Abstract
The ability of eukaryotes to adapt to an extreme range of temperatures is critically important for survival. Although adaptation to extreme high temperatures is well understood, reflecting the action of molecular chaperones, it is unclear whether these molecules play a role in survival at extremely low temperatures. The recent genome sequencing of the yeast Glaciozyma antarctica, isolated from Antarctic sea ice near Casey Station, provides an opportunity to investigate the role of molecular chaperones in adaptation to cold temperatures. We isolated a G. antarctica homologue of small heat shock protein 20 (HSP20), GaSGT1, and observed that the GaSGT1 mRNA expression in G. antarctica was markedly increased following culture exposure at low temperatures. Additionally, we demonstrated that GaSGT1 overexpression in Escherichia coli protected these bacteria from exposure to both high and low temperatures, which are lethal for growth. The recombinant GaSGT1 retained up to 60 % of its native luciferase activity after exposure to luciferase-denaturing temperatures. These results suggest that GaSGT1 promotes cell thermotolerance and employs molecular chaperone-like activity toward temperature assaults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life in Antarctica is considered extreme due to several lethal barriers that most organisms cannot tolerate, such as extremely low-energy environments, low nutrients, and high ultraviolet radiation (UV). Studies on cold adaptation and tolerance to heat have been extensively conducted in bacteria compared with eukaryotes, but knowledge of higher level organisms remains limited. The most prominent finding in cold adaptation is the presence of heat shock proteins (HSPs), which primarily function as molecular chaperones that facilitate protein folding and prevent protein degradation and aggregation upon thermal assault (Boshoff et al. 2004). Glaciozyma antarctica, formerly known as Leucosporidium antarcticum, is psychrophilic yeast first isolated from the surface of sea ice in Antarctica (Fell et al. 1969). In 2011, Turchetti et al. reclassified this yeast to G. antarctica based on phylogenic analysis and the absence of lenticular bodies. G. antarctica is classified as obligate psychrophilic based on its optimum temperature, reported as 12 °C, and tolerance of temperatures up to 20 °C (Boo et al. 2013). The Whole Genome Sequencing Project on Extremophiles Malaysia has sequenced the genome of this organism. The size of the G. antarctica genome is 20.03 Mb, with 7857 protein-coding genes. The study of G. antarctica has revealed new platforms and perspectives on the cold adaptation strategies of Antarctic yeast via the characterization of novel proteins.
In cells, ubiquitous small heat shock proteins (sHSP) play important roles in balancing protein homeostasis upon exposure to stress. With small monomeric masses ranging from 12 to 42 kDa, sHSPs are observed in nearly all organisms from archaea, bacteria, and eukarya and have been implicated in diverse cellular functions, such as stress tolerance, protein degradation, protein folding, and apoptosis (Bakthisaran et al. 2015). All known sHSPs harbor a conserved α-crystallin domain of approximately 90 amino acids, flanked by variable amino- and carboxyl-terminal extensions (Kriehuber et al. 2010) that form compact a barrel-like β-sheet sandwich fold (Kim et al. 1998). Despite the divergence of organisms across all domains, many structural features of sHSPs, such as the C-terminal region, referred to as the α-crystalline domain, and anti-parallel β-sandwich motifs, which form hollow ball-shaped oligomers (Waters et al. 1996), are highly conserved. In vitro, sHSPs function as shields that prevent the irreversible aggregation of non-native proteins, while in vivo these molecules are akin as the protein protector that maintain the protein unfolding and disassembly (Van Montfort et al. 2001). sHSPs ranging from 15 to 22 kDa are members of the HSP20 protein family (Waters et al. 1996). The identification of an SGT1-like protein from G. antarctica (GaSGT1) containing an HSP20-like chaperone domain that mimics the function of HSPs has revealed new findings in protein cold adaptation. The suppressor of the G2 allele of SKP1 (SGT1) protein interacts with HSP90 and is important for the G1/S and G2/M transitions in the cell cycle (Kitagawa et al. 1999). The sequence analysis of SGT1 proteins from yeast, human, barley, rice, and Arabidopsis thaliana has revealed three conserved domains, tetratricopeptide repeats (TPRs), CHORD-containing proteins, and SGT1- (CS) and SGT1-specific (SGS) proteins (Azevedo et al. 2002). The N-terminal TPR domain, known as the heat shock protein-binding domain, plays an important role as a mediator of HSP90 interactions. The C-terminal SGS domain interacts with S100 calcium-binding proteins (Nowotny et al. 2003). Similar to HSP20 or the α-crystallin domain of the human p23 co-chaperone family, the CS domain, the central motif, plays an important role in HSP90 interaction, particularly as a co-chaperone (Dubacq et al. 2002; Garcia-Ranea et al. 2002). p23 and the HSP20/α-crystallin family of heat shock proteins share the same three-dimensional folding function and exhibit a pattern of conserved residues that suggest a common origin in the evolution of both protein domains. The p23 and HSP20/α-crystallin phylogenetic relationship and similar roles in chaperone activity suggest a common function for proteins containing p23-like domains, such as SGT1 (Garcia-Ranea et al. 2002). However, the precise function and structure of SGT1 remain relatively unknown.
The present study is the first to explore the role of SGT1 in adaptation to lower temperatures via the characterization of SGT1 from G. antarctica. We analyzed the GaSGT1 gene expression in cells exposed to different temperatures to determine the induction of this gene and characterized the molecular chaperone activity of GaSGT1. Parallel to the functional analysis, a structural study was performed to determine how this protein adapts to the low-energy environment.
Materials and methods
G. antarctica culture and exposure to different heat and cold shock temperatures
G. antarctica cells were cultured in yeast peptone dextrose broth (10 % (w/v) yeast extract, 20 % (w/v) peptone, and 20 % (w/v) dextrose) at 12 °C until the OD600 reached approximately 0.6–0.8. Subsequently, the cultures were exposed to different temperatures:−12, 0, 5, 12, and 20 °C. The cells were harvested after exposure to each temperature for 6 h.
RNA extraction
The following materials were treated overnight with diethylpyrocarbonate (DEPC) 0.1 % (v/v) and subsequently autoclaved: pestle and mortar, microcentrifuge tubes, pipette tips, and spatula. The RNA extraction was performed using TRIzol® reagent (Invitrogen, USA) according to Bharudin et al. (2014). The concentration and purity of total RNA was measured using a Nanodrop spectrophotometer at 260 nm (ThermoScientific, USA). The RNA quality was determined after running total RNA on a 1 % (w/v) agarose gel at 120 V for 1 h. Total RNA was stored at −80 °C.
Cloning and sequence analysis
GaSGT1 complementary DNA (cDNA) was amplified using specific primers that promoted the ligation-independent cloning of target genes into the pET30 Ek/LIC (Merck-Millipore, Germany) vector (Table 1). The direct ligation of amplified GaSGT1 cDNA with the pET30 Ek/LIC vector was prepared according to the manufacturer’s instructions. Moreover, the annealed product was transformed into an E. coli BL21 expression host. The positive transformants were identified using sequencing analysis. The isoelectric point was determined using the ProtParam tool (Gasteiger et al. 2005). The sequence domain was analyzed using the InterPro Scan (Quevillon et al. 2005) and Pfam (Sonnhammer et al. 1997). The sequence alignment was performed using the ClustalW (Thompson et al. 1994). The GaSGT1 sequence was deposited in GenBankTM under accession number KT220749.
