Abstract
The Antarctic krill, Euphausia superba, is a Southern Ocean endemic species of proven ecological importance to the region. In the context of predicted global warming, it is particularly important to understand how classic biomarkers of heat stress function in this species. In this respect, Hsp70s are acknowledged as good candidates. However, previous studies of expression kinetics have not been able to demonstrate significant upregulation of these genes in response to heat shocks at 3 °C and 6 °C for 3 and 6 h. The current work complements these previous results and broadens the prospects for the use of Hsp70s as a relevant marker of thermal shock in this krill species. New experiments demonstrate that induction of Hsp70 isoforms was not detected during exposure to heat shock, but increased expression was observed after several hours of recovery. To complete the analysis of the expression kinetics of the different isoforms, experiments were carried out over short time scales (1 and 2 h at 3 °C and 6 °C) as well as at higher temperatures (9 °C, 12 °C, and 15 °C for 3 h), without any significant response. A 6-week monitoring of animals at 3 °C showed that the time factor is decisive in the establishment of the response. CTmax experiments with incremental times of 1 °C per day or 1 °C every 3 days have shown a particularly high resilience of the animals. The demonstration of the abundance of Hsp70s present before thermal stress in various species of krill, as well as in specimens of E. superba of various origins, showed that the delay in the response in expression could be related to the high constitutive levels of Hsp70 available before the stress experiments. The alternative labelling of the two main isoforms of Hsp70 according to the origin of the animals allowed hypotheses to be put forward on the functioning of thermoregulation in Antarctic krill as well as ice krill.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global warming constitutes a Grand Challenge for Biology as defined by Schwenk et al. (2009) and Denny and Helmuth (2009). It represents a major, unsolved problem that requires a significant improvement in capability or a fundamental change in perspective to solve. In this respect, the pivotal role of physiology has been highlighted in confronting the complex problems posed by global climatic change, and the need to strive toward a common focus, the whole organism: “It is physiology - our understanding of how individual organisms function and interact with their environment - that presents the largest challenge. Without a better mechanistic understanding of how plants and animals work, we can never be assured of an accurate warning of what lies ahead for life on earth” (Denny and Helmuth 2009).
To fit with this strategy, the choice of the biological model is extremely important. The Southern Ocean is an important breeding and/or foraging location for a wide range of charismatic megafauna such as whales, seals, penguins, and other sea birds including albatrosses. While these species fascinate the public, they represent the apex of a complex food chain, the keystone species of which is the Antarctic krill Euphausia superba (Trivelpiece et al. 2011). These shrimp-like crustaceans are not only a major prey item for these animals but are also significant consumers, grazing on the phytoplankton bloom in the austral summer and on algae under the sea ice in winter for the most southern ones. In a more global context, the Antarctic krill has been suggested to be the most abundant eukaryotic species in the World’s Oceans existing in large schools or swarms with densities that may be between 10,000 and 30,000 individual animals per square meter (Macaulay et al. 1984). In addition, it is an increasingly profitable fisheries resource. It is therefore essential to have maximum information on the thermal resilience of these animals in order to best manage this stock (Atkinson et al. 2004; Everson 2000; Flores et al. 2012; Schiermeier 2010). Given the central role of this key species in the Southern Ocean ecosystem, detailed physiological and molecular studies are essential to understand this species and its responses to a changing environment.
This work continues a previous study focused on the capacity of Antarctic krill to respond to a rise in environmental temperature (Cascella et al. 2015). The Cascella study included the CTmax values of two krill species from the Antarctic zone Euphausia superba and Euphausia crystallorophias, as well as the first measurements of the expression of different Hsp70 isoforms in response to thermal shocks of 3 and 6 h to 3 °C and 6 °C. These first results showed, especially in E. superba, a very weak response in terms of Hsp70 expression during the shock at these different temperatures. Furthermore, a second study on the arcto-boreal krill Thysanoessa inermis, which lives in warmer waters and should be less stenothermal, has shown a similar thermal resilience (CTmax) to the Antarctic species, but with different expression kinetics, especially in terms of isoform reactivity (Huenerlage et al. 2016).
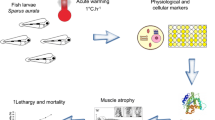
In the current study, further experiments were performed to interrogate in more detail the question of whether E. superba is capable of an HSP response. Measurements were performed on the expression of the different Hsp70 isoforms in the recovery period (post-shock), but also after short shocks of 1 and 2 h and more intense thermal shocks at 9 °C, 12 °C, and 15 °C. In addition, a study of the expression in response to a long shock at 3 °C for 6 weeks was conducted in an aquarium under controlled conditions. Finally, organismal heat tolerance was assayed using a slower rate of warming than prior studies to provide an ecologically relevant measure of tolerance.
Material and methods
Animal collection
E. superba were obtained from different sources, depending on the experiment (see Fig. 1).
E. superba for acute trials at 3 °C and 6 °C were fished off the French base Dumont d’Urville (DDU) in Terre Adélie just beyond the continental plateau (65.30° S, 139.57° E). Fishing was carried out using an Isaacs-Kidd midwater trawl (IKMT) from the ship « L’Astrolabe » at a maximum speed of 2 knots for 10–15 min. The sampling depth was determined by sonar observations of swarms, taking samples from those with a depth of between 20 and 50 m. After fishing, the animals were moved to a refrigerated flow-through tank on board, which was continuously supplied with seawater at − 0.5 to + 0.5 °C. Animals were acclimated for 24 h to aquarium conditions prior to experimentation to minimize holding stress. A circulation pump was used to set up a vertical current maintaining the animals in the water column.
