elucidated. The role of these proteins stabilizing membranes under stress conditions, their capabilities of sensing stress, modulating the movement of subcellular vesicles, their potential participation in cellular membrane biogenesis, and their role in several pathologies have created new excitement that is likely to increase in the upcoming years. However, it is clear that there is still more to be explored.
References
Alder GM, Austen BM, Bashford CL, Mehlert A, Pasternak CA (1990) Heat shock proteins induce pores in membranes. Biosci Rep 10:509–518
Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK (1996) Tumor-specific cell surface expression of the KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer 69:340–349
Ananthan J, Goldberg AL, Voellmy R (1986) Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232:522–524
Angelidis CE, Lazaridis I, Pagoulatos GN (1999) Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem 259:505–512
Aprile FA, Dhulesia A, Stengel F et al (2013) Hsp70 oligomerization is mediated by an interaction between the interdomain linker and the substrate-binding domain. PLoS One 8(6):e67961
Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R (2004) Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell 6:275–284
Arispe N, De Maio A (2000) ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem 275:30839–30843
Arispe N, Doh M, De Maio A (2002) Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones 7:330–338
Arispe N, Doh M, Simakova O, Kurganov B, De Maio A (2004) Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J 18:1636–1645
Armijo G, Okerblom J, Cauvi DM et al (2014) Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones 19:877–886
Asea A, Kraeft SK, Kurt-Jones EA et al (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435–442
Bahl H, Echols H, Straus DB, Court D, Crowl R, Georgopoulos CP (1987) Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda cIII protein. Genes Dev 1:57–64
Balakrishnan K, De Maio A (2006) Heat shock protein 70 binds its own messenger ribonucleic acid as part of a gene expression self-limiting mechanism. Cell Stress Chaperones 11:44–50
Balogh G, Peter M, Glatz A et al (2013) Key role of lipids in heat stress management. FEBS Lett 587:1970–1980
Balogi Z, Multhoff G, Jensen TK et al (2019) Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog Lipid Res 74:18–30
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546
Bausero MA, Page DT, Osinaga E, Asea A (2004) Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol 25:243–251
Belles C, Kuhl A, Nosheny R, Carding SR (1999) Plasma membrane expression of heat shock protein 60 in vivo in response to infection. Infect Immun 67:4191–4200
Benaroudj N, Triniolles F, Ladjimi MM (1996) Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem 271:18471–18476
Berger CL, Dong Z, Hanlon D, Bisaccia E, Edelson RL (1997) A lymphocyte cell surface heat shock protein homologous to the endoplasmic reticulum chaperone, immunoglobulin heavy chain binding protein BIP. Int J Cancer 71:1077–1085
Bilog AD, Smulders L, Oliverio R, Labanieh C, Zapanta J, Stahelin RV, Nikolaidis N (2019) Membrane Localization of HspA1A, a Stress Inducible 70-kDa Heat-Shock Protein, Depends on Its Interaction with Intracellular Phosphatidylserine. Biomolecules 9(4):152
Black RA, Blosser MC (2016) A self-assembled aggregate composed of a fatty acid membrane and the building blocks of biological polymers provides a first step in the emergence of protocells. Life (Basel) 6(3):33
Borchman D, Tang D (1996) Binding capacity of alpha-crystallin to bovine lens lipids. Exp Eye Res 63:407–410
Botzler C, Issels R, Multhoff G (1996) Heat-shock protein 72 cell-surface expression on human lung carcinoma cells in associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol Immunother 43:226–230