Quantitative real-time PCR analysis
cDNA was synthesized from 10 ng of total RNA from each sample using specific primers and the QuantiFast SYBR® Green RT-PCR Kit (QIAGEN, USA) in a 20-μL reaction. The GaSGT expression pattern was analyzed using a Thermal cycler (Eppendorf, USA). Each reaction contained 12.5 μL of 1X Master Mix, 1 μM GaSGT1F-RT primer, 1 μM GaSGT1R-RT primer, 0.25 μL of Quantifast Mix, and 2 μL of template. A standard curve was constructed using tenfold serial dilutions (100, 10, 1, 0.1, and 0.01 ng) of RNA amplified using the 18S reference gene and GaSGT1 primers (Table 1). The analysis was performed in triplicate. Statistical significance was assessed using a two-tailed paired Student’s t test. The GaSGT1 expression profiles were normalized to the 18S reference gene according to Hashim et al. (2013) to compensate for any variation in the amount of starting material between samples. A melting curve analysis was performed to analyze the specificity of the PCR reaction.
Expression, purification, and detection of recombinant GaSGT1
Expression was assessed in LB containing 50 μg/mL of kanamycin. Induction was performed using 0.5 mM isopropyl β-D-thiogalactopyrosidase (IPTG) after reaching an OD600 of ∼0.8 and inducing for 16 h at 16 °C. Purification was performed using prepacked Ni-NTA columns, followed by anion exchange chromatography and gel filtration on a S200 16/60 column (GE Healthcare, USA) according to the manufacturer’s instructions. The purified GaSGT1 was analyzed for purity using denatured and native gel electrophoresis. The Western blot analysis was performed using the primary antibody, monoclonal anti-histidine (Novagen, USA), at a ratio of 1:10,000 and the secondary antibody, anti-goat IgG horse peroxidase antibody, at a ratio of 1:25,000, each for an hour with agitation at room temperature, followed by detection with Luminate Forte Western HRP (Merck, USA).
Thermotolerance experiments with transformed E. coli
For the thermotolerance experiments, E. coli MC4100 ΔibpA/B lacking the small heat shock proteins IbpA and IbpB and its parent strain MC4100 (Kuczynska-Wisnik et al. 2002) were used. The expression plasmid was transformed into E. coli MC4100 ΔibpA/B to generate E. coli ΔIbpA/B_GaSGT1. The transformed E. coli cell cultures, E. coli MC4100 ΔibpA/B, and E. coli MC4100 were grown as described above. IPTG was added to mid-log phase cultures (OD600 = ∼0.8) at a final concentration of 1 mM, and incubation was continued at 37 °C for 2 h. The cultures were transferred to 50 °C. Samples (100 μL) were obtained at 0, 15, 30, 45, 60, and 75 min after 50 °C treatment, diluted using serial dilutions and plated onto LB supplemented with 50 μg/mL kanamycin in triplicate. The plates were incubated overnight at 37 °C prior to scoring colony formation to determine the percentage of survivors. The same methods were used for cells exposed to 0 °C, and the samples exposed to 0 °C were analyzed on days 0, 2, 4, 6, and 8.
Luciferase heat-induced aggregation assay
Luciferase (Promega, USA) was diluted to 1 μg/μL using stability buffer (25 mM Tris-HCl, pH 8, 8 mM MgSO4, 0.1 mM EDTA, 1 mg/mL bovine serum (BSA), 10 % glycerol, and 1 % Triton X-100) and incubated with purified 10 μg/μL GaSGT1 in a tube. The samples were heated to 41 °C for 2 min, and reaction buffer (0.5 mM D-luciferin, 0.1 mM adenosine 5′-trisphosphate (ATP), 1 mM DTT, 10 % glycerol, and 10 % PEG 4 K) was added in a 200-μL reaction. Unheated luciferase, incubated with and without purified GaSGT1, was used as a positive control, whereas heated luciferase without any incubation with purified GaSGT1 was used as a negative control. Luciferase aggregation was measured at 560 nm using a SynergyHT luminometer (Research Instrument, USA) for 7 min.
Modeling GaSGT1 tertiary structures
The three-dimensional GaSGT1 structures were modeled to the human CS domain (PDB: 1RL1) using the SWISS-MODEL program (Guex and Peitsch 1997; Schwede et al. 2003). The structure quality was evaluated using PROCHECK (Laskowski et al. 1993), Verify3D (Eisenberg et al. 1997) and ANOLEA (Melo et al. 1997). The superimposed GaSGT1 model and template and comparative analysis were performed using sCHIMERA USCF (Pettersen et al. 2004).