A second series of acute thermal trials and longer thermal shocks were performed in the temperature-controlled aquariums of the Australian Antarctic Division in Kingston (Tasmania) (Kawaguchi et al. 2010). Animals used for these experiments were fished at 64.11° S, 111.12° E and kept in aquaria for 8 months before being challenged with heat. Samples of E. superba from western Antarctica (62.29° S, 139.59 E) were kindly provided by Drs. Juan Höfer (IDEAL, Chile) and Marcelo Gonzalez-Aravena (INACH, Chile) for the Western blots.
Experiments
Thermal shocks at 3 °C and 6 °C (onboard L’Astrolabe)
After around 24 h in the main aquarium, the most mobile animals were selected and put into two aquaria containing seawater at the same temperature as the main stock aquarium. These aquaria were placed within a larger tank containing fresh water. A copper coil linked to a thermostat and pump was added to the system. The coil was used to pump hot water around the system to warm up the tank water for experiments. A circulating pump ensured that the warming was equally distributed around the tanks and air pumps were used to maintain the oxygen levels in the aquaria and also facilitate water mixing.
The protocol of the experiments is depicted in Fig. 1a. For each thermal shock, animals from the same trawl were treated at the same time for 3 or 6 h before being returned to 0 °C seawater for recovery. The animals were put directly into the warmed water. At the same time, a set of control animals from the main aquarium were snap frozen in liquid nitrogen. Animals having undergone heat shock at 3 °C or 6 °C were sampled after 3 or/and 6 h of shock and 2, 4, 6 h of recovery (post-shock (PS)) and deep-frozen. Each sampling comprised 10 animals.
Acute shocks at 3 °C and 6 °C (Australian Antarctic Division)
E. superba were directly transferred from the tank at 0 °C to the tanks at 3 °C and 6 °C. Ten animals were collected after 1 and 2 h and immediately snap frozen in liquid nitrogen and then kept at − 80 °C until required.
Acute shocks at 9 °C, 12 °C, and 15 °C for 3 h (Australian Antarctic Division)
E. superba were directly transferred from the tank at 0 °C to the tanks at 9 °C, 12 °C or 15 °C. Ten animals were used at each temperature. Heat-shocked animals were sampled after 3 h and directly frozen in liquid nitrogen before storage at − 80 °C.
Longer thermal trials at 3 °C for 6 weeks (Australian Antarctic Division)
One hundred twenty E. superba were warmed from 0 to 3 °C at 1 °C/h. Ten animals were sampled at 3, 6, 12, 24 h and then, at the same time, every day for 2 days and finally every week until the sixth week. Animals were snap frozen and samples kept at − 80 °C. Ten more animals from the original tank were directly frozen and used as control (Fig. 1b).
CTmax experiments
CTmax experiments were carried aboard the Astrolabe using increments of 1 °C/10 min according to the protocol set out in Cascella et al. (2015). Additional CTmax experiments were carried out at rates of 1 °C per day and 1 °C every 3 days. These experiments were carried out in the AAD aquaria in Kingston (Tasmania). Animals no longer responding to a tactile stimulus during this thermal trial were removed from the aquarium. The CTmax was considered the temperature at which 50% of experimental animals were still mobile. It was determined though the non-linear curve fitting option in JMP10 (SAS). The survival curve used was as follows: Survival = c/(1 + (T/CTmax)b) where c is the plateau value before the sharp decrease, CTmax the temperature at which 50% of mobile animals is reached, and b a sigmoidicity coefficient. The program explores the different values of these three parameters and calculates a chi-square value. While exploring the different parameter values, the program aims to minimize this chi-square value and converges toward a value for each parameter (provided with a standard error). The numbers of animals used for these CTmax experiments were 43, 30, and 30 for 1 °C per 10 min, 1 °C per day, and 1 °C per 3 days, respectively.
qPCR analysis
mRNA levels of the Hsp70 isoforms were determined by quantitative RT-PCR amplification, on 6 to 10 individuals for each experimental condition according to the same protocol in the previous publication (Cascella et al. 2015). A dissociation curve was generated and PCR efficiency was estimated for each primer pair. All primer pairs tested generated a single peak in the dissociation curve and a PCR efficiency of 80–100%. Data were analyzed with the LightCycler 480 software. The 18S gene was chosen as a reference gene using the BestKeeper algorithm (Pfaffl et al. 2004) after testing EF1α, 18S, RPL8, and GAPDH as potential normalizing housekeeping sequences. Hsp70 expression was subsequently normalized to this reference. Differences of the mean normalized expression (MNE) of the five Hsp70 isoform genes (A, B, C, D, and E) over the time course of the experiment were analyzed using a non-parametric Kruskal–Wallis test and a Dunn’s multiple comparison test to determine significant differences between control and heat-shocked samples. Relative gene expression (fold Hsp70 expression) was calculated from the MNE of test specimens (heat shock at 3 °C or 6 °C for 3 or 6 h and subsequent recovery at 0 °C) divided by the MNE of control specimens (kept at 0 °C control temperature).
Statistical analyses were carried out using GraphPad Prism 6 (GraphPad Software, Inc., USA). The significance level was set at p < 0.05.