Botzler C, Li G, Issels RD, Multhoff G (1998) Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones 3:6–11
Boyle DL, Takemoto L (1996) EM immunolocalization of alpha-crystallins: association with the plasma membrane from normal and cataractous human lenses. Curr Eye Res 15:577–582
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172
Calderwood SK, Mambula SS, Gray PJ Jr (2007a) Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci 1113:28–39
Calderwood SK, Theriault J, Gray PJ, Gong J (2007b) Cell surface receptors for molecular chaperones. Methods 43:199–206
Camins A, Diez-Fernandez C, Prieto P (1999) Cell-surface expression of heat shock proteins in dog neutrophils after oxidative stress. Toxicol in Vitro 13:437–443
Carlos AJ, Ha DP, Yeh DW, Van Krieken R, Gill P, Machida K, Lee AS (2021) GRP78 binds SARS-CoV-2 spike protein and ACE2 and GRP78 depleting antibody blocks viral entry and infection in vitro. bioRxiv. https://doi.org/10.1101/2021.01.20.427368
Carra S, Alberti S, Arrigo PA, Benesch JL, Benjamin IJ, Boelens W, Bartelt-Kirbach B (2017) The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones 22:601–611
Cenedella RJ, Fleschner CR (1992) Selective association of crystallins with lens ‘native’ membrane during dynamic cataractogenesis. Curr Eye Res 11:801–815
Chang CP, Lin G, Chen SJ, Chiu WC, Chen WH, Wang CC (2008) Promoting the formation of an active synthetase/tRNA complex by a nonspecific tRNA-binding domain. J Biol Chem 283:30699–30706
Chanteloup G, Cordonnier M, Isambert N, Bertaut A, Marcion G, Garrido C, Gobbo J (2020) Membrane-bound exosomal HSP70 as a biomarker for detection and monitoring of malignant solid tumours: a pilot study. Pilot Feasibility Stud 6:35
Chouchane L, Bowers FS, Sawasdikosol S, Simpson RM, Kindt TJ (1994) Heat-shock proteins expressed on the surface of human T cell leukemia virus type I-infected cell lines induce autoantibodies in rabbits. J Infect Dis 169:253–259
Chu H, Chan CM, Zhang X et al (2018) Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J Biol Chem 293:11709–11726
Cid C, Alvarez-Cermeno JC, Camafeita E, Salinas M, Alcazar A (2004) Antibodies reactive to heat shock protein 90 induce oligodendrocyte precursor cell death in culture. Implications for demyelination in multiple sclerosis. FASEB J 18:409–411
Cid C, Alvarez-Cermeno JC, Salinas M, Alcazar A (2005) Anti-heat shock protein 90beta antibodies decrease pre-oligodendrocyte population in perinatal and adult cell cultures. Implications for remyelination in multiple sclerosis. J Neurochem 95:349–360
Cid C, Regidor I, Poveda PD, Alcazar A (2009) Expression of heat shock protein 90 at the cell surface in human neuroblastoma cells. Cell Stress Chaperones 14:321–327
Cobb BA, Petrash JM (2002) alpha-Crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry 41:483–490
Cornell CE, Black RA, Xue M et al (2019) Prebiotic amino acids bind to and stabilize prebiotic fatty acid membranes. Proc Natl Acad Sci U S A 116:17239–17244
Craig EA, McCarthy BJ, Wadsworth SC (1979) Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell 16:575–588
Crile G Jr (1963) The effects of heat and radiation on cancers implanted on the feet of mice. Cancer Res 23:372–380
Csoboz B, Balogh GE, Kusz E et al (2013) Membrane fluidity matters: hyperthermia from the aspects of lipids and membranes. Int J Hyperthermia 29:491–499
Currie RW, White FP (1983) Characterization of the synthesis and accumulation of a 71-kilodaton protein induced in rat tissues after hyperthermia. Can J Biochem Cell Biol 61:438–446
Dai S, Tang Z, Cao J, Zhou W, Li H, Sampson S, Dai C (2015) Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J 34:275–293
Damer B, Deamer D (2015) Coupled phases and combinatorial selection in fluctuating hydrothermal pools: a scenario to guide experimental approaches to the origin of cellular life. Life (basel) 5:872–887