Results
GaSGT1 sequence analysis
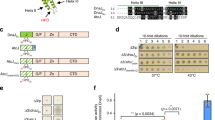
The GaSGT1 sequence analysis revealed that this protein is related to SGT1 proteins from R. norvegicus (acc. no. B0BN85.1), M. musculus (acc. no. Q9CX34.3), H. sapiens (acc. no. Q9Y2Z0.3), and B. taurus (acc. no. Q2KIK0.1) (Fig. 1). The size of the cDNA was 639 bp, and no signal peptide sequence was detected. The protein contains two major domains: the central CHORD-SGT1 (CS) domain, similar to the HSP20/α-crystallin domain of the human p23 co-chaperone family, and a C-terminal SGT1-specific (SGS) domain, which is structurally less well defined, although highly conserved relative to the other SGT1 domains (Dubacq et al. 2002; Garcia-Ranea et al. 2002). However, no TPR domain was observed, suggesting that GaSGT1 might not interact with HSP90 in G. antarctica. Additionally, high residue substitution of charged side chains and bulky polar residues to alanine (7.5 %) were observed in the GaSGT1 sequence, which might be important for proper functioning at lower temperatures.
Sequence alignment of GaSGT1 with the SGT1 from R. norvegicus, M. musculus, H. sapiens, and B. taurus. The domain analysis revealed three domains: TPR (blue box), CS (red box), and SGS (green box). GaSGT1 only contained two conserved domains, the CS and SGS domains. The amino acid sequences of the GaSGT1 CS domain were aligned relative to human p23 and other CS domain-containing proteins. Highly conserved residues that stabilized the β-sheets in the p23 and CS domain-containing proteins are highlighted in red. Alanine substitutions of charged side chains and bulky polar residues are indicated using steric. Highly conserved residues are highlighted in black, and partially conserved residues are shown in gray
GaSGT1 mRNA expression levels at different temperatures
The purity of the RNA was 1.9 to 2.0, indicating the high purity of the RNA. The melting curve analysis showed the single amplification of 18S and GaSGT1 genes. The PCR efficiency of the primer sets used to amplify 18S and GaSGT1 genes was acceptable, as the slopes were within −3 to −3.3. The R 2 values ranged from 0.97 to 0.99, and the E values for both primer sets were 110 %. Thus, the template purity and PCR efficiency fulfilled the standard characteristics for accurate and reliable PCR data according to the general MIQE quantitative PCR guidelines (Bustin et al. 2009). The gene expression results were normalized to 18S expression as the reference gene (Fig. 2). GaSGT1 messenger RNA (mRNA) expression in G. antarctica was measured at different temperatures to determine the induction of GaSGT1. GaSGT1 mRNA expression strongly increased in cells exposed to temperatures far from the optimal growth of 12 °C, i.e., −12 and 20 °C, showing 12.54- and 16.28-fold increases, respectively. The increases in GaSGT1 mRNA expression at 5 and 0 °C were 1.71- and 2.93-fold, respectively.