Western blot
Samples of krill abdomens without their cuticle were ground in liquid nitrogen, and the powder was homogenized in 1 ml of extraction buffer (20 mM Tris/HCl, pH 7.5: 40 μl protease inhibitor cocktail (Roche, France)). The homogenates were centrifuged at 10,000g for 15 min at 4 °C. The extracted proteins were quantified in the supernatant with the Bio-Rad Protein Assay (Bio-Rad, Marnes-la-Coquette, France) using bovine serum albumin (Sigma) as a standard. Proteins of the total supernatant were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE; 7.5% acrylamide: 0.3% bisacrylamide (w/v); 20 μg·protein·well−1). After running the electrophoresis (15 min at 15 mA gel−1, 1 h at 20 mA gel−1), the bands were stained with Coomassie Blue. Protein molecular mass standards from 10 to 250 kDa (All Blue, Bio-Rad) were used to evaluate the apparent Mr of the separated bands.
For Western blotting, the proteins were transferred from the SDS–PAGE gel (20 μg·protein·well−1) to a nitrocellulose membrane by semi-dry blotting at 25 mA for 2 h (Trans-Blot semi-dry cell; Bio-Rad). Membranes were blocked in milk/BSA/Tris-buffered saline pH 7.5 (TBST: Tris-buffered saline with Tween 20) (2.5% w/v each) for 1 h, rinsed in TBST, and incubated overnight with a polyclonal antibody (1/1000 Mouse anti-chicken HSC70/HSP70 monoclonal antibody; Enzo Life Sciences, Lausen, Switzerland) at room temperature (Ravaux et al. 2003). Subsequently, a 10-min washing step with TBST with 2.5% milk pH 7.5 was repeated four times, and the membranes were incubated with a polyclonal secondary antibody coupled to horseradish peroxydase (HRP) (1/3000 donkey anti-mouse IgG-HRP: sc-2314, Santa Cruz Biotechnology Inc.) for 1 h at room temperature. After another 4 × 10 min washing step with TBST, the antibody complex was detected using the Luminol reagent substrate (SuperSignal West Femto Maximum Sensitivity Substrate, Thermo Scientific, Redford, USA) incubated at room temperature for 1 min, and read with a Fusion FX (Vilber Lourmat, Marne la Vallée, France).
Results and discussion
Upper critical thermal limits vary according to temperature increment rate
As a preliminary analysis to understand the thermal resilience of E. superba in the Southern Ocean, short-term acute exposures were first undertaken, using a temperature increase of 0.1 °C/min (Cascella et al. 2015). The interpretation of the CTmax curve of E. superba gave an unexpectedly high value of 15.8 ± 0.1 °C for a stenothermal Antarctic ectotherm (Peck et al. 2009). As the thermal shocks applied in these first sets of experiments were relatively short and represented very rapid temperature increases, thermal shocks with longer increment times were conducted to confirm or refute the apparent thermal resistance highlighted by these previous experiments. Indeed, Peck et al. showed that CTmax varied with the rate of experimental warming, more precisely CTmax declined at slower rates of warming (Peck et al. 2009; Peck et al. 2014). Thus, the time elapsed between two temperature increments of 1 °C was significantly increased since the curves presented were obtained for intervals of 1 and 3 days (Fig. 2).
Observed CTmax values were 14.7 ± 0.1 °C and 13.9 ± 0.03 °C for increments of 1 °C per 1 and 3 days, respectively. These values appear particularly high compared with what has been regularly observed in other polar invertebrates (Peck et al. 2014). More especially, the small difference between the CTmax as a function of the time between each temperature increase was surprising. Overall, the decrease in CTmax was approximately 1 °C between the different rates of change experiments. This small decrease highlights a particularly high temperature resilience. This result makes sense when comparing these values with those found with another polar eucarid, the shrimp Chorismus antarcticus, a species phylogenetically closer than most of the Antarctic invertebrates studied so far, with CTmax values of 11.6 °C and 6.7 °C for increments of 1 °C every 10 min or every day, respectively (personal and unpublished data). In this example, the CTmax for a 1 °C/10 min was already much lower than that of E. superba but drops significantly with the extension of the warming interval. These differences are certainly partially attributable to the differences in living environment, which is pelagic beyond the continental shelf for E. superba versus benthic on the continental shelf for C. antarcticus (Peck et al. 2009).
Kinetics of Hsp70 expression
Post-shock kinetics
In a previous publication (Cascella et al. 2015), the expression kinetics of five E. superba Hsp70 isoforms (A, B, C, D, E) were analyzed in response to moderate thermal shocks at 3 °C or 6 °C for 3 or 6 h. Results showed that this response in Antarctic krill was very weak when compared with the fold changes observed in temperate species (Feder and Hofmann 1999; Ravaux et al. 2012). In this study, the initial heat shock experiments of Cascella et al. (2015) were expanded to include a recovery period at 0 °C (Fig. 3). According their structural characteristics and phylogenetic position, the A, B, and E isoforms were most similar to the traditional constitutive form gene cluster. The C isoform is most similar to inducible isoforms in other species. These four isoforms are cytoplasmic while the D isoform is most similar to the group of mitochondrial Hsp70s (Baringou et al. 2016; Cascella et al. 2015; Huenerlage et al. 2016).
a–d Mean normalized expression ratios of five hsp70 isoforms in the muscle tissue of adult E. superba (n = 6) during 3 and 6 h continuous heat shock at 3 °C or 6 °C followed by 6 h of recovery at control temperature (0 °C). Thermal shocks are presented on a white background, post shocks on a gray background. Values relate to the control group (specimens continuously kept at 0 °C). Asterisk (*): significant MNE difference to 0 °C control temperature evaluated with a Kruskal–Wallis test. Figure was created using GraphPad Prism 6.0
The rapid transition from 0 to 3 °C did not invoke an upregulation of Hsp70 in E. superba (Fig. 3a). Actually, there was a general trend for a reduction in Hsp70 gene expression after 6 h compared with the controls (Fig. 3b). However, if the upregulation of expression recorded during the actual heat shock itself was weak, the analyses of post-shock showed much higher levels of expression, which increased with the length of the initial shock. The most responsive isoform was the E form, both in terms of speed of response and intensity. Nevertheless, even though this form was the only one showing significant upregulation during the recovery phase after 3 h of shock (Fig. 3a), the duration of the shock greatly influenced the amplitude of the responses of the other isoforms. The expression kinetics were clearly distinct according to the Hsp70 isoforms. However, similar to the results in Cascella et al. (2015), there was no correlation between the expression levels and the category of isoform group to which they belonged, i.e., the group of constitutive forms (theoretically no upregulation to heat shock) or the group of inducible forms (theoretically strong upregulation to heat shock).
