Abstract
The crenarchaeon Sulfolobus acidocaldarius, growing optimally at temperatures between 75 and 80 °C, thrives in volcanic hot spring habitats that are typified by large temperature gradients, which impose frequent temperature stresses on the cells. Heat shock response is characterized by an upregulation of heat shock proteins, but similar to most (hyper-)thermophilic archaea, S. acidocaldarius seems to be able to bear supra-optimal temperatures with a restricted repertoire of chaperones. Here, we study the physiological consequences of continuous high-temperature stress and rapid heat shock for S. acidocaldarius. Growth experiments and cell viability assays demonstrate that temperatures of 85 °C and higher result in a decreased growth rate and, when the cells are rapidly subjected to a heat shock, a dynamic increase in mRNA levels of all relevant heat shock proteins and a subset of transcription regulators is observed. When exponentially growing cultures are exposed to a heat shock, the survival tipping point is situated around 90 °C, and the rate of heating determines whether cells are able to cope with this stress or whether the defense mechanism immediately fails, leading to extensive cell death. In conclusion, S. acidocaldarius does not seem to be better equipped to handle sudden supra-optimal temperature stress than mesophilic organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For all living organisms, high-temperature stress compromises cellular and molecular integrity. Therefore, an adequate response to a sudden, rapid temperature rise above the optimal physiological temperature is crucial for the survival and fitness of a species. This so-called heat-shock (HS) response is characterized by an upregulation of HS proteins (HSPs), which protect cellular constituents from irreversible damage. The most prominent class of HSPs are chaperones, protein complexes that bind a wide variety of unfolded proteins to either refold or degrade them (Richter et al. 2010; Lemmens et al. 2018). Remarkably, while bacteria and eukaryotes typically have a quite extensive set of chaperones, (hyper-)thermophilic archaea have a more limited repertoire lacking the HSP70-, HSP90- and HSP100-type chaperones and only harboring small HSPs (sHSPs), prefoldins (PFDs) and HSP60-type chaperones (Lemmens et al. 2018).
Archaeal HSP60, also referred to as the thermosome, is the major chaperone complex of thermoacidophilic Crenarchaeota belonging to the phylogenetic order Sulfolobales (Rani et al. 2018). The thermosome enables an ATP-dependent refolding of partially refolded proteins (Lopez et al. 2015; Chaston et al. 2016). Substrate specificity of the thermosome depends on its subunit composition, which in turn is dependent on the environmental temperature (Chaston et al. 2016). In contrast, sHSPs have no intrinsic refolding abilities themselves. However, in the presence of denaturing proteins, the sHSP-1 oligomer of Sulfolobus acidocaldarius undergoes dissociation into an active dimeric form that acts as a molecular ‘trap’ for unfolded proteins thereby preventing them from aggregation (Laksanalamai and Robb 2004; Kocabiyik 2009; Roy et al. 2018). In addition, sHSP-1 stabilizes membrane lipids, counteracting the effect of temperature on membrane permeability (Roy et al. 2018). Similar to sHSPs, PFDs are ATP-independent chaperones that bind a large variety of denaturing proteins and typically transfer these to the thermosome complex for refolding (Vainberg et al. 1998; Siegert et al. 2000; Okochi et al. 2004; D’Amaro et al. 2008; Ohtaki et al. 2010; Sahlan et al. 2018). The set of HSPs present in Sulfolobales is relatively well conserved, in terms of protein sequence identity and of gene synteny and genomic location of the respective genes (Supplementary Figure S1).
In contrast to bacteria and eukaryotes, the effects of HS on cellular physiology and the associated molecular response resulting in an active HSP machinery are understudied in (hyper-)thermophilic archaea. To our knowledge, the only experiments investigating physiological effects of HS on Sulfolobus spp. were performed with S. shibatae, for which it was observed that shifting the incubation temperature from 70 to 95 °C for 90 min causes cell death of half of the population (Trent et al. 1994). This deleterious effect was strongly diminished when subjecting cells to an adaptive pretreatment at 88 °C, which could be associated with the upregulation of the thermosome β subunit (Thβ) protein (Trent et al. 1990, 1994). Transcriptomic analyses in Archaeoglobus fulgidus, Pyrococcus furiosus and S. solfataricus revealed that a significant genome-wide transcriptional reprogramming is taking place upon HS, with 14%, 26% and 33%, respectively, of all genes exhibiting differential expression (Rohlin et al. 2005; Tachdjian and Kelly 2006; Esteves et al. 2019). This is reflective of a large-scale, dynamic process. Currently, two transcription factors (TFs) involved in regulating HS response are identified in Archaea, HSR1 in A. fulgidus and Phr in P. furiosus, although they do not seem to be the major HS-response regulating TFs since their regulons do not include the thermosome genes (Vierke et al. 2003; Rohlin et al. 2005; Kanai et al. 2009).
