Abstract
In the context of climate change, there is a sustained interest in understanding better the functional mechanisms by which marine ectotherms maintain their physiological scope and define their ability to cope with thermal changes in their environment. Here, we present evidence that the variable shrimp Palaemonetes varians shows genuine acclimation capacities of both the thermal limit (CTmax) and the heat shock response (hsp70 induction temperature). During cold acclimation to 10 °C, the time lag to adjust the stress gene expression to the current environmental temperature proved to exceed 1 week, thereby highlighting the importance of long-term experiments in evaluating the species’ acclimation capacities. Cold and warm-acclimated specimens of P. varians can mobilise the heat shock response (HSR) at temperatures above those experienced in nature, which suggests that the species is potentially capable of expanding its upper thermal range. The shrimp also survived acute heat shock well above its thermal limit without subsequent induction of the HSR, which is discussed with regard to thermal adaptations required for life in highly variable environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many ecosystems are at the present time warming faster than they have for thousands of years (IPCC 2007, http://www.ipcc.ch/), and the biological consequences of these temperature changes will depend on the ways in which species can adapt to thermal increase and heterogeneity (Angilletta 2009). All organisms possess some capacity to modify their behavioural, physiological or morphological characteristics in response to environmental temperature, this phenotypic plasticity being referred to as ‘thermal acclimation’ (Angilletta 2009). The thermal acclimation potential differs among species, and could thereby establish species’ relative degrees of stenothermy and eurythermy and sensitivity to climate change (Somero 2010). Recent surveys of marine animals propose that species living near their thermal limit with limited abilities to increase their thermal tolerance through acclimation, as well as extreme stenotherms, may be the most susceptible to local extinctions (Somero 2010; Tomanek 2010). When referring to the ecosystems, this was also enounced as species either from very stable or highly variable thermal environments are likely to be more affected by global climate change than species from moderately variable thermal environments (Tomanek 2010).
We address here the capacities for acclimation of the thermal tolerance, and of modulation of gene expression in the shrimp Palaemonetes varians. This species occurs in areas where seasonal thermal fluctuations range from 0 to 30 °C, and daily variations of the water temperature reach about 10 °C in summer (Lofts 1956; Jefferies 1964; Healy 1997; Troccaz 1996; Nielsen and Hagerman 1998). According to Tomanek (2010), such a thermal environment would fall into the category of moderately variable environments, where temperature does not fluctuate by more than 10 °C. Although previous physiological studies have shown this species to be highly tolerant to severe hypoxia and salinity fluctuations in the range of fresh water to seawater (Hagerman and Uglow 1983; Nielsen and Hagerman 1998), the thermal biology per se has not yet been studied in P. varians. Since this species raises both commercial and scientific interests (see “Materials and methods”), we examined its potential sensitivity to warming in the view of the recent assumptions for marine species.
We therefore assessed, through analyses in cold- versus warm-acclimated specimens, the plasticity of a common index of thermal tolerance, the critical thermal maximum (CTmax), as well as the plasticity of a widespread and conserved molecular response to stress, the heat shock response (HSR) (Lindquist 1986). The HSR comprises the cellular induction of heat shock proteins (Hsp) under elevated temperature, including the Hsp70 that is commonly used as a biomarker of heat stress and plays a central role in tolerance to high temperatures by allowing cell survival during and after thermal stress (reviewed in Parsell and Lindquist 1993). Since the characteristics of the heat shock response also proved to contribute to setting the acute upper thermal limits of most organisms (see Tomanek 2010 for review), our study aimed at defining the set points of the HSR in P. varians. Finally, comparisons of thermal acclimation and thresholds between shrimp living in different habitats allowed us to reflect on potential for marine ectotherms from diversely variable thermal environments to respond and adapt to thermal changes.
Materials and methods
Sampling and acclimation
Palaemonetes varians (Leach 1814) is a shallow water brackish shrimp (Decapoda, Caridea) native to Western Europe. This species is frequently harvested for human consumption, used as fishing bait, and it has recently received attention as a potential aquaculture crop (Palma et al. 2008, 2009). Moreover, P. varians previously served as a model species for comparison with deep living shrimps for their adaptation strategies to temperature (Cottin et al. 2010), pressure (Oliphant et al. 2011) and metal toxicity (Gonzalez-Rey et al. 2007, 2008).