It is also clear that, if the duration of the shock influences the importance of the expression, the intensity of the shock impacts this response as well. Indeed, a shock of 3 h at 6 °C produced a larger response for isoforms A and B than a shock of identical duration at 3 °C, with a maximum observed at 6 h post-shock. This response was measured paradoxically only for these so-called constitutive forms (Fig. 3c). However, the upregulation patterns are very different for 6 h at 6 °C, where the response is far more muted with a trend in overall decreases in the responses recorded so far (Fig. 3d). This lack of upregulation may indicate that the animal needs more time for recovery or has reached its limits and no longer has the ability to compensate for variation in the environment.
Be that as it may, even if the upregulation in Hsp70 in response to thermal shock did not reach the values observed in other ectotherms, especially from temperate regions, the recovery period values were much higher than those measured during the shock itself. E. superba is therefore unquestionably capable of responding to a thermal shock via upregulation of its Hsp70s. The very low level or the absence of response observed during the heat shock is curious. Several reasons could explain the values observed: (i) the timing of first samplings was too late and did not allow for a very rapid and early response, within the first 3 h; (ii) the applied temperatures were too low to generate a response; (iii) Antarctic krill has a large constitutive level of Hsp70 proteins, which it can use as an available pool to cope with the molecular perturbations induced by the increase in temperature, thus explaining the late upregulation of mRNAs. Each of these hypotheses was tested in the following experiments.
Short heat shock kinetics
In order to follow the kinetics of expression over shorter times, a new series of heat shock experiments (+ 3 °C and + 6 °C) were conducted according to an identical protocol but with sampling points at 1 and 2 h (Fig. 4).
Mean normalized expression levels of five hsp70 genes obtained by qPCR in E. superba during a heat shock of 3 °C and 6 °C whereas 0 h group is control at 0 °C. Heat shocks were carried out during 1 and 2 h, and hsp70 expressions measured at those times on n = 6 individuals per group. Hsp70 expressions were normalized by the 18S gene expression. To compare values, a Kruskal–Wallis test followed by a Dunn’s test was used to compare control groups (0 h) and shocked groups (1 h and 2 h). Significant differences between mean normalized expressions are indicated by asterisks (*). Figure was created using GraphPad Prism 6.0
No significant upregulation was observed except for E isoform after 2 h of heat shock. The values obtained were similar to the trends observed with 3 h of heat shock. However, it is interesting to note significant downregulation of expression after 1 h for the A isoform and after 1 and 2 h for the B isoform. The results are similar for both temperatures. This decrease in expression could be attributed to a massive recruitment of mRNAs, originally present before the heat shock, for protein synthesis. Much of the response would then partly consist of replacing the mRNAs involved in translation, when the shock remains moderate in duration and intensity. On the other hand, the potentially inducible form C does not show any significant expression variation. Again, as demonstrated throughout the heat shocks, the so-called constitutive forms are the most involved in the response of E. superba to temperature.
In summary, the hypothesis of an upregulation in Hsp70s prior to the original 3-h sampling period was not validated.
Heat shock kinetics
In order to test the hypothesis that the weak response observed after thermal shocks at 3 °C and 6 °C was, or was not, related to the fact that the intensity of these shocks was insufficient, the animals were immersed for 3 h in water at 9 °C, 12 °C, and 15 °C, then expression of Hsp70s were measured (Fig. 5).
Mean normalized expression levels of five hsp70 genes obtained by qPCR in E. superba during a heat shock of 9 °C and 12 °C whereas 0 h group is control at 0 °C. Heat shocks were carried out during 3 h, and hsp70 expressions measured at those time on n = 6 individuals per group. Hsp70 expressions were normalized by the 18S gene expression. To compare values, a Kruskal–Wallis test followed by a Dunn’s test was used to compare control groups (0 h) and shocked groups (9 °C and 12 °C). Significant differences between mean normalized expressions are indicated by asterisks (*). Figure was created using GraphPad Prism 6.0
As a first observation, none of the animals immersed in water at 15 °C survived the experiment and only half of the individuals resisted for 3 h in water at 12 °C; thus, the 12 °C results represent a resisting population, which may explain why expression levels are slightly higher at 12 °C for some isoforms. This observation highlights the importance of the implementation of the thermal shock. These animals were directly immersed in water at the shock temperature, with no gradual warming. The gradual raising of temperature is an essential component in the ability of animals to manage the increase in environmental temperature, as occurs with potential acclimation. Indeed, the CTmax curves (Fig. 2) demonstrate for example that no loss was recorded at the temperature of 12 °C with increments of 1 °C every 10 min or even 1 °C every day.