Similar to most hyperthermophilic archaea, Sulfolobales seem to be able to bear supra-optimal temperatures with a restricted set of molecular chaperones, a finding that might seem contradictory (Lemmens et al. 2018). It has been hypothesized that (hyper-)thermophilic archaea rely on a less extensive chaperone repertoire upon heat stress because they are already “well-adapted” by having an increased membrane stability and inherent protein stability at high temperatures (Lemmens et al. 2018). This raises the question whether (hyper-)thermophilic archaea such as Sulfolobus spp. can survive larger temperature increases as compared to mesophilic microorganisms. However, a clear definition of HS conditions for Sulfolobus spp. is still missing. Furthermore, although a large-scale dynamic reprogramming is taking place, the molecular mechanisms underlying this HS response are still elusive: for example, a HS-response regulating TF has not been described in Sulfolobus spp.
In this work, we aim to obtain a better understanding of the effect of high-temperature stress on S. acidocaldarius, a model species of the Sulfolobales, in terms of cellular physiology, survival and of transcriptional regulation. To this end, a robust experimental set-up will be established enabling to narrow down the HS temperature range that still enables cell survival. In addition, the effect of a mild 10 °C-HS on transcriptional expression levels of the most important HSPs and a subset of (putative) TFs, hypothesized to be involved in the regulation of the HS response, will be examined. This will lead to a better definition of which conditions elicit a HS response in S. acidocaldarius.
Materials and methods
Strains and growth conditions
Liquid cultures of the uracil-auxotrophic Sulfolobus acidocaldarius strain MW001 (Wagner et al. 2012) were grown aerobically in 20 ml Brock basal salts medium (Brock et al. 1972) supplemented with 0.2% sucrose, 0.1% NZ-Amine and 20 µg ml−1 uracil and acidified to a pH between 3.0 and 3.5 with sulfuric acid, at 75 °C while shaking, unless stated otherwise. Growth was followed by measurement of optical density at 600 nm (OD600). For growth on plates, 0.6% gelrite was used as a solidifying agent of Brock medium with addition of 3 mM CaCl2 and 10 mM MgCl2.
Growth curve measurements
Duplicates or triplicates of 25-ml S. acidocaldarius MW001 cultures were inoculated at an initial OD600 of 0.01 and incubated for at least 110 h in a shaking incubator. Growth kinetics were continuously monitored by the Cell Growth Quantifier (Aquila Biolabs), allowing for non-invasive, real-time and continuous growth measurements by monitoring backscatter every 20 s at 520 nm, with backscatter intensity (measured in arbitrary units) being converted to OD600 by scaling to the initial and final OD600 measurements. Obtained data were fitted to quantify growth parameters. The start- and endpoint for the exponential growth phase was manually determined based on plots of log OD600 versus time, thereby covering a maximal range. As two distinct diauxic growth phases were observed during the exponential growth phase, linear fits were determined using R, based on identifying the ‘switching timepoint’ by maximizing the lowest R2 of both linear fits for each biological replicate (BR) separately (Supplementary Script S1). The specific growth rate (µ) was determined as the slope of these linear fits and the doubling time (td) was determined as ln(2)/µ.
Selection of heat shock set-up
For HS experiments, S. acidocaldarius culture aliquots were transferred to a ThermoMixer® C heat block (Eppendorf) closed with a ThermoTop® (Eppendorf) lid in a 6-well plate (SmartBlock™ plates, Eppendorf) while shaking at 300 rpm (Supplementary Figure S2). Growth at 75 °C was also validated by using 1.5-ml tubes at 500 rpm. Temperatures inside the 6-well plate were measured with a digital pocket thermometer (Traceable, with an accuracy of ± 1 °C), of which the K-type probe was secured in 5 ml Basic Brock medium by a small hole in the lid of the plate in the upper left well.