Specimens of Palaemonetes varians were collected from Bay of Mont Saint-Michel (France; 48°38′N, 1°30′W) in October 2009 and 2010 and kept for 4 months in an aquarium. The water temperature was gradually decreased from 17 °C (field temperature at the time of collection) to 10 °C or increased to 20 °C, at a rate of 1 °C per week. The salinity of the water during acclimation was 35 g l−1. The photoperiod was set to 12:12 h light:dark and the animals were regularly fed with granules (Novo Prawn) and mussels (Mytilus edulis) ad libitum during the 4 months prior to the experiments.
CTmax determination
Palaemonetes varians specimens of similar size (cephalothorax length 10.8 ± 1.0 mm, n = 40) were placed individually in a beaker filled with 80 ml of seawater and capped with a transparent lid to allow observation throughout the experiment. The beaker was placed in a water bath (Polystat 22 or 37, Bioblock Scientific, Illkirch, France), which regulated the temperature of the surrounding water. The water temperature was monitored to the nearest 0.1 °C using an electronic thermometer. The initial temperature was 13 °C for 10 °C-acclimated specimens and 20 °C for the 20 °C-acclimated specimens, and this was increased at a constant rate of 0.92 °C min−1 (10 °C-acclimated specimens) and 0.93 °C min−1 (20 °C-acclimated specimens). Behaviour in response to increasing temperature was observed and classified into three categories according to previous studies (Ravaux et al. 2003; Shillito et al. 2006; Oliphant et al. 2011) as follows:
-
‘Active moving’: when the shrimp moved (walked or swam) along a distance exceeding their own length in less than 30 s.
-
‘Loss of equilibrium’ (LOE): when the shrimp rested on the bottom in either an “upside-down” or a “sideways” position for more than 2 s.
-
‘Spasms’: vibrations of the pleopods and/or sudden contraction of the abdomen without any coordinated movement.
CTmax was defined as the first temperature at which coordinated movements were lost, i.e. appearance of either spasmodic motions or loss of equilibrium. The experiment ended when the shrimp experienced LOE for more than 30 s. The trial was repeated 20 times for each batch of shrimp (10 and 20 °C-acclimated), which corresponds to a total of 20 individual shrimp for each batch (2n = 40). Following the CTmax experiment, the shrimp were quickly returned to their acclimation temperature and survived for several weeks thereafter.
Heat-shock experiments
A total of 19 independent experiments were conducted to determine the effect of acclimation temperature, temperature and duration of heat exposure on the induction threshold of hsp70 expression (Table 1). For heat shock, shrimp were transferred from the aquarium in which they had been acclimated to 20-l tanks set to maintain the desired temperature. After the heat exposure, shrimp were transferred back to their previous acclimation temperature (10 or 20 °C) in floating cages for 2-h recovery. Shrimp sampled from both acclimation groups without exposure to heat shock served as controls. The tissues from gill, nervous chain, digestive tract and abdomen muscles were dissected out, subsequently frozen and stored in liquid nitrogen until further analysis.
RNA extraction and reverse transcription
Total RNA was extracted from ground tissues using RNeasy Mini kit (Qiagen) and QIAshredder (Qiagen) in accordance with the manufacturer’s protocols. RNA (0.5 μg) was treated to remove DNA contamination using the Turbo-DNAse kit (Ambion) and then reversely transcribed to cDNA with the oligo(dT)18 primer and Superscript II reverse transcriptase kit (Invitrogen) according to the manufacturer’s instructions.