As second observation, no significant upregulation was recorded at these temperatures. However, as observed during the acute heat shocks, there was a downregulation of isoforms forms A and B at 9 °C only. This decrease in the amount of mRNAs encoding the constitutive Hsp70s appears to be a first step in the response process. It was present at 3 °C and 6 °C but seems to extend to 9 °C before a trend of upregulation as observed during the recovery period (Fig. 3). Finally, when the length of the heat shock is extended, there might be a neosynthesis of mRNA coding for these chaperones following the disappearance of feedback inhibition generated by native Hsp70 proteins. Thus, the absence of upregulation during heat shocks and its appearance during post-shock recovery periods would not be linked to an absence of response or a potential recovery of animals but rather to a delayed response related to a native abundance of functional proteins, which is supported by large constitutive quantities of mRNAs of A and B isoforms in particular. These reflections lead us to postulate that E. superba has naturally high quantities of HSP70 proteins, but to date, HSP70 abundance in this species has only been evaluated at the mRNA level.
Long-term heat shock
The objective of this experiment was to estimate the ability of E. superba to acclimate to 3 °C. The aim was to follow the expression of the different isoforms during this process, which would correspond to a scenario in agreement with a future global warming or simply to a sudden change of geographical zone or of water layer. The scenario of global warming is obviously difficult to achieve since it implies a gradual increase over several years. The second scenario is more likely, both because of the longevity of the members of the species and their migrations through geographical areas with waters of different temperatures.
The first notable result was the total absence of mortality in this experiment. Clearly, on this sole criterion of survival, the transition to 3 °C seemed to have no impact on the animals and they appear to have acclimated.
Secondly, in view of the previous results, the implementation of a long heat shock was of great interest. Indeed, it should allow us to determine unequivocally if the responses observed during the post-shock recovery experiments could be due to a return to the original conditions and was therefore due to a recovery or rather to a belated response as suggested above. The isoform D was not evaluated because of the absence of a measurable signal in the majority of sampled times.
In general, the expression levels did not remain constant for the isoforms (Fig. 6). Their kinetics of expression showed peaks at different times highlighting the presence of Hsp70 regulation beyond 6 h and therefore their activity of repairing cellular damage caused by chronic temperature challenge. The most important expression variations were recorded from 12-h onwards. It is therefore clear that post-shock conditions are not solely responsible for increasing the HSP70 response. The time factor is also fundamental.
Mean normalized expression ratios of four hsp70 isoforms in the muscle tissue of adult E. superba (n = 6) during continuous heat shock at 3 °C for 6 weeks. Values relate to the control group (specimens continuously kept at 0 °C). Asterisk (*): significant MNE difference to 0 °C control temperature evaluated with a Kruskal–Wallis test. Figure was created using GraphPad Prism 6.0
The different isoforms showed very different kinetics in amplitude and chronology. The E isoform remained the most reactive and confirmed the data obtained during short heat shocks. The C isoform, which is structurally affiliated to inducible Hsp70s, showed a significant increase in expression after 12 h, which is therefore rather late compared with 3 °C animals measured after acute shocks and post-shock recovery. Similarly, the so-called constitutive isoforms, A and B, underwent a significant decrease on day 1 before recording an upregulation on day 3. The comparison of the kinetics of expression of these isoforms also reveals variations in response between the animals in recovery and those maintained under extended heat shock conditions. The latter showed significant downregulation, especially around day 1 probably related to mRNA recruitment in these chronic stress conditions, followed by upregulation. Overall, the patterns of upregulation were delayed, compared with acute heat shocks, probably because the isoforms were originally present in large quantities, either as protein or mRNA.
It is clear that the kinetics of expression vary on the hourly scale at least during the first day. This observation is also valid on a day-to-day basis with respect to the differences recorded during the first 3 days of heat shock, although it is perfectly reasonable to think that a daily sampling smoothed the data and obscured probable short-term variations. This reflection is even truer at the scale of the week when the probability that there was a synchronism between the variations of expression and the sampling time remains low. Nevertheless, the 6-week heat shock expression data appeared to show a general decrease until a slight downregulation was observed in the last week. This raises the question as to whether this overall decrease in expression was due to a metabolic decline linked to the duration of the heat shock or to a process of acclimatization that is gradually taking place, which was not possible during the course of this experiment. A posteriori, it would be interesting in the future to finalize this study by a daily, or even better, an hourly monitoring of isoform expressions.
What about constitutive HSP70 quantities?
PCR monitoring of RNA expression provides information on the impact of a heat shock, but this approach, although powerful, considers only a part of the metabolic cascade involved. It is obvious that monitoring the production of proteins in response to a heat shock would also be useful as proteins are the end production of transcription/translation. Unfortunately, technical barriers related to the difficulty of quantification but also of identification and discrimination of the different isoforms by antibodies as all the Hsp70s described here have high sequence identities. Nevertheless, a Western blot approach should allow a first quantitative assessment of Hsp70 constitutive protein levels. In order to determine whether there is a large native pool Hsp70 proteins in E. superba, which could explain the delayed Hsp70 responses described above, Western blots were performed abdomen extracts from different species of eucarids from the Antarctic or Arctic regions. This was performed on the krill species E. superba (fished from different areas), E. crystallorophias, Thysanoessa inermis, and the shrimp Chorismus antarcticus (Fig. 7).