GaSGT1 mRNA expression in G. antarctica. GaSGT1 levels were measured in cells exposed to the indicated temperatures for 6 h and normalized to 18S (reference gene) levels. mRNA expression at 12 °C was set to 1, and other values were normalized against this value. The data are representative of three trials with standard deviations of the mean, and statistical significance was assessed using a two-tailed t test
Thermotolerance in E. coli expressing GaSGT1
E. coli cells expressing the GaSGT1 protein demonstrated more thermotolerance than cells without GaSGT1 expression. As shown in Fig. 3, after exposing cells to 50 °C for 45 min, the survival of mutant E. coli ΔIbpA/B_GaSGT1 cells was 48.5 %, whereas mutant E. coli ΔIbpA/B cell survival decreased to 40.5 %. After incubation at 50 °C for 75 min, E. coli ΔIbpA/B_GaSGT1 cells retained up to 23.5 % viability, whereas E. coli ΔIbpA/B cells showed a 13 % decrease in viability. In addition, at 0 °C, E. coli ΔIbpA/B_GaSGT1 showed 20.7 % viability after incubation for 8 days compared with E. coli ΔIbpA/B cells, which showed a total cell loss. These results demonstrated that the survival rate of E. coli ΔIbpA/B_GaSGT1 was higher than that of E. coli ΔIbpA/B cells at 50 and 0 °C, with and without IPTG induction (data not shown). Thus, these results did not reflect an effect of IPTG, but they more likely reflected the presence of GaSGT1 protein, which promotes thermotolerance in cells.
E. coli thermotolerance experiments. The experiments were performed at a lethal E. coli heat shock at a 50 °C and cold shock at b 0 °C. E. coli MC4100 (WT) and mutated E. coli ΔibpA/B cells harboring Ga SGT1 (ΔIbpA/B_GaSGT1) and without Ga SGT1 (ΔIbpA/B) were cultured and subjected to incubation at 50 °C for 75 min and 0 °C for 8 days. After heat and cold shock treatments, the samples were collected at the indicated times, diluted, and plated on LB plates supplemented with kanamycin. Statistical significance is expressed using a two-tailed t test where P < 0.01
GaSGT1 protects luciferase from heat-induced thermoinactivation
Another characteristic of molecular chaperones is the inhibition of non-native protein aggregation. To examine whether GaSGT1 exhibits this chaperone activity, a quantitative chaperone assay was performed using luciferase as a substrate (Fig. 4). The GaSGT1 purification has been previously described (Yusof et al. 2013). The purified GaSGT1 effectively protected luciferase against heat-induced thermoinactivation. GaSGT1 reduced heat-induced thermoinactivation 60 % after incubation at 41 °C, whereas the luciferase without any prior incubation with GaSGT1 decreased activity 10 % compared with the unheated native luciferase. These results demonstrate that GaSGT1 displays chaperone activity in vitro.
Luciferase aggregation assay. For these experiments, native luciferase was exposed to 41 °C with and without prior incubation with purified GaSGT1. GaSGT1 prevented the total aggregation of luciferase exposed to heat, whereas heated luciferase without any prior incubation with GaSGT1 exhibited an excessive loss
3D structure analysis of GaSGT1
The CS domain of the GaSGT1 (GaSGT1_CS) model was evaluated using PROCHECK, Verify3D and ANOLEA. PROCHECK analysis demonstrated that the constructed GaSGT1_CS model fulfilled the Ramachandran plot, with 97.8 % in the favored region and 2.2 % in the allowed regions. In addition, model verification using Verify3D demonstrated that the constructed GaSGT1_CS model had a perfect score of 100 %. The third evaluation involved the energy calculation of a protein chain using ANOLEA. A low amino acid content of 7.45 % with high energy was observed. The GaSGT1_CS model was acceptable for tertiary structure analysis. The proposed model comprised seven β-sheets in an anti-parallel configuration. When the backbone atoms of the CS domains of GaSGT1 and human SGT1 were superimposed, the RMSD was 0.371 Å. The CS domain in GaSGT1 and human SGT1 were structurally compared according to the tertiary structures of these proteins. The superimposed CS domains from GaSGT1 and human SGT1 (PDB: 1RL1) exhibited alanine substitutions in the “connector” regions. Alanine substitutions are criteria for psychrophilic enzymes, and these substitutions primarily occur at exposed sites (Gianese et al. 2001). Figure 5 shows the amino acid sequence changes in the three-dimensional structure of GaSGT1_CS, which has been modeled on the human CS domain. For the model analysis, the CS domain of GaSGT1 was further analyzed because the CS domain is the main binding domain mediating the interactions between SGT1 and HSP90. Based on the structure of the human CS domain, the K25A, E63A, and T72A (human CS domain residues/position/substitution residue) substitutions were located in the twist region, enabling a 90° turn of the adjacent β-sheet, whereas the H57A and E88A substitutions were located in the loop regions connecting the β-sheets. These substitutions reduced the hydrogen bonds and ionic and aromatic interactions, resulting in a net increase in molecular flexibility. The substitution of lysine with alanine at residue 25 in