Real-time quantitative RT-PCR
The expression of hsp70 gene was assessed by qPCR. Three reference genes were selected for the analysis: β-actin, GAPDH and RpL8. Specific primers for the reference and hsp70 genes were described in Cottin et al. (2010). Each reaction of qPCR was run in triplicate and corresponded to 3–6 individuals (n = 3–6) for each experimental condition. All reactions were performed on the LightCycler® 480 II Real-Time PCR Detection System (Roche, France), using Sybr Green I Master (Roche, France). The PCR program consisted of an initial 13.5 min step at 95 °C, followed by 45 cycles consisting of 30 s of denaturation at 94 °C, 30 s of annealing at the optimal annealing temperature (56 °C) and 30 s at 72 °C. The measurement of fluorescence during the 70–95 °C melting curve showed a single and discrete peak for all primers tested. One negative control and one dilution series protocol of pooled cDNA were included in each run. The dilution series were used to construct a relative standard curve to determine the PCR efficiencies and for further quantification analysis. In all experiments, all primers gave amplification efficiencies of 90–100 %. Data were analysed with the LightCycler® 480 software and the BestKeeper program. Using the algorithms, the GAPDH (gills and nervous chain) and RpL8 (digestive tract and muscles) genes can be considered as displaying consistent expression and are suitable for downstream analysis. The hsp70 expression was subsequently normalized to this reference.
A Kruskall–Wallis test and a non-parametric Bonferroni-type multiple comparison of the mean normalized expressions of hsp70 gene were performed (http://chiryo.phar.nagoya-cu.ac.jp/javastat/JavaStat-e.htm). The first exposure temperature at which the hsp70 mean normalized expression in test shrimp significantly (P < 0.05) exceeded that in controls (acclimated at 10 or 20 °C) was designed as the ‘threshold temperature’ for induction of hsp70.
Results
CTmax determination
Critical thermal temperature experiments used shrimp acclimated at either 10 or 20 °C. CTmax was determined by changing the temperature at constant rate of 0.9 °C min−1 from the acclimation temperature until the first occurrence of loss of equilibrium and spasms, i.e. when shrimp lose the ability to escape the conditions which may ultimately lead to death. Enhanced activity of 10 °C-acclimated shrimp was observed when the temperature increased. Indeed, from 20.6 ± 0.21 °C onwards, more than 50 % of the shrimp were observed actively moving (Fig. 1). The peak of this activity, where 100 % of the shrimp were actively moving, corresponds to approximately 31.3 ± 0.19 °C and was followed by a fast decrease in activity from 32.4 ± 0.12 °C until the end of the experiment. The 20 °C-acclimated shrimp were in general more active than the 10 °C-acclimated group, i.e. they were swimming and eating more during the acclimation period. During the CTmax experiment, more than 90 % of the individuals from the 20 °C-acclimated group were actively swimming from the beginning of the experiment until 33.0 ± 0.5 °C, and the activity then decreased gradually until the end of the experiment.
Determination of the critical thermal maximum (CTmax) of Palaemonetes varians acclimated at 10 or 20 °C. Distribution of behavioural categories of n = 20 shrimp (totals for behavioural categories of 20 trials) related to mean temperature (temperature averaged across all 20 trials) throughout exposure to an increasing water temperature. LOE loss of equilibrium. For an explanation of the different behaviours, see “Materials and methods”. Each behavioural point represents the observations for a 30-s period. For each observation time, the maximal error with regard to the corresponding temperature is approximately ±0.5 °C. Results for ten acclimated specimens were modified from Oliphant et al. (2011)
An apparent loss of locomotor coordination, expressed as spasmodic movements of the pleopods and/or abdomen with no resulting displacement, was first observed at 30.9 ± 1.0 °C for 10 °C-acclimated shrimp (Oliphant et al. 2011) and 35.8 ± 0.8 °C for 20 °C-acclimated shrimp. This disorder of locomotor activity was also observed as the shrimp lost their balance (LOE response) upon reaching 30.9 ± 1.3 °C for 10 °C-acclimated shrimp or 35.9 ± 0.6 °C for 20 °C-acclimated shrimp.
Tissue-specific expression of hsp70
An induction of hsp70 expression following a 28 °C-shock (10 °C-acclimated shrimp) was measured in the respiratory, nervous, digestive and locomotory systems (Supplementary material). The respiratory and digestive systems showed more variable values and lower fold of induction, i.e. 50 for the gill and 130 for the digestive tract, compared with 325 for the nervous chain and 1,230 for the muscle. The muscle was chosen as the target tissue for all the other heat shock response analyses, since it showed the highest over-expression and the most homogeneous values.