Spot tests and plating assays
Triplicates of S. acidocaldarius MW001 cultures were grown to the first phase in the diauxic exponential growth (OD600 between 0.3 and 0.5) in a shaking incubator at 75 °C. Per replicate, 5 ml was transferred to two wells of a 6-well plate, placed inside the preheated, closed ThermoMixer at 75 °C while shaking at 300 rpm. After an acclimatization period of between 10 and 15 min, 700-µl aliquots were taken as control samples. After another 10 min, a HS was administered by increasing the temperature of the ThermoMixer. Timepoint zero corresponded to the time at which it was indicated on the ThermoMixer display that the installed temperature was reached. Subsequently, 700-µl samples were quickly taken at defined post-HS timepoints (5, 15, 30 and 60 min), thereby alternating between the two wells in order not to have a profound impact on the culture volume in the wells, and spotted on plates. To this end, all samples were diluted to OD600 0.1 in Basic Brock medium, from which a ten-fold dilution series (10−1–10−6) was made. Spotting was performed by dropping 10 µl of each dilution on a plate. For plating assays, 200 µl of the dilutions of interest was spread on a plate. Plates were incubated during 5–6 days at 75 °C to enable colony formation. Colonies were manually counted at the highest cell concentrations for which single colonies were observed. Based on these counts, colony-forming units (CFUs) per ml were calculated and the percentage of relative survival was derived by calculating the number of surviving cells in the initial OD600 0.1 dilutions and normalizing this to the pre-HS control sample of 75 °C.
Gene expression analysis by quantitative RT-PCR (qRT-PCR)
Triplicates of S. acidocaldarius MW001 cultures were grown to the first phase in the diauxic exponential growth (OD600 0.3–0.4) in a shaking incubator. Per replicate, 5 ml was transferred to two wells of a 6-well plate, placed inside the preheated, closed ThermoMixer at 75 °C while shaking at 300 rpm. After an acclimatization period of between 10 and 15 min, 5 ml of culture was taken out and after another 10 min, the temperature of the ThermoMixer was set to 85 °C, taking about 3.5 min until the installed temperature was given on the display (timepoint zero). After a specific HS duration, another 5-ml aliquot was taken out. Cells were pelleted after stabilization with an equal volume of RNAProtect Reagent (Qiagen) and stored at − 80 °C until RNA extraction. RNA was extracted with the SV Total RNA Isolation System (Promega), followed by removal of residual genomic DNA using a Turbo DNase kit (Ambion Life Technologies). Next, cDNA was prepared from 1 µg RNA with the iScript Select cDNA Synthesis Kit (Bio-Rad). qRT-PCR primers (Supplementary Table S1) were designed with Primer3 software (Untergasser et al. 2012) and tested for efficiency using gDNA as a template.
qRT-PCR reactions of 20 µL were performed in a Bio-Rad iCycler with each reaction mixture containing 1 µl of a 1:10 cDNA dilution, 12.5 μl GoTaq qPCR Master Mix (Promega) and 1.6 pmol of each primer. The following program was performed: 3 min at 95 °C, 40 cycles of 10 s at 95 °C and 30 s at 55 °C. Quantification cycles (CT) were determined with Bio-Rad iQ5 software. In each plate, no-RT controls and no-template controls were analyzed. For each gene, two or three technical replicates were incorporated in the analysis, which in most cases differed no more than 0.5 CT, by taking their average. Relative expression ratios were calculated using the Pfaffl-method (Pfaffl, 2001) with respect to the expression levels of the reference gene Saci_1336 (encoding TATA-binding protein), which was tested for expression stability during HS conditions by employing Bestkeeper software (Pfaffl et al. 2004). Genes with fold changes of ≥ 2 or ≤ 0.5 were considered differentially expressed. Raw data and fold change calculations are given in Supplementary Files 1 and 2.
Results and discussion
Growth curve characterization at different cultivation temperatures
To investigate the effect of high-temperature stress on the initiation and kinetics of growth of S. acidocaldarius, growth was continuously monitored in liquid culture in a non-invasive way at four different temperatures (Fig. 1). We observed a diauxic exponential growth behavior, not only at 75 °C, but also at elevated temperatures with the first exponential growth phase being characterized by shorter doubling times (tds) (Table 1; Supplementary Figure S3). This can be attributed to the preferential utilization of the complex NZ-Amine substrate over defined sugars, as previously observed for S. acidocaldarius (Quehenberger et al. 2019). Growth kinetics were comparable at temperatures of 75 °C and 80 °C (Table 1), both of which were previously reported to be optimal for growth (Brock et al. 1972), however with the total exponential growth phase being slightly extended at 80 °C. For both temperatures, the first exponential growth phase was initiated at around 30 h after inoculation and was characterized by a mean td of 14.8 and 16.1 h at 75 °C and 80 °C, respectively (Table 1). After about 45 h, the second exponential growth phase was initiated (at OD600 of 0.50–0.55), reflected by an increased td of 42.4 and 63.0 h at 75 °C and 80 °C, respectively. The stationary phase was reached at an OD600 of approximately 1.4 after 64 and 74 h at 75 °C and 80 °C, respectively. Whereas cultivation at 80 °C did not elicit a substantial phenotypical response in growth behavior, apart from a slightly increased td in the second exponential phase, it was previously shown to induce a molecular stress response, as demonstrated by the observation of a transcriptional upregulation of the sHSP-1 homolog in S. solfataricus, when cultivated at 80 °C instead of 75 °C (Li et al. 2012).