GaSGT1 (in human SGT1, the position was 162) hindered the lysine-162 hydrogen and aromatic interactions with residues in a distant region of the polypeptide, including phenylalanine-203, lysine-204, and valine-205. The lysine-162 side chains also interacted with glutamic acid-161, asparagine-163, and valine-165 via hydrogen bonds. The substitution of lysine with alanine at position 25 in GaSgt_CS only enabled a hydrogen bond between adjacent residues of alanine-25 and glutamic acid-26. The other alanine substitution occurred at position 57 of the GaSGT1_CS structure (position 194 in the human CS domain) in the loop region between β-sheet 5 and β-sheet 6, which could also introduce modest changes that enhance structural flexibility, likely facilitating conformational adjustments associated with protein binding and decreasing the strength of the inter-protein association. A comparative analysis of the interactions resulting from the alanine substitutions in the CS domain of GaSGT1 was performed through comparisons with the human CS domain (PDB: 1RL1) homologue (Table 2).
Tertiary structures and residue substitutions of the GaSGT1_CS domain. Substitutions in the GaSGT1_CS are labeled in green. The GaSGT1_CS model is colored in blue, whereas the human CS template is shown in red. The dotted boxes represent alanine substitutions in the GaSGT1_CS model and the corresponding residues in the human CS template. This figure was prepared using UCSF Chimera
Discussion
In the present study, we described the thermotolerance and molecular chaperone properties of an SGT1-like protein from G. antarctica against thermal assaults. GaSGT1 mRNA expression was markedly increased following culture at temperatures far from the G. antarctica optimal growth temperature of 12 °C, suggesting that GaSGT1 mRNA expression is stress-induced and might play a protective role in G. antarctica against heat and cold shock stress. Previous studies on the E. coli small heat shock proteins IbpA and IbpB have shown that these proteins play important roles in the protection of heat-denatured proteins against irreversible aggregation (Kuczynska-Wisnik et al. 2002). The results of the analysis conducted in the present study showed that GaSGT1 expression in mutant E. coli ΔibpA/B enhances cell thermotolerance to heat and cold shock assaults, suggesting that GaSGT1 might play a vital role in protecting cells from thermal killing and adopting the properties of HSPs to prevent protein aggregation. Moreover, GaSGT1 prevents luciferase from total thermoinactivation and retains functional activity after thermal assault. Interestingly, GaSGT1 exhibits functional features similar to the E. coli small heat shock proteins IbpA and IbpB, members of the ATP-independent sHSP chaperone family, which protect proteins from thermoinactivation and decrease the efficacy of aggregation in response to temperature assault. However, unlike IbpA/B chaperones, GaSGT1 prevents protein aggregation during temperature assaults as low as 0 °C. GaSGT1 also shows the upregulation of gene expression in cell cultures exposed to 0 °C and negative temperatures. These results might reflect the fact that GaSGT1 possesses molecular chaperone activity and likely plays the same role as HSPs, which recognize and bind unfolded proteins, thus preventing aggregation and functional loss at high and low temperature assaults. Moreover, the amino acid sequences of GaSGT1 lacked the TPR domain, which is highly conserved among nearly all eukaryotic organisms. However, human SGT1 binds to HSP90 and client proteins via the CS domain (Lee et al. 2004), suggesting functional SGT1 without the presence of a TPR domain. Because the TPR domain comprises 3 to 16 tandem repeats of 34 amino acid residues that mediate protein-protein interactions and multiprotein complex assembly (D’Andrea and Regan 2003), the absence of a TPR domain might contribute to structural flexibility by enabling the efficient protein binding and folding of client proteins in a low-energy environment. The sequence alignment of GaSGT1 and SGT1 from other organisms indicated high alanine substitutions, replacing bulky polar residues and charged amino acids. Previous studies have shown that alanine substitutions primarily occur within loops or turns and favor the flexibility of the chain connecting adjacent secondary structures (Feller et al. 2006). In GaSGT1, the alanine substitutions primarily occurred in the loop segments linking the β-sheets or in the twist region, where the adjacent β-sheet turns 90°. These regions act as hinge regions or “connectors” between the two anti-parallel β-sheets, which likely enhance the flexibility of the chains connecting adjacent secondary structures, thereby increasing the protein-binding efficiency and folding of substrates. The results of these analyses indicate that the structure and function of the GaSGT1_CS domain involved in substrate binding might acquire cold adaptation criteria, and this knowledge is beneficial for the rational design of mutations for engineering mesophilic proteins to function optimally in cold temperatures and vice versa.