Threshold temperature of hsp70 expression induction in 10- versus 20 °C-acclimated shrimps
While the relative abundance of hsp70 transcripts was comparable in the control specimens of each acclimation group, with 4.8 × 10−3 for 10 °C control shrimp and 3.3 × 10−3 for 20 °C control shrimp, an acclimation of the heat shock response was evidenced by the threshold temperature of hsp70 induction (Fig. 2). The first temperature that triggers a significant induction of hsp70 mRNA expression relatively to the corresponding control specimens at 10 and 20 °C (P < 0.05) was 28 °C for 10 °C-acclimated shrimp and 31 °C for 20 °C-acclimated shrimp (Fig. 2). When normalized to the expression levels of the control shrimp, this corresponds to an average fold of induction of 1,850 for 10 °C-acclimated shrimp, and 570 for 20 °C-acclimated shrimp. Besides these high overexpressions, a relatively small induction of hsp70 expression was also observed at each lower temperature of shock, which correspond to fold of induction ranging from 5 to 44 (Fig. 2; fold not indicated in figure).
Determination of the threshold temperature for hsp70 expression induction in Palaemonetes varians. Levels of hsp70 mRNA were measured after a 1-h heat shock followed by a 2-h recovery period, and are expressed as means for n individuals (±SEM) of relative hsp70 amount normalized to RPL8 abundance (reference gene); n = 3–4 for 10 °C-acclimated specimens (except n = 1 at 25 °C), and n = 3–5 for 20 °C-acclimated specimens. Asterisks indicate significant differences (P ≤ 0.05) with the 10 °C reference group (*) or 20 °C reference group (**). Numbers beside the points indicate the hsp70 levels in the heat shock individuals relative to the 10 or 20 °C-control group and therefore correspond to the increase (x-fold) of hsp70 expression in the heat shock sample
Threshold duration of heat exposure for hsp70 expression triggering
Both groups of 10 and 20 °C acclimated shrimp were subjected to a decreasing duration range of heat exposure from 60 to 1 min. The subsequent hsp70 expression was quantified and showed a significant induction relative to the control (specimens kept at their acclimation temperature) for heat exposures that last 30 min or more (Fig. 3). The temperature of shock was chosen 2–3 °C below the CTmax for each acclimation group, and induced a similar expression of hsp70 for both 30- and 60-min exposures (Fig. 3), which corresponds to an over-expression of 810-fold (30 min) and 1,850-fold (60 min) for 10 °C-acclimated shrimp, and 1,540-fold (30 min) and 2,250-fold (60 min) for 20 °C-acclimated shrimp.
Determination of the threshold duration for hsp70 expression induction in Palaemonetes varians. Levels of hsp70 were measured after a shock at 28 °C (10 °C-acclimation) or 34 °C (20 °C acclimation), followed by a 2-h recovery period, and are expressed as means (±SEM) normalized to RPL8 expression; n = 3–6 for 10 °C-acclimated specimens and n = 3–5 for 20 °C-acclimated specimens. Asterisks indicate significant differences (P ≤ 0.05) with the 10 °C reference point (*) or 20 °C reference point (**) at t = 0. Numbers beside the points indicate the hsp70 levels in the heat shock individuals relative to the 10 or 20 °C-control group and therefore correspond to the increase (x-fold) of hsp70 expression in the heat shock sample
Heat shock response beyond thermal limits
Specimens of P. varians acclimated to 20 °C were exposed to acute heat shocks exceeding their thermal limit (35.8 °C CTmax), to explore the boundaries of the HSR. The 5-s shock at 34 and 40 °C did not trigger any detectable HSR (amount of hsp70 mRNA similar to that of the control at t = 0), and only yielded a very weak HSR at 45 °C similar to the hsp70 expression in the 1-min heat shock individuals at 34 °C (reported from Fig. 3), i.e. well below the induction threshold for hsp70 expression defined in Figs. 2 and 3 (Fig. 4). The shrimp were all alive and showed a normal activity level during the 2 h following the exposure at 45 °C.