Growth curves of S. acidocaldarius MW001 in basic Brock medium supplemented with 0.2% sucrose, 0.1% NZ-amine and 20 µg ml−1 uracil at four different growth temperatures (75 °C, 80 °C, 85 °C and 90 °C) as measured by the Aquila Biolabs’ Cell Growth Quantifier. To reduce erroneous measurements originating from high-temperature exposure of the sensor plates, OD600 values were averaged over a time period of five hours for each BR and subsequently for every temperature and plotted in the middle of the time interval (Supplementary Script S2). OD600 values are averages of biological triplicates (75 °C) or duplicates (80 °C, 85 °C and 90 °C). Error bars represent the standard deviation. Vertical dotted lines indicate the ‘switching timepoint’ in the diauxic exponential growth (Table 1; Supplementary Figure S3)
At 85 °C, growth was considerably impaired, with an extended exponential growth phase with an almost doubled td as compared to 75 °C (Fig. 1; Table 1). Although cells grown for 5 days at 85 °C were able to continue growth at 75 °C with improved growth kinetics (µ1 = 0.037 h−1 and td,1 = 19.1 h, µ2 = 0.015 h−1 and td,2 = 48.1 h), long-term exposure to 85 °C caused a long-lasting growth aberration (Supplementary Figure S4). At 90 °C, S. acidocaldarius was unable to initiate growth during the course of five days. These cultures were subsequently incubated for a week at 75 °C, during which no further growth was observed either (Supplementary Figure S4), indicating that the viability of the cells was entirely compromised.
Development of an experimental set-up for heat shock characterization
In contrast to performing growth experiments under constant high-temperature stress, the study of cellular response to dynamic temperature changes poses considerable technical difficulties, especially at high temperatures, because of the following reasons: (i) large temperature differences between the incubator and the room could cause a quick unintended decrease of culture temperature; (ii) small-volume cultures are characterized by high evaporation rates; and (iii) heating of a large-volume incubator requires a significant amount of time. In other words, a critical aspect in the reproducibility of all HS-based experiments is the manner by which the cells are subjected to this high-temperature stress, the so-called ‘HS-set up’. Early HS experiments were typically performed by switching cultures between different water baths or oil baths that were preheated to a particular temperature (Trent et al. 1990, 1994), but this does not allow for precise installations of a particular temperature. Furthermore, it is difficult to maintain a constant temperature in such a set-up, especially during sample-taking. Therefore, we aimed to develop an optimized HS protocol for S. acidocaldarius and opted to use a shaking heat block in combination with tubes or plates (Supplementary Figure S2), to better control the temperature and to minimize the interference of sample taking on the temperature.
To validate this HS-set up, we confirmed active cell growth of S. acidocaldarius at the optimal growth temperature of 75 °C using different culturing methods (Supplementary Figure S5). Transferring 1 ml of an exponentially growing culture to a closed 1.5-ml tube in a preheated, shaking heat block at 75 °C, immediately led to a stagnation of growth, most likely due to lack of aeration. Nevertheless, plating assays revealed that cellular viability was not impaired after culturing the cells during 3 h at 75 °C in these closed tubes (Supplementary Figure S5a). Growing the cultures in tubes with a small hole in the lid resolved the issue of oxygen depletion, demonstrated by active cell growth and an unaffected viability (Supplementary Figure S5b). Nevertheless, evaporation at high temperatures (especially upon rather harsh HS conditions) cannot be excluded. Therefore, by employing another HS-set up, namely a 6-well plate in the shaking heating block, it was possible to increase the culture volume to approximately 5 ml, while at the same time limiting the effects of evaporation. This experimental set-up also resulted in active cell growth with unaffected viability and was selected as the preferred set-up to perform further HS experiments (Supplementary Figure S5c and S8a).