It is clear that the strongest labelling is present in the protein sample extracted from E. superba fished near Dumont d’Urville Station (lane 3). The other extracts are weakly stained or almost absent, as shown by the results for the Antarctic shrimp (Chorismus antarcticus). These bands were globally at or slightly above the human HSPA control band estimated at 72 kDa. Due to their positions and according the amino acid sequences deduced from transcriptomic data (Cascella et al. 2015; Clark et al. 2011), the bands shown in Fig. 7 can be attributed to A isoform for the Antarctic species (EusHspA = 72.46 kDa, EucHspA = 72.56 kDa, as the B isoform molecular weight is under 72 kDa, 71.64 kDa, and 71.84 kDa, respectively) and isoform B for Thysanoessa inermis (72.03 kDa), in which no A isoform was characterized (Huenerlage et al. 2016). The other isoforms do not appear to have been translated in sufficient quantity to allow visible labelling by the antibody. This observation, without constituting definitive proof, supports the hypothesis that E. superba maintains high constitutive levels of Hsp70 protein. The labelling is all the weaker as the species of krill considered have a significant early response in terms of expression of the mRNAs encoding these isoforms. In fact, T. inermis showed much faster and more Hsp70 intense responses to thermal shocks than Antarctic species, even though the corrected CTmax values were very similar (Huenerlage et al. 2016). In the same way, E. crystallorophias appeared to be more reactive to temperature than Antarctic krill. The kinetics of response to thermal shock have not yet been carried out on C. antarcticus; however, the CTmax values were found to be much lower than those measured on E. superba and were particularly sensitive to the time factor, demonstrating a greater sensitivity to temperature variation (unpublished personal data).
The potential Hsp70A band could be preferentially involved in the response to cold shock in the natural environment (Peck et al. 2014). Interestingly, there is less native Hsp70A in E. crystallorophias, a species adapted to the colder environment of the Antarctic continental shelves. On the other hand, this protein is absent in the surveyed T. inermis, which lives in the warmer waters of the Arctico-boreal region. But most interesting is the labelling observed on the protein extract from E. superba specimens fished on the edge of the Antarctic Peninsula (Fig. 7, lane 7). The water temperature in this region is regularly positive and it would have been reasonable to expect, on the basis of the stated hypothesis, that this Hsp70A band would have been less stained if the high quantity of Hsp70A in the sample in lanes 3 and 4 is due to cold acclimation. However, the difference is much more drastic, since the A band disappeared in favor of the potential band B (Fig. 7, lane 7), similar to T. inermis (Fig. 7, lane 6).
General discussion and conclusion
The high quantity of these Hsp70 isoforms in Euphausia superba constitutes a significant advantage in response to either a heat or cold shock. The hypothesis that ectothermic animals living in cold environments favor the accumulation of Hsps to correct the damage directly linked to the problems of protein folding at low temperatures could be an explanation for this physiological behavior (Peck 2016; Place and Hofmann 2005). Thus, the delay in gene expression would find its origin in the presence of large constitutive amounts of Hsp proteins in Antarctic animals, in quantities that would be sufficient to manage the immediate damage caused by a moderate or an acute temperature shock. When the shock is prolonged and quantities and/or degradation of available Hsp70s become critical to correct cell damage caused by a thermal shock, the available mRNAs could be recruited via translation and at that moment new mRNAs would need to be transcribed. As a consequence, the higher the amounts of Hsp present as either protein or mRNA within the cell under normal conditions, the longer delay expected in the heat shock response (Bedulina et al. 2010). These assumptions correspond well with what was observed with acute heat shocks. Short shocks of 1, 2, 3, or 6 h did not cause significant upregulation of Hsps whatever the intensity of the heat treatment. These results showed a general decrease in the mRNAs encoding the constitutive forms, probably involved in the synthesis of new Hsp70 proteins. However, there was significant upregulation during post-shock recovery. This upregulation of HSP70 transcripts, which could, in the first instance, be attributed to recovery, was as likely to be due to the natural abundance levels of Hsp70s that have delayed the response, as demonstrated by the Western blot results.
Nevertheless, the presence of large amounts of Hsp70, particularly in comparison with the benthic shrimp (Chorismus antarcticus), questions its justification as a universal adaptation of Southern Ocean endemic species. Reasoning that the large amount of Hsp70 protein in control animals would be linked to a potential protection against the effects of cold, Antarctic krill fished near the French base at Dumont d’Urville would suffer a permanent cold stress if an optimum temperature at 0.5 ± 1 °C is considered (Atkinson et al. 2006; Brown et al. 2010; Cuzin-Roudy et al. 2014; Mackey et al. 2012). Similarly, the labelling shown in E. superba from the Antarctic Peninsula could be attributed to a heat shock. Each of the animals studied would be outside its comfort zone and would therefore be subject to thermal stress. This result is a new evidence highlighting the role of HSPs in the adaptation of organisms to temperature other than through thermotolerance (Banerjee et al. 2014; Ulmasov et al. 1992). The species-specific differences in HSP70 isoforms may be more likely related to variations in thermal tolerance (Yamashita et al. 2004), and isoform expression may vary with regard to thermotolerance (Hightower et al. 1999). In this context, the comparison of the two sister species, E. crystallorophias and E. superba, illustrates this duality in adaptive choices (Cascella et al. 2015; Papot et al. 2016). Indeed, there are differences in terms of CTmax, response, and antibody staining intensity of Hsp70s and therefore of the amount of proteins, but also in terms of selection pressure experienced by the two sister species E. superba and E. crystallorophias (Papot et al. 2016). These differences may be related to their geographic distribution, with ice krill preferring cold continental shelf waters with low temperature variation (Mackey et al. 2012), whereas E. superba lives in deep oceanic waters (Atkinson et al. 2008) and is more exposed to variable temperatures. E. crystallorophias would favor molecular thermotolerance to cold and thus a weak degradation of proteins in a cold environment and a limited involvement of Hsp70, whereas E. superba, due to a wider and more variable distribution, would be less cold adapted and would have developed a greater HSP response.