The heat shock response following temperature spikes above the thermal limit of Palaemonetes varians. Levels of hsp70 mRNA were measured, on 20 °C-acclimated specimens, after a 5-s shock at 34, 40 or 45 °C, followed by a 2-h recovery period, and are expressed as means for four individuals (±SEM) of relative hsp70 amount normalized to RPL8 abundance (reference gene). For comparison, values for hsp70 expression of 20 °C control specimens (t = 0) and 1 min-shocked at 34 °C specimens were plotted from Fig. 3. A control group, which corresponds to the hsp70 expression triggered by a potential manipulation stress during the experimental procedure without temperature variation, is also represented (white triangle)
Discussion
Acclimation of thermal limit (CTmax) and heat shock response (HSR)
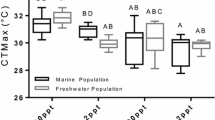
Critical thermal limits have shown to acclimate in various taxa, generally following a linear relationship with the environmental or body temperature, with the CTmax plasticity being equivalent to the slope of the function (Angilletta 2009). This particular phenotypic plasticity was also named acclimation response ratio (ARR), which defines the change in the CTmax per degree change in acclimation temperature (i.e. ΔCTmax/ΔT; Claussen 1977). The relationship between CTmax and acclimation temperature was previously reported in crustacean species (see Re et al. 2006; Hopkin et al. 2006) and is illustrated in Fig. 5 for eight species of shrimp from different climate zones, including P. varians. Despite the lack of data for acclimation temperatures below 20 °C, it appears that CTmax is generally higher in warm-acclimated and summer-captured specimens and that the acclimation potential varies among species (Fig. 5). In P. varians, the thermal acclimation was evidenced as a shift in the CTmax from approximately 31 °C (10 °C acclimation) to 36 °C (20 °C acclimation). This corresponds to a calculated ARR of 0.50 (in the 10–20 °C range), which ranks in the high-range values reported for tropical and sub-tropical shrimp species (Re et al. 2006; Fig. 5). Shrimp from these different habitats, i.e. saline marsh pools and tropical freshwater/seawater, would thus have similar acclimation capacities for their upper thermal limit, and in any case these species can readily extend their thermal tolerance when the environmental temperature is warming.
Critical thermal maxima related to the acclimation temperature in shrimp from marine and freshwater habitats. Black circles, Palaemonetes varians (this study for the CTmax of 20 °C-acclimated specimens, and Oliphant et al. 2011 for the CTmax of 10 °C-acclimated specimens). White diamonds, Macrobrachium acanthurus (Díaz et al. 2002); grey diamonds, Macrobrachium rosenbergii (Manush et al. 2004; Díaz et al. 1998); white squares, Macrobrachium tenellum (Hernandez et al. 1996); grey squares, Palaemonetes kadiakensis (Nelson and Hooper 1982); white triangles, Macrobrachium malcolmsonii (Selvakumar and Geraldine 2005); light grey triangles, Farfantepenaeus aztecus (Re et al. 2005); dark grey triangles, Litopenaeus stylirostris (Re et al. 2006)
But this acclimation of thermal limits does not necessarily imply a shift in the thermal optimum for performance (considered here in terms of locomotory activity), since the latter rarely acclimates to changes in environmental temperature (Angilletta 2009). In P. varians, a high locomotory activity was observed for both acclimation groups from about 20 °C to approximately 32–33 °C, where a similar decrease of this activity appeared (Fig. 1; see also Oliphant et al. 2011). This would mean that the shrimp could not maintain normal levels of activity (active moving) above 32 °C, whatever their acclimation temperature. We can conclude that this shrimp would be able to extend its performance breadth to higher temperatures without shifting the thermal optimum and would then show less efficiency for swimming activity near the thermal limit (actively moving individuals dropped from 95–100 to 50–60 % between 32 and 36 °C). This species can thus probably not cope with sustained water temperatures above 32 °C in its habitat, since long-term effects of heat stress, while not acutely lethal, may nevertheless ultimately lead to the disappearance of a species from its ecosystem if fitness is impacted due to diminished physiological or behavioural performance at elevated temperatures (Somero 2010).