De Maio A (1999) Heat shock proteins: facts, thoughts, and dreams. Shock 11:1–12
De Maio A (2011) Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage. Cell Stress Chaperones 16:235–249
De Maio A, Santoro MG, Tanguay RM, Hightower LE (2012) Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: a new view of biology, a new society, and a new journal. Cell Stress Chaperones 17:139–143
De Maio A, Vazquez D (2013) Extracellular heat shock proteins: a new location, a new function. Shock 40:239–246
De Maio A (2014) Extracellular Hsp70: export and function. Curr Protein Pept Sci 15:225–231
De Maio A, Cauvi DM, Capone R, Bello I, Egberts WV, Arispe N, Boelens W (2019) The small heat shock proteins, HSPB1 and HSPB5, interact differently with lipid membranes. Cell Stress Chaperones 24:947–956
De Maio A, Hightower LE (2020) Heat shock proteins and the biogenesis of cellular membranes. Cell Stress Chaperones 26:15–18
Delpino A, Castelli M (2002) The 78 kDa glucose-regulated protein (GRP78/BIP) is expressed on the cell membrane, is released into cell culture medium and is also present in human peripheral circulation. Biosci Rep 22:407–420
Dhanasekaran M, Komal KG, Kumar P, Mandal SS (2020) Critical insights into the interactions of heat shock protein 70 with phospholipids. Phys Chem Chem Phys 22:19238–19248
DiDomenico BJ, Bugaisky GE, Lindquist S (1982) The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell 31:593–603
Dores-Silva PR, Cauvi DM, Kiraly VTR, Borges JC, De Maio A (2020a) Human HSPA9 (mtHsp70, mortalin) interacts with lipid bilayers containing cardiolipin, a major component of the inner mitochondrial membrane. Biochim Biophys Acta Biomembr 1862:183436
Dores-Silva PR, Cauvi DM, Coto ALS, Kiraly VTR, Borges JC, De Maio A (2020b) Interaction of HSPA5 (Grp78, BIP) with negatively charged phospholipid membranes via oligomerization involving the N-terminal end domain. Cell Stress Chaperones 25:979–991
Dores-Silva PR, Cauvi DM, Coto ALS, Silva NSM, Borges JC, De Maio A (2021) Human heat shock cognate protein (HSC70/HSPA8) interacts with negatively charged phospholipids by a different mechanism than other HSP70s and brings HSP90 into membranes. Cell Stress Chaperones [In Press]
Edington BV, Whelan SA, Hightower LE (1989) Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: additional support for the abnormal protein hypothesis of induction. J Cell Physiol 139:219–228
Elfiky AA and Ibrahim MI (2021) Host-cell recognition through GRP78 is enhanced in the new UK variant of SARS-CoV-2, in silico. J Infect Jan 22;S0163–4453(21)00038–4 [Online ahead of print]
Ellis RJ (1996) Discovery of molecular chaperones. Cell Stress Chaperones 1:155–160
Elmallah MIY, Cordonnier M, Vautrot V, Chanteloup G, Garrido C, Gobbo J (2020) Membrane-anchored heat-shock protein 70 (Hsp70) in cancer. Cancer Lett 469:134–141
Evdonin AL, Martynova MG, Bystrova OA, Guzhova IV, Margulis BA, Medvedeva ND (2006) The release of Hsp70 from A431 carcinoma cells is mediated by secretory-like granules. Eur J Cell Biol 85:443–455
Farkas B, Hantschel M, Magyarlaki M et al (2003) Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res 13:147–152
Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S (1992) The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev 6:1402–1413
Ferrarini M, Heltai S, Zocchi MR, Rugarli C (1992) Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 51:613–619
Fong-Ngern K, Sueksakit K, Thongboonkerd V (2016) Surface heat shock protein 90 serves as a potential receptor for calcium oxalate crystal on apical membrane of renal tubular epithelial cells. J Biol Inorg Chem 21:463–474
Friedrich MG, Truscott RJ (2010) Large-scale binding of alpha-crystallin to cell membranes of aged normal human lenses: a phenomenon that can be induced by mild thermal stress. Invest Ophthalmol vis Sci 51:5145–5152