A similar case has been reported in amphipod species with different vertical distributions and thermotolerance capacities (Bedulina et al. 2013; Bedulina et al. 2010). The hypothesis put forward was that one of the two isoforms would only be synthesized under normal physiological conditions, whereas the second could be synthesized under normal conditions but also induced by heat stress. It seems similar in the case of krill, but the difference is more pronounced since although the two isoforms Hsp70A and B can be expressed together, they are mostly represented in the transcriptomes; they do not seem to be translated simultaneously. It was surprising, in view of the similar values of the FPKMs recorded for the two isoforms in the transcriptome of E. superba (Cascella et al. 2015), that the Western blot revealed only one band in each of the samples of E. superba from east or west Antarctica. This should not be a recognition problem with the Western blot since clearly both Hsp70 A and B isoforms can be recognized by the heterologous antibody. This difference could be attributed to the fact that the original E. superba transcriptome was made from a mixture of control and thermally stressed individuals. Nevertheless, the values obtained for qPCR for the controls are comparable for the two isoforms A and B. It could also be envisaged that the differences could be related to the genetic diversity of two populations. However, Antarctic krill is particularly genetically homogeneous despite its large population size and wide range, limiting differences potentially related to local conditions (Deagle et al. 2015). Based on these observations, differential translation as a function of environmental temperature would therefore remain a reasonable explanation for the results in this study. The experiment carried out by Western blot was conducted on control animals only, i.e., without the application of a heat shock. It will obviously be interesting to continue this study on animals having undergone heat stress in order to validate the hypothesis and to estimate the kinetics of appearance of the different isoforms. The E. superba sample (Fig. 7, lane 4) was kept in an aquarium for 8 months at a temperature of 0.5 °C before freezing, which corresponds to its theoretical thermal optimum (Kawaguchi et al. 2010). Although the Hsp70A staining seems less strong than for krill fished near Dumont d’Urville on the east coast, which is to be confirmed on a larger sample of individuals, it is nevertheless present and well-marked.
In summary, this differentiation of Hsp70 isoform protein expression between animals from West and East Antarctica raises more questions than it answers. However, it represents a new opportunity in the study of Hsp70s, which until now were not considered strong markers of thermal stress in Antarctic krill species. Understanding the differential mechanisms of these thermoregulatory actors in E. superba is a prerequisite in the search for indicators of the biological impact of climate change on this global source of fisheries protein.
References
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103. https://doi.org/10.1038/Nature02950
Atkinson A et al (2006) Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol Oceanogr 51:973–987
Atkinson A et al (2008) Oceanic circumpolar habitats of Antarctic krill. Mar Ecol Prog Ser 362:1–23
Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh, G J, Polley S, Mukherjee A, Das TK, de S (2014) Seasonal variation in expression pattern of genes under HSP70 : Seasonal variation in expression pattern of genes under HSP70 family in heat- and cold-adapted goats (Capra hircus). Cell Stress Chaperones 19:401–408. https://doi.org/10.1007/s12192-013-0469-0
Baringou S, Rouault JD, Koken M, Hardivillier Y, Hurtado L, Leignel V (2016) Diversity of cytosolic HSP70 heat shock protein from decapods and their phylogenetic placement within Arthropoda. Gene 591:97–107. https://doi.org/10.1016/j.gene.2016.06.061
Bedulina DS, Zimmer M, Timofeyev MA (2010) Sub-littoral and supra-littoral amphipods respond differently to acute thermal stress. Comp Biochem Physiol B Biochem Mol Biol 155:413–418. https://doi.org/10.1016/j.cbpb.2010.01.004
Bedulina DS, Evgen'ev MB, Timofeyev MA, Protopopova MV, Garbuz DG, Pavlichenko VV, Luckenbach T, Shatilina ZM, Axenov-Gribanov DV, Gurkov AN, Sokolova IM, Zatsepina OG (2013) Expression patterns and organization of the hsp70 genes correlate with thermotolerance in two congener endemic amphipod species (Eulimnogammarus cyaneus and E. verrucosus) from Lake Baikal. Mol Ecol 22:1416–1430. https://doi.org/10.1111/mec.12136
Brown M et al (2010) Temperature effects on the growth and maturation of Antarctic krill (Euphausia superba). Deep-Sea Res II Top Stud Oceanogr 57:672–682. https://doi.org/10.1016/J.Dsr2.2009.10.016
Cascella K et al (2015) Diversification, evolution and sub-functionalization of 70kDa heat-shock proteins in two sister species of antarctic krill: differences in thermal habitats, responses and implications under climate change. PLoS One 10:1–23. https://doi.org/10.1371/journal.pone.0121642
Clark MS, Thorne MA, Toullec JY, Meng Y, Guan LL, Peck LS, Moore S (2011) Antarctic krill 454 pyrosequencing reveals chaperone and stress transcriptome. PLoS One 6:e15919. https://doi.org/10.1371/journal.pone.0015919
Cuzin-Roudy J et al (2014) Southern Ocean Euphausiids. In: De Broyer CKP, Griffiths HJ, Raymond B, Udekem d’Acoz C’ et al (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 309–320