Since adaptive modification of the phenotype during acclimation involves large-scale alterations in gene expression, the capacities of species to cope with temperature change would also lie in their relative abilities to modify transcriptional processes in response to thermal stress (Somero 2010). The heat shock response (HSR) is a modification of heat shock gene expression that is commonly used to assess levels of thermal stress and thermal tolerance limits (see Tomanek 2010 for review). Acclimation of the HSR was evidenced in P. varians as a shift of about 3 °C of the threshold temperature for hsp70 induction between 10 and 20 °C-acclimated shrimp (28–31 °C; Fig. 2). This shift is a common phenomenon of acclimation of the HSR, the temperature set-points of the HSR indeed exhibit considerable plasticity in response to rearing temperature, particularly in mobile and stenotherm organisms (Barua and Heckathorn 2004). For P. varians, the magnitude of acclimation, estimated as the change in HSR induction temperature per unit change in rearing temperature, is 0.30 (change of 3 °C for a 10 °C interval in acclimation temperature), which ranks in the upper range of values reported for marine invertebrates (change in T on for hsp70: 0 to 0.30; Barua and Heckathorn 2004). This is consistent with the assumption that organisms from moderately variable thermal environments can shift their response to a higher onset temperature (Tomanek 2010). However, increasing the incidence of HSR activation due to increasing temperatures in these organisms may incur costs that are detrimental to the long-term fitness of the species and restrictive of the thermal niches in which the organisms can occur (Tomanek 2010).
Acclimation requires both time and energy for physiological changes to occur (see Angilletta 2009 for review). The costs of acclimation are expected to differ between species and would in part depend on time that is required to adjust their physiology to the current environment. A first clue of this time lag in P. varians can be deduced from comparison of HSR between specimens acclimated for 4 months or 7 days at 10 °C (this study and Cottin et al. 2010). The hsp70 mRNA relative amount, following a 28 °C-shock, is more than 100-fold higher in specimens acclimated for 4 months in comparison with specimens acclimated for 7 days. This means that changes have occured in the characteristics of the heat shock response, involving a shift of the maximal hsp70 synthesis to lower temperatures in long-term acclimated specimens. In P. varians, the time lag to retune the stress gene expression to the new environmental temperature would therefore exceed 7 days.
HSR in variable thermal environments
There is evidence that marine organisms from thermally distinct habitats (i.e. stable, moderately or highly variable) vary in their heat shock response in a way that suggests that some use the response more frequently than others (Tomanek 2010). Due to this variation in the HSR, species from different thermal habitats would unequally cope with global climate change, with organisms from moderately variable environments being the least affected.
In P. varians, the onset for hsp70 expression was 28 °C for cold-acclimated shrimp and 31 °C for warm-acclimated shrimp (Fig. 2), those threshold temperatures being well above the winter and summer maxima reported in its natural environment (Lofts 1956; Jefferies 1964; Healy 1997; Troccaz 1996). This is consistent with HSR of organisms from moderately variable environments, which have the option to induce the response over a wide temperature range above the one they are currently experiencing in the field (Tomanek 2010). These organisms would then very rarely trigger hsp70 synthesis under natural conditions, an assumption that should be confirmed through further studies of HSR in natural populations. Another question that should be addressed in natural populations is the level of hsp70 expression that can be considered as physiologically relevant. Here, we report statistically significant thresholds, which correspond to huge folds of induction, in the hundreds to thousands range. However, more moderate levels of hsp70 expression were obtained below these thresholds that may be physiologically important.