Gangalum RK, Schibler MJ, Bhat SP (2004) Small heat shock protein alphaB-crystallin is part of cell cycle-dependent Golgi reorganization. J Biol Chem 279:43374–43377
Gangalum RK, Bhat SP (2009) AlphaB-crystallin: a Golgi-associated membrane protein in the developing ocular lens. Invest Ophthalmol vis Sci 50:3283–3290
Gangalum RK, Bhat AM, Kohan SA, Bhat SP (2016) Inhibition of the expression of the small heat shock protein alphaB-crystallin inhibits exosome secretion in human retinal pigment epithelial cells in culture. J Biol Chem 291:12930–12942
Gao B, Eisenberg E, Greene L (1996) Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate. J Biol Chem 271:16792–16797
Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65:5238–5247
Gehrmann M, Liebisch G, Schmitz G et al (2008) Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS One 3(4):e1925
Gobbo J, Marcion G, Cordonnier M, et al (2016) Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst 108
Gomez-Pastor R, Burchfiel ET, Thiele DJ (2018) Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol 19:4–19
Gronthos S, McCarty R, Mrozik K et al (2009) Heat shock protein-90 beta is expressed at the surface of multipotential mesenchymal precursor cells: generation of a novel monoclonal antibody, STRO-4, with specificity for mesenchymal precursor cells from human and ovine tissues. Stem Cells Dev 18:1253–1262
Guidon PT Jr, Hightower LE (1986a) Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry 25:3231–3239
Guidon PT Jr, Hightower LE (1986b) The 73 kilodalton heat shock cognate protein purified from rat brain contains nonesterified palmitic and stearic acids. J Cell Physiol 128:239–245
Hantschel M, Pfister K, Jordan A et al (2000) Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 5(5):438–442
Heufelder AE, Wenzel BE, Bahn RS (1992) Cell surface localization of a 72 kilodalton heat shock protein in retroocular fibroblasts from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab 74:732–736
Hightower LE (1980) Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol 102:407–427
Hightower LE, White FP (1981) Cellular responses to stress: comparison of a family of 71–73-kilodaton proteins rapidly synthesized in rat tissue slices and canavanine-treated cells in culture. J Cell Physiol 108:261–275
Hightower LE, Guidon PT Jr (1989) Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol 138:257–266
Hightower LE (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66:191–197
Honda T, Horie M, Daito T, Ikuta K, Tomonaga K (2009) Molecular chaperone BiP interacts with Borna disease virus glycoprotein at the cell surface. J Virol 83:12622–12625
Horvath I, Multhoff G, Sonnleitner A, Vigh L (2008) Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta 1778:1653–1664
Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH (2004) Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun 324:511–517
Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA (2020) COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect 80:554–562
Ifeanyi F, Takemoto L (1991) Involvement of the N-terminal region in alpha-crystallin-lens membrane recognition. Exp Eye Res 53:305–308
Iosefson O, Sharon S, Goloubinoff P, Azem A (2012) Reactivation of protein aggregates by mortalin and Tid1—the human mitochondrial Hsp70 chaperone system. Cell Stress Chaperones 17:57–66
Johnson RF, Witzel II, Perkins ND (2011) p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-kB. Cancer Res 71:5588–5597
Juhasz K, Thuenauer R, Spachinger A et al (2013) Lysosomal rerouting of Hsp70 trafficking as a potential immune activating tool for targeting melanoma. Curr Pharm Des 19:430–440
Kakimura J, Kitamura Y, Takata K et al (2002) Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J 16:601–603
Kang BR, Yang SH, Chung BR, Kim W, Kim Y (2016) Cell surface GRP78 as a biomarker and target for suppressing glioma cells. Sci Rep 6:34922