Deagle BE, Faux C, Kawaguchi S, Meyer B, Jarman SN (2015) Antarctic krill population genomics: apparent panmixia, but genome complexity and large population size muddy the water. Mol Ecol 24:4943–4959. https://doi.org/10.1111/mec.13370
Denny M, Helmuth B (2009) Confronting the physiological bottleneck: a challenge from ecomechanics. Integr Comp Biol 49:197–201. https://doi.org/10.1093/icb/icp070
Everson I (2000) Krill biology, ecology and fisheries. In. Blackwell Science, Oxford
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. https://doi.org/10.1146/annurev.physiol.61.1.243
Flores H et al (2012) Impact of climate change on Antarctic krill. Mar Ecol Prog Ser 458:1–19
Hightower LE et al (1999) Heat shock responses of closely related species of tropical and desert fish. Am Zool 39:877–888
Huenerlage K, Cascella K, Corre E, Toomey L, Lee CY, Buchholz F, Toullec JY (2016) Responses of the arcto-boreal krill species Thysanoessa inermis to variations in water temperature: coupling Hsp70 isoform expressions with metabolism. Cell Stress Chaperones 21:969–981. https://doi.org/10.1007/s12192-016-0720-6
Kawaguchi S et al. (2010) An experimental aquarium for observing the schooling behaviour of Antarctic krill (Euphausia superba). Deep-Sea Research Part Ii-Topical Studies in Oceanography 57:683-692. Doi:Doi https://doi.org/10.1016/J.Dsr2.2009.10.017
Macaulay MC et al (1984) Acoustic characterization of swarms of Antarctic krill (Euphausia superba) from Elephant Island and Bransfield Strait. J Crustac Biol 4:16–44
Mackey AP et al (2012) Antarctic macrozooplankton of the southwest Atlantic sector and Bellingshausen Sea: baseline historical distributions (discovery investigations, 1928-1935) related to temperature and food, with projections for subsequent ocean warming. Deep-Sea Res II Top Stud Oceanogr 59:130–146
Papot C, Cascella K, Toullec JY, Jollivet D (2016) Divergent ecological histories of two sister Antarctic krill species led to contrasted patterns of genetic diversity in their heat-shock protein (hsp70) arsenal. Ecol Evol 6:1555–1575. https://doi.org/10.1002/ece3.1989
Peck LS (2016) A cold limit to adaptation in the sea. Trends Ecol Evol 31:13–26. https://doi.org/10.1016/j.tree.2015.09.014
Peck LS et al (2009) Animal temperature limits and ecological relevance: effects of size, activity and rates of change. Funct Ecol 23:248–256. https://doi.org/10.1111/j.1365-2435.2008.01537.x
Peck LS, Morley SA, Richard J, Clark MS (2014) Acclimation and thermal tolerance in Antarctic marine ectotherms. J Exp Biol 217:16–22. https://doi.org/10.1242/jeb.089946
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper - Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Place SP, Hofmann GE (2005) Comparison of Hsc70 orthologs from polar and temperate notothenioid fishes: differences in prevention of aggregation and refolding of denatured proteins. Am J Physiol-Reg I 288:R1195–R1202
Ravaux J, Gaill F, le Bris N, Sarradin PM, Jollivet D, Shillito B (2003) Heat-shock response and temperature resistance in the deep-sea vent shrimp Rimicaris exoculata. J Exp Biol 206:2345–2354
Ravaux J, Léger N, Rabet N, Morini M, Zbinden M, Thatje S, Shillito B (2012) Adaptation to thermally variable environments: capacity for acclimation of thermal limit and heat shock response in the shrimp Palaemonetes varians. J Comp Physiol B 182:899–907. https://doi.org/10.1007/s00360-012-0666-7
Schiermeier Q (2010) Ecologists fear Antarctic krill crisis. Nature 467:15. https://doi.org/10.1038/467015a
Schwenk K, Padilla DK, Bakken GS, Full RJ (2009) Grand challenges in organismal biology. Integr Comp Biol 49:7–14. https://doi.org/10.1093/icb/icp034
Trivelpiece WZ, Hinke JT, Miller AK, Reiss CS, Trivelpiece SG, Watters GM (2011) Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc Natl Acad Sci U S A 108:7625–7628. https://doi.org/10.1073/pnas.1016560108
Ulmasov KA et al (1992) Heat-shock proteins and thermoresistance in lizards. Proc Natl Acad Sci USA 89:1666–1670
Yamashita M, Hirayoshi K, Nagata K (2004) Characterization of multiple members of the HSP70 family in platyfish culture cells: molecular evolution of stress protein HSP70 in vertebrates. Gene 336:207–218
Acknowledgments
KC received a PhD grant from the Emergence-UPMC 2011 research program and the “Région Bretagne.” JYT benefited from funding provided by Institut Paul Emile Victor (IPEV) (KREVET program) and also from the “Région Bretagne” (SAD-1 - DRAKAR program). JYT would like to thank all the IPEV staff at the base Dumont d’Urville and Astrolabe’s crew for their help during different Antarctic campaigns in Terre Adélie. JYT would also like to thank Dr. So Kawaguchi and Rob King for their invaluable advice and support in setting up the aquarium experiments at the AAD in Kingston, Tasmania. Many thanks to Drs. Juan Höfer (IDEAL, Chile) and Marcelo Gonzalez-Aravena (INACH, Chile) for providing krill samples from western Antarctica. The authors would like to thank also the reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This project (IPEV-1039) was approved by IPEV (Institut Paul Emile Victor, the French Polar Institute) review committee and was declared to, and approved by, the “Terres Australes et Antarctiques Françaises” in 2009 according the Annex I of the Madrid Protocol and the French Decret No 2005-403. No endangered or protected species were used.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toullec, JY., Cascella, K., Ruault, S. et al. Antarctic krill (Euphausia superba) in a warming ocean: thermotolerance and deciphering Hsp70 responses. Cell Stress and Chaperones 25, 519–531 (2020). https://doi.org/10.1007/s12192-020-01103-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-020-01103-2