The HSR depends on the length of the heat shock, and in P. varians the response was triggered only when the shrimp were submitted to a sustained elevated temperature for at least 30 min (Fig. 3). Short-term exposures, in the seconds to minutes range, at temperatures from near and above the CTmax did not induce any significant hsp70 expression (Figs. 3, 4). This could either mean that the temperature spikes do not last long enough to cause any significant cellular damages, or that the damages are restricted to very peripheral tissues and do not ultimately result in a massive HSR. These temperature spikes beyond CTmax, if they are not ecologically relevant for P. varians are, however, very similar to the thermal challenges experienced by the deep-sea hydrothermal shrimp Rimicaris exoculata. This species dwells in a very unstable thermal environment and mobilises the HSR within the temperature range currently encountered in its habitat, i.e. following a 30 °C-exposure and even a 25 °C-exposure, while this shrimp is supposed to face temperature of up to 40 °C in the field (Ravaux et al. 2003, 2009; Cottin et al. 2010). R. exoculata clearly belongs to the highly variable environments; however, the thermal fluctuations of the habitat temperature were categorised as moderate (<10 °C) and high (>20 °C) on a daily basis, which would place the hydrothermal species in a special category since the temporal variability (several degrees over minutes) is much higher in vent habitats than in any other aquatic habitats (Bates et al. 2010). The characteristics of the HSR in such a particular environment still have to be investigated, but the first attempts of HSR measurements beyond the thermal limit of the shallow shrimp P. varians provide insights into the HSR boundaries, which may ultimately help to understand how shrimp have come to colonize and adapt to the highly fluctuating hydrothermal environment. Indeed, since exposure to spikes of extreme temperature did not trigger a significant stress response in P. varians, we might well question if these shocks can still be considered as being stressful for the animals. Referring to the hydrothermal shrimp, this could be consistent with the low levels of the heat-inducible hsp70 mRNA measured in natural populations of R. exoculata (Ravaux et al. 2009), meaning that the shrimp would not respond to acute thermal variations of their environment. The hydrothermal environment could then be termed stressful rather due to the unpredictably thermal fluctuations than to the occurrence of temperature spikes. This is in the line of a recent study that demonstrates that hydrothermal species avoid temperatures well within their tolerated range to maintain a safety margin against rapid temperature fluctuations (Bates et al. 2010).
Conclusion
This study showed genuine acclimation capacities for P. varians through plasticity of both the thermal limit (CTmax) and the HSR (hsp70 induction temperature). This species is readily able to expand its thermal range since it can shift its thermal maximum to higher temperatures and also mobilise the HSR over a wide range of temperatures above those experienced in nature. Further studies should aim at examining the natural levels of hsp70 in P. varians populations across its wide range in distribution and evaluating the HSR regulation in response to seasonal changes in temperature. Because of their great acclimation capacity, marine species, like P. varians, inhabiting moderately variable thermal environments, are assumed to be the least affected by global warming. However, the time required for implementation of acclimation may impact the fitness of the organisms, and further inter-species comparisons of time lags will help to evaluate their ability to cope with environmental warming.
References
Angilletta MJ (2009) Thermal adaptation. Oxford University Press, Oxford
Barua D, Heckathorn SA (2004) Acclimation of the temperature set-points of the heat-shock response. J Therm Biol 29:185–193
Bates AE, Lee RW, Tunnicliffe V, Lamare MD (2010) Deep-sea hydrothermal vent animals seek cool fluids in a highly variable thermal environment. Nat Commun 1:14. doi:10.1038/ncomms1014
Claussen DL (1977) Thermal acclimation in ambystomatid salamanders. Comp Biochem Physiol A 58:333–340
Cottin D, Shillito B, Chertemps T, Thatje S, Léger N, Ravaux J (2010) Comparison of heat-shock responses between the hot vent shrimp Rimicaris exoculata and the related coastal shrimp Palaemonetes varians. J Exp Mar Biol Ecol 393:9–16
Díaz HF, Uribe ES, Ramirez LFB, Mora AG (1998) Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: Palaemonidae). J Therm Biol 23(6):381–385
Díaz HF, Sierra E, Re AD, Rodríguez L (2002) Behavioural thermoregulation and critical thermal limits of Macrobrachium acanthurus (Wiegman). J Therm Biol 27:423–428