Kaur J, Srivastava A, Ralhan R (1998) Expression of 70-kDa heat shock protein in oral lesions: marker of biological stress or pathogenicity. Oral Oncol 34:496–501
Kim D, Lee YJ, Corry PM (1992) Constitutive HSP70: oligomerization and its dependence on ATP binding. J Cell Physiol 153(2):353–361. https://doi.org/10.1002/jcp.1041530215
Kiraly VTR, Dores-Silva PR, Serrao VHB, Cauvi DM, De Maio A, Borges JC (2020) Thermal aggregates of human mortalin and Hsp70-1A behave as supramolecular assemblies. Int J Biol Macromol 146:320–331
Kirkegaard T, Roth AG, Petersen NH et al (2010) Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463:549–553
Kirkegaard T, Gray J, Priestman DA et al (2016) Heat shock protein-based therapy as a potential candidate for treating the sphingolipidoses. Sci Transl Med 8(355):355ra118
Kore RA, Abraham EC (2016) Phosphorylation negatively regulates exosome mediated secretion of cryAB in glioma cells. Biochim Biophys Acta 1863:368–377
Kottom TJ, Hebrink DM, Limper AH (2018) Binding of Pneumocystis carinii to the lung epithelial cell receptor HSPA5 (GRP78). J Med Microbiol 67:1772–1777
Lamprecht C, Gehrmann M, Madl J, Romer W, Multhoff G, Ebner A (2018) Molecular AFM imaging of Hsp70-1A association with dipalmitoyl phosphatidylserine reveals membrane blebbing in the presence of cholesterol. Cell Stress Chaperones 23:673–683
Landry J, Chretien P, Bernier D, Nicole LM, Marceau N, Tanguay RM (1982) Thermotolerance and heat shock proteins induced by hyperthermia in rat liver cells. Int J Radiat Oncol Biol Phys 8:59–62
Laskey RA, Honda BM, Mills AD, Finch JT (1978) Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature 275:416–420
Laskowska E, Bohdanowicz J, Kuczynska-Wisnik D, Matuszewska E, Kedzierska S, Taylor A (2004) Aggregation of heat-shock-denatured, endogenous proteins and distribution of the IbpA/B and Fda marker-proteins in Escherichia coli WT and grpE280 cells. Microbiology (reading) 150:247–259
Lasunskaia EB, Fridlianskaia I, Arnholdt AV, Kanashiro M, Guzhova I, Margulis B (2010) Sub-lethal heat shock induces plasma membrane translocation of 70-kDa heat shock protein in viable, but not in apoptotic, U-937 leukaemia cells. APMIS 118:179–187
LaThangue NB (1984) A major heat-shock protein defined by a monoclonal antibody. EMBO J 3:1871–1879
Lauwers E, Wang YC, Gallardo R et al (2018) Hsp90 mediates membrane deformation and exosome release. Mol Cell 71(5):689-702 e689
Leventis PA, Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39:407–427
Li GC, Werb Z (1982) Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A 79:3218–3222
Li P, Zhang M, Zou Y et al (2018) Interaction of heat shock protein 90 B1 (Hsp90B1) with liposome reveals its potential role in protection the integrity of lipid membranes. Int J Biol Macromol 106:1250–1257
Li P, Wang J, Zou Y et al (2019) Interaction of Hsp90AA1 with phospholipids stabilizes membranes under stress conditions. Biochim Biophys Acta Biomembr 1861:457–465
Lindquist S (1986) The heat shock response. Annu Rev Biochem 55:1151–1191
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Livak KJ, Freund R, Schweber M, Wensink PC, Meselson M (1978) Sequence organization and transcription at two heat shock loci in Drosophila. Proc Natl Acad Sci U S A 75:5613–5617
Lo Cicero A, Stahl PD, Raposo G (2015) Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol 35:69–77
Lopez V, Cauvi DM, Arispe N, De Maio A (2016) Bacterial Hsp70 (DnaK) and mammalian Hsp70 interact differently with lipid membranes. Cell Stress Chaperones 21:609–616
Macazo FC, White RJ (2014) Monitoring charge flux to quantify unusual ligand-induced ion channel activity for use in biological nanopore-based sensors. Anal Chem 86:5519–5525
Mahalka AK, Kirkegaard T, Jukola LT, Jaattela M, Kinnunen PK (2014) Human heat shock protein 70 (Hsp70) as a peripheral membrane protein. Biochim Biophys Acta 1838:1344–1361