References
Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K, Schulze-Lefert P (2002) The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295(5562):2073–2076. doi:10.1126/science.1067554
Bakthisaran R, Tangirala R, Rao CM (2015) Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta 1854(4):291–319. doi:10.1016/j.bbapap
Bharudin I, Zaki NZ, Bakar FDA, Mahadi NM, Najimudin N, Illias RM, Murad AMA (2014) Comparison of RNA extraction methods for transcript analysis from the psychrophilic yeast, Glaciozyma antarctica. Malays Appl Biol 43(2):71–79
Boo SY, Wong CMVL, Rodrigues KF, Najimudin N, Murad AMA, Mahadi NM (2013) Thermal stress responses in Antarctic yeast, Glaciozyma antarctica PI12, characterized by real-time quantitative PCR. Polar Biol 36:381–389. doi:10.1007/s00300-012-1268-2
Boshoff A, Nicoll WS, Hennessy F, Ludewig M, Daniel S, Modisakeng KW, Shonhai A, McNamara C, Bradley G, Blatch GL (2004) Molecular chaperone in biology, medicine and protein biotechnology. S Afr J Sci 100:665–677, ISSN: 00382353
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:4. doi:10.1373/clinchem.2008.112797
D’Andrea LD, Regan L (2003) TPR proteins: the versatile helix. Trends Biochem Sci 28(12):655–662. doi:10.1016/j.tibs.2003.10.007
Dubacq C, Guerois R, Courbeyrette R, Kitagawa K, Mann C (2002) Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot Cell 1(4):568–582
Eisenberg D, Luthy R, Bowie JU (1997) Verify3D: assessment of protein models with three-dimensional profiles. Methods Enzymol 277:396–404. doi:10.1016/S0076-6879(97)77022-8
Fell JW, Statzell AC, Hunter IL, Phaff HJ (1969) Leucosporidium gen. n., the heterobasidio mycetous stage of several yeasts of the genus Candida. Antonie Van Leeuwenhoek 35(1):433–462
Feller G, Narinx E, Arpigny JL, Aittaleb M, Baise E, Genicot S, Gerday C (2006) Enzymes from psychrophilic organisms. FEM Microbiol Reviews 18(2–3):189–202. doi:10.1111/j.1574-6976.1996.tb00236.x
Garcia-Ranea JA, Mirey G, Camonis J, Valencia A (2002) p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett 529:162–167. doi:10.1016/S0014-5793(02)03321-5
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: John WM (ed) The proteomics protocols handbook. Humana Press, New Jersey, pp 571–607
Gianese G, Argos P, Pascarella S (2001) Structural adaptation of enzymes to low temperatures. Protein Eng 14:141–148. doi:10.1093/protein/14.3.141
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723. doi:10.1002/elps.1150181505
Hashim N, Bharudin I, Nguong D, Higa S, Bakar F, Nathan S, Rabu A, Kawahara H, Illias R, Najimudin N, Mahadi N, Murad A (2013) Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles 17:63–73. doi:10.1007/s00792-012-0494-4
Kim KK, Kim R, Kim SH (1998) Crystal structure of a small heat shock protein. Nature 394:595–599. doi:10.1038/29106
Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P (1999) SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol Cell 4:21–33. doi:10.1016/S1097-2765(00)80184-7
Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J (2010) Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J 24(10):3633–3642. doi:10.1096/fj.10-156992