Gonzalez-Rey M, Serafim A, Company R, Bebianno MJ (2007) Adaptation to metal toxicity: a comparison of hydrothermal and coastal shrimps. Mar Ecol 28:100–107
Gonzalez-Rey M, Serafim A, Company R, Gomes T, Bebianno MJ (2008) Detoxification mechanisms in shrimp: comparative approach between hydrothermal vent fields and estuarine environments. Mar Environ Res 66:35–37
Hagerman L, Uglow RF (1983) The influence of temperature on the osmoregulation of the brackish-water shrimp Palaemonetes varians Leach. Ophelia 22(2):229–236
Healy B (1997) Long-term changes in a brackish lagoon, Lady’s Island Lake, South-east Ireland. Biol Environ Proc R Irish Acad 97B:33–51
Hernandez RM, Bückle RLF, Diaz HF (1996) Critical thermal maximum of Macrobrachium tenellum. J Therm Biol 21:139–143
Hopkin RS, Qari S, Bowler K, Hyde D, Cuculescu M (2006) Seasonal thermal tolerance in marine Crustacea. J Exp Mar Biol Ecol 331:74–81
Jefferies DJ (1964) The moulting behaviour of Palaemonetes varians (Leach) (Decapoda; Palaemonidae). Hydrobiology 24:457–488
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191
Lofts B (1956) The effects of salinity changes on the respiratory rate of the prawn Palaemonetes varians (Leach). J Exp Biol 33:730–736
Manush SM, Pal AK, Chatterjee N, Das T, Mukherjee SC (2004) Thermal tolerance and oxygen consumption of Macrobrachium rosenbergii acclimated to three temperatures. J Therm Biol 29:15–19
Nelson DH, Hooper DK (1982) Thermal tolerance and preference of the freshwater shrimp Palaemonetes kadiakensis. J Therm Biol 7(3):183–187
Nielsen A, Hagerman L (1998) Effects of short-term hypoxia on metabolism and haemocyanin oxygen transport in the prawns Palaemon adspersus and Palaemonetes varians. Mar Ecol Prog Ser 167:177–183
Oliphant A, Thatje S, Brown A, Morini M, Ravaux J, Shillito B (2011) Pressure tolerance of the shallow-water caridean shrimp, Palaemonetes varians, across its thermal tolerance window. J Exp Biol 214:1109–1117
Palma J, Bureau DP, Andrade JP (2008) Effects of binder type and binder addition on the growth of juvenile Palaemonetes varians and Palaemon elegans (Crustacea: Palaemonidae). Aquac Int 16:427–436
Palma J, Bureau DP, Correia M, Andrade JP (2009) Effects of temperature, density and early weaning on the survival and growth of Atlantic ditch shrimp Palaemonetes varians larvae. Aquac Res 40:1468–1473
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Ravaux J, Gaill F, Le Bris N, Sarradin PM, Jollivet D, Shillito B (2003) Heat shock response and temperature resistance in the deep-sea vent shrimp Rimicaris exoculata. J Exp Biol 206:2345–2354
Ravaux J, Cottin D, Chertemps T, Hamel G, Shillito B (2009) Hydrothermal vent shrimps display low expression of heat-inducible hsp70 gene in nature. Mar Ecol Prog Ser 396:153–156
Re AD, Díaz HF, Sierra E, Rodriguez J (2005) Effect of salinity and temperature on thermal tolerance of brown shrimp Farfantepenaeus aztecus (Ives) (Crustacea, Penaeidae). J Therm Biol 30:618–622
Re AD, Díaz HF, Valdez G (2006) Effect of salinity on the thermoregulatory behavior of juvenile blue shrimp Litopenaeus stylirostris Stimpson. J Therm Biol 31:506–513
Selvakumar S, Geraldine P (2005) Heat shock protein induction in the freshwater prawn Macrobrachium malcolmsonii: acclimation-influenced variations in the induction temperatures for Hsp70. Comp Biochem Physiol A Mol Integr Physiol 140(2):209–215
Shillito B, Le Bris N, Hourdez S, Ravaux J, Cottin D et al (2006) Temperature resistance studies on the deep-sea vent shrimp Mirocaris fortunata. J Exp Biol 209:945–955
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Tomanek L (2010) Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J Exp Biol 213:971–979
Troccaz O (1996) Evolution de la dynamique d’un marais salé: Processus fonctionnels internes et relations avec le milieu côtier, la baie du Mont Saint-Michel. PhD thesis, University Rennes I
Acknowledgments
This work was funded through a research grant (Abyss 2100) from the Total Foundation to S.T. The funders (Total Foundation) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The qPCR analyses were performed at the Real Time PCR service (IFR 83 Biologie Intégrative, Université Pierre et Marie Curie). This study benefited from helpful discussions with Samuel Bornens, Thomas Chertemps and Delphine Cottin, and also from fishing skills of Arnaud Schwarz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravaux, J., Léger, N., Rabet, N. et al. Adaptation to thermally variable environments: capacity for acclimation of thermal limit and heat shock response in the shrimp Palaemonetes varians . J Comp Physiol B 182, 899–907 (2012). https://doi.org/10.1007/s00360-012-0666-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0666-7