Mambula SS, Calderwood SK (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol 177:7849–7857
Mamelak D, Mylvaganam M, Whetstone H et al (2001) Hsp70s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic hsp70 family members. Biochemistry 40:3572–3582
McCallister C, Kdeiss B, Nikolaidis N (2016) Biochemical characterization of the interaction between HspA1A and phospholipids. Cell Stress Chaperones 21:41–53
Mills DR, Haskell MD, Callanan HM, Flanagan DL, Brilliant KE, Yang D, Hixson DC (2010) Monoclonal antibody to novel cell surface epitope on Hsc70 promotes morphogenesis of bile ducts in newborn rat liver. Cell Stress Chaperones 15:39–53
Miyake T, Araki S, Tsuchido T (1993) Synthesis and sedimentation of a subset of 15 kDa heat shock proteins in Escherichia coli cells recovering from sublethal heat stress. Biosci Biotech Biochem 57:578–583
Mizzen LA, Welch WJ (1988) Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol 106:1105–1116
Morgner N, Schmidt C, Beilsten-Edmands, et al (2015) Hsp70 forms antiparallel dimers stabilized by post-translational modifications to position clients for transfer to Hsp90. Cell Rep 11:759–769
Morozova K, Clement CC, Kaushik S et al (2016) Structural and biological interaction of hsc-70 protein with phosphatidylserine in endosomal microautophagy. J Biol Chem 291:18096–18106
Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD (1995) A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 61:272–279
Multhoff G, Hightower LE (2011) Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones 16:251–255
Multhoff G, Seier S, Stangl S et al (2020) Targeted natural killer cell-based adoptive immunotherapy for the treatment of patients with nsclc after radiochemotherapy: a randomized phase II clinical trial. Clin Cancer Res 26:5368–5379
Naaby-Hansen S, Herr JC (2010) Heat shock proteins on the human sperm surface. J Reprod Immunol 84:32–40
Negulyaev YA, Vedernikova EA, Kinev AV, Voronin AP (1996) Exogenous heat shock protein hsp70 activates potassium channels in U937 cells. Biochim Biophys Acta 1282:156–162
Nickel W, Seedorf M (2008) Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol 24:287–308
Nimmervoll B, Chtcheglova LA, Juhasz K et al (2015) Cell surface localised Hsp70 is a cancer specific regulator of clathrin-independent endocytosis. FEBS Lett 589:2747–2753
Noonan E, Giardina C, Hightower L (2008) Hsp70B’ and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res 314:2468–2476
Nylandsted J, Gyrd-Hansen M, Danielewicz A et al (2004) Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 200:425–435
Pelham HR (1982) A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell 30:517–528
Petersen RB, Lindquist S (1989) Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul 1:135–149
Pfaffenbach KT, Lee AS (2011) The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol 23:150–156
Pockley AG, Henderson B, Multhoff G (2014) Extracellular cell stress proteins as biomarkers of human disease. Biochem Soc Trans 42:1744–1751
Ramos A, Pastore A (2001) Dissecting nucleases into their structural and functional domains. Mapping the RNA-binding surface of RNase III by NMR. Methods Mol Biol 160:237–248
Rayner K, Chen YX, McNulty M et al (2008) Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res 103:133–141
Rayner K, Sun J, Chen YX et al (2009) Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: role of selective estrogen receptor beta modulation. Arterioscler Thromb Vasc Biol 29:1751–1756
Ritossa F (1962) A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 18:571–573
Ritossa F (1996) Discovery of the heat shock response. Cell Stress Chaperones 1:97–98
Robert J, Menoret A, Cohen N (1999) Cell surface expression of the endoplasmic reticular heat shock protein gp96 is phylogenetically conserved. J Immunol 163:4133–4139