Kuczynska-Wisnik D, Kçdzierska S, Matuszewska E, Lund P, Taylor A, Lipinska B, Laskowska E (2002) The Escherichia coli small heat-shock proteins IbpA and IbpB prevent the aggregation of endogenous proteins denatured in vivo during extreme heat shock. Microbiology 148(6):1757–1765. doi:10.1099/00221287-148-6-1757
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. doi:10.1107/S0021889892009944
Lee YT, Jacob J, Michowski W, Nowotny M, Kuznicki J, Chazin WJ (2004) Human Sgt1 binds HSP90 through the CHORD-Sgt1 domain and not the tetratricopeptide repeat domain. J Biol Chem 16(279):16511–16517. doi:10.1074/jbc.M400215200
Melo F, Devos D, Depiereux E, Feytmans E (1997) ANOLEA: a www server to assess protein structures. Proc Int Conf Intell Syst Mol Biol 5:187–190. doi:10.1093/nar/gkh440
Nowotny M, Spiechowicz M, Jastrzebska B, Filipek A, Kitagawa K, Kuznicki J (2003) Calcium-regulated interaction of Sgt1 with S100A6 (calcyclin) and other S100 proteins. J Biol Chem 278:26923–26928. doi:10.1074/jbc.M211518200
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. doi:10.1002/jcc.20084
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33(2):116–120. doi:10.1093/nar/gki442
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 3113:3381–3385. doi:10.1093/nar/gkg520
Sonnhammer EL, Eddy SR, Durbin R (1997) Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28(3):405–420. doi:10.1002/(SICI)1097-0134(199707)28:3<405::AID-PROT10>3.0.CO;2-L
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680. doi:10.1093/nar/22.22.4673
Turchetti B, Hall SRT, Connell LB, Branda E, Buzzini P, Theelen B, Mu¨ller WH, Boekhout T (2011) Psychrophilic yeasts from Antarctica and European glaciers: description of Glaciozyma gen. nov., Glaciozyma martini sp. nov. and Glaciozyma watsonii sp. nov. Extremophiles 15:573–586. doi:10.1007/s00792-011-0388-x
Van Montfort R, Slingsby C, Vierling E (2001) Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem 59:105–156, PMID:11868270
Waters ER, Lee GJ, Vierling E (1996) Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot 47(3):325–338. doi:10.1093/jxb/47.3.325
Yusof NA, Abu Bakar FB, Beddoe T, Murad AMA (2013) Cloning, expression and crystallisation of SGT1 co-chaperone protein from Glaciozyma antarctica. AIP Conf Proc 1571:292. doi:10.1063/1.4858671
Acknowledgments
The authors would like to thank the Ministry of Science, Technology and Innovation, Malaysia (MOSTI), for funding this project under grant numbers 10-05-16-MB002 and 07-05-MGI-GMB014. The authors would also like to thank Prof. Jamie Rossjohn for the use of his lab at Monash University, Australia. Special thanks to Prof. Ewa Laskowska from the Department of Biochemistry, University of Gdansk, Poland, for generously supplying MC4100 wild-type strain and mutated ΔIbpA/B MC4100 for the thermotolerance analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yusof, N.A., Hashim, N.H.F., Beddoe, T. et al. Thermotolerance and molecular chaperone function of an SGT1-like protein from the psychrophilic yeast, Glaciozyma antarctica . Cell Stress and Chaperones 21, 707–715 (2016). https://doi.org/10.1007/s12192-016-0696-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0696-2