Sadis S, Hightower LE (1992) Unfolded proteins stimulate molecular chaperone Hsc70 ATPase by accelerating ADP/ATP exchange. Biochemistry 31:9406–9412
Sahu R, Kaushik S, Clement CC et al (2011) Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20:131–139
Schedl P, Artavanis-Tsakonas S, Steward R et al (1978) Two hybrid plasmids with D. melanogaster DNA sequences complementary to mRNA coding for the major heat shock protein. Cell 14:921–929
Schilling D, Gehrmann M, Steinem C et al (2009) Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J 23:2467–2477
Sedlackova L, Nguyen TT, Zlacka D, Sosna A, Hromadnikova I (2009) Cell surface and relative mRNA expression of heat shock protein 70 in human synovial cells. Autoimmunity 42:17–24
Segre D, Ben-Eli D, Deamer DW, Lancet D (2001) The lipid world. Orig Life Evol Biosph 31:119–145
Shevtsov MA, Komarova EY, Meshalkina DA, Bychkova NV, Aksenov ND, Abkin SV, Margulis BA, Guzhova IV (2014) Exogenously delivered heat shock protein 70 displaces its endogenous analogue and sensitizes cancer cells to lymphocytes-mediated cytotoxicity. Oncotarget 5:3101–3114
Shevtsov M, Balogi Z, Khachatryan W, Gao H, Vigh L, Multhoff G (2020) Membrane-associated heat shock proteins in oncology: from basic research to new theranostic targets. Cells 9:1263
Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E (2004) Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J Biol Chem 279:45379–45388
Simcox AA, Cheney CM, Hoffman EP, Shearn A (1985) A deletion of the 3’ end of the Drosophila melanogaster hsp70 gene increases stability of mutant mRNA during recovery from heat shock. Mol Cell Biol 5:3397–3402
Subjeck JR, Sciandra JJ, Johnson RJ (1982) Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol 55:579–584
Suzuki CK, Bonifacino JS, Lin AY, Davis MM, Klausner RD (1991) Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol 114:189–205
Takashima S, Sato N, Kishi A et al (1996) Involvement of peptide antigens in the cytotoxicity between 70-kDa heat shock cognate protein-like molecule and CD3+, CD4-, CD8-, TCR-alpha beta- killer T cells. J Immunol 157:3391–3395
Takemoto H, Yoshimori T, Yamamoto A, Miyata Y, Yahara I, Inoue K, Tashiro Y (1992) Heavy chain binding protein (BiP/GRP78) and endoplasmin are exported from the endoplasmic reticulum in rat exocrine pancreatic cells, similar to protein disulfide-isomerase. Arch Biochem Biophys 296:129–136
Tang D, Borchman D, Yappert MC, Cenedella RJ (1998) Influence of cholesterol on the interaction of alpha-crystallin with phospholipids. Exp Eye Res 66:559–567
Tani F, Ohno M, Furukawa Y, Sakamoto M, Masuda S, Kitabatake N (2009) Surface expression of a C-terminal alpha-helix region in heat shock protein 72 on murine LL/2 lung carcinoma can be recognized by innate immune sentinels. Mol Immunol 46:1326–1339
Tezgin D, Giardina C, Perdrizet GA, Hightower LE (2020) The Effect of Hyperbaric Oxygen on Mitochondrial and Glycolytic Energy Metablolism: the Caloristasis Concept 25:667–677
Theodorakis NG, Morimoto RI (1987) Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol 7:4357–4368
Theodorakis NG, Drujan D, De Maio A (1999) Thermotolerant cells show an attenuated expression of Hsp70 after heat shock. J Biol Chem 274:12081–12086
Tissieres A, Mitchell HK, Tracy UM (1974) Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol 84:389–398
Tjondro HC, Xi YB, Chen XJ, Su JT, Yan YB (2016) Membrane insertion of alphaA-crystallin is oligomer-size dependent. Biochem Biophys Res Commun 473:1–7
Torok Z, Horvath I, Goloubinoff P, Kovacs E, Glatz A, Balogh G, Vigh L (1997) Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci U S A 94:2192–2197
Torok Z, Crul T, Maresca B et al (2014) Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications. Biochim Biophys Acta 1838:1594–1618
Toyoda Y, Akarlar B, Sarov M, Ozlu N, Saitoh S (2018) Extracellular glucose level regulates dependence on GRP78 for cell surface localization of multipass transmembrane proteins in HeLa cells. FEBS Lett 592:3295–3304
Tsai YL, Zhang Y, Tseng CC, Stanciauskas R, Pinaud F, Lee AS (2015) Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface. J Biol Chem 290:8049–8064
Tseng CC, Zhang P, Lee AS (2019) The COOH-terminal proline-rich region of GRP78 is a key regulator of its cell surface expression and viability of tamoxifen-resistant breast cancer cells. Neoplasia 21:837–848
Tsutsumi S, Neckers L (2007) Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci 98:1536–1539
Tsvetkova NM, Horvath I, Torok Z et al (2002) Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A 99:13504–13509
Tytell M, Greenberg SG, Lasek RJ (1986) Heat shock-like protein is transferred from glia to axon. Brain Res 363:161–164
Vega VL, De Maio A (2005) Increase in phagocytosis after geldanamycin treatment or heat shock: role of heat shock proteins. J Immunol 175:5280–5287
Vega VL, Rodriguez-Silva M, Frey T et al (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 180:4299–4307
Vega VL, Charles W, De Maio A (2010) A new feature of the stress response: increase in endocytosis mediated by Hsp70. Cell Stress Chaperones 15:517–527
Velazquez JM, DiDomenico BJ, Lindquist S (1980) Intracellular localization of heat shock proteins in Drosophila. Cell 20:679–689
Velazquez JM, Lindquist S (1984) hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell 36:655–662
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7:309–316
Welch WJ, Feramisco JR (1982) Purification of the major mammalian heat shock proteins. J Biol Chem 257:14949–14959
Welch WJ, Feramisco JR (1984) Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem 259(7):4501–4513
Welch WJ, Suhan JP (1985) Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J Cell Biol 101:1198–1211
Welch WJ, Suhan JP (1986) Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol 103:2035–2052
Whittaker R, Glassy MS, Gude N, Sussman MA, Gottlieb RA, Glembotski CC (2009) Kinetics of the translocation and phosphorylation of alphaB-crystallin in mouse heart mitochondria during ex vivo ischemia. Am J Physiol Heart Circ Physiol 296:H1633-1642
Wiener MC, White SH (1992) Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III Complete Structure Biophys J 61:434–447
Wimley WC, Hristova K, Ladokhin AS, Silvestro L, Axelsen PH, White SH (1998) Folding of beta-sheet membrane proteins: a hydrophobic hexapeptide model. J Mol Biol 277:1091–1110
Wu C (1984) Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature 311(5981):81–84
Zhang M, Wang D, Li P et al (2018) Interaction of Hsp90 with phospholipid model membranes. Biochim Biophys Acta Biomembr 1860:611–616
Zhang Y, Liu R, Ni M, Gill P, Lee AS (2010) Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem 285:15065–15075
Zhang Y, Tseng CC, Tsai YL, Fu X, Schiff R, Lee AS (2013) Cancer cells resistant to therapy promote cell surface relocalization of GRP78 which complexes with PI3K and enhances PI(3,4,5)P3 production. PLoS One 8(11):e80071
Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173:2026–2034
Acknowledgements
We would like to recognize the outstanding contribution of many talented investigators that participated in this long and fantastic adventure. They are in order of appearance: Peter T. Guidon, Jr., Michael Tytell, Nelson Arispe, Virginia Vega, Gabriele Multhoff, David M. Cauvi, Jonathan Okerblom, Gabrielle Armijo, Victor Lopez, Wilbert Boelens, Ricardo Capone, and Paulo R. Dores-Silva. The excellent editorial assistance of Barbara Rho is appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Maio, A., Hightower, L. The interaction of heat shock proteins with cellular membranes: a historical perspective. Cell Stress and Chaperones 26, 769–783 (2021). https://doi.org/10.1007/s12192-021-01228-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-021-01228-y