To verify whether the temperature indicated on the heat block’s display corresponds to the temperature sensed by the cells, the actual temperature was monitored by a digital pocket thermometer secured inside the medium (Fig. 2; Supplementary Figures S6 and S7). After acclimatization of the medium at 75 °C, a HS was applied by increasing the temperature setting of the heat block device, thereby avoiding a short cold shock (room temperature) as is often the case when transferring cultures to a water bath. We defined timepoint zero in all HS-experiments as the time at which the desired temperature was reached on the heat block’s display, which varied between 3.5 and 8.5 min, depending on the installed temperature (between 85 and 99 °C). However, the temperature profiles as measured by the pocket thermometer revealed a clear discrepancy between the installed and actual temperature (Fig. 2; Supplementary Figure S7), confirming the complexity of performing the experiment accurately. During the first 15–20 min after it was displayed by the heat block device that the HS-temperature was reached, the temperature of culture medium inside the wells was still increasing (Fig. 2; Supplementary Figure S7). It cannot be excluded that during this period of heating an adaptive response is already initiated. Nevertheless, it has previously been shown that no enhanced survival occurs at higher HS-temperatures after pretreatment of Sulfolobus cells at 88 °C during 15 and 30 min (Trent et al. 1990), therefore, we expect the influence of this heating phase neglectable on the HS response. After between 15 and 20 min, the maximum temperature Tmax inside the culture was reached, after which a relatively stable temperature was maintained (Fig. 2; Supplementary Figure S7). The Tmax temperature was consistently lower than the temperature that was set in the heat block device. Notably, at the highest HS temperatures, 96 °C//92.5 °C and 99 °C//93.5 °C, more fluctuations were observed of the actual temperature. In all the following experiments, we will refer to these HS-temperature profiles based on the actual Tmax in the liquid cultures in the 6-well plate.
Actual HS-temperature profiles of 5 ml basic Brock culture medium in a 6-well plate in a shaking heat block, preheated at 75 °C as measured by a digital pocket thermometer at different timepoints in the experimental set-up. Preheated basic Brock medium (75 °C) was transferred to all wells of a 6-well plate in a shaking, preheated heat block installed at 75 °C. After a 15-min acclimatization period, HS was applied by increasing the temperature of the heat block. Timepoint zero corresponds to the timepoint at which the heat block indicated that the installed temperature was reached, although the actual temperature inside the medium still increases at this timepoint. The symbol legend represents the installed HS-temperature of the device (Tinstalled) and the measured maximal temperature (Tmax) of the medium reached after between 15 and 25 min post HS (Tinstalled//Tmax)
Exploring cellular survival in response to heat shock
Using the developed experimental set-up, we further examined the response of exponentially growing S. acidocaldarius to HS on the phenotypical level of viability by means of spot tests, with the aim of defining the limits of HS-survival and narrowing down the range of viable HS conditions (Fig. 3; Supplementary Figure S8). In contrast to our previous observations at 90 °C, at which we observed no growth over the course of 110 h and no recovery afterwards at 75 °C (Fig. 1; Supplementary Figure S4), the OD600 continues to increase for at least 60 min (comparable to growth at 75 °C) when exponentially growing cultures are gradually heated to a Tmax of 90.5 °C over the course of 20 min (Supplementary Figure S9a), according to the temperature profile in Fig. 2. Spot tests indeed revealed only a subtle effect on the cell viability with still 70% of viable cells after 60 min of HS exposure (Fig. 3a, d), indicating that the defense mechanism of S. acidocaldarius is capable of protecting cellular integrity for a period of up to at least 40 min at 90.5 °C when cells were first actively growing.
Phenotypical response of exponentially growing S. acidocaldarius MW001 cultures exposed to HS, according to the temperature profiles given in Fig. 2. Spot tests were performed at different timepoints (5, 15, 30 and 60 min) after applying a HS. All samples were diluted in a 10-fold dilution series with the starting concentration (10−1) corresponding to OD600 0.1 and 10 µl of each dilution was spotted on one plate per BR. A control sample, grown at 75 °C before HS treatment, is spotted in the first line. Plates were incubated during 5–6 days at 75 °C before colony counting and analysis. The applied HS profiles were, respectively, 90.5 °C (a); 92.5 °C (b) and 93.5 °C (c). A representative BR is shown. d Graphical representation of cell viability tests, with the survival fraction being standardized to the pre-HS sample of 75 °C. Error bars represent the standard deviation
In contrast, when cells are being heated to 92.5 °C over the course of 15-20 min, already two-thirds of cells are killed off (Fig. 3b, d), reflected by a stagnation of growth according to OD600 measurements (Supplementary Figure S9b). Maintaining a plateau temperature of about 92–92.5 °C for another 15 min (timepoint 30 min) results in a further decrease of the relative survival to 17%. Although the temperature inside the cultures dropped to 90.5 °C at 60 min, possibly as a result of evaporation due to the deformation of the lid of the 6-well plate, it can be observed that survival is greatly affected upon continued high-temperature stress of 90.5–92 °C, with no cells surviving at 60 min (Fig. 3b, d). A further increase of the HS temperature with 1 °C from 92.5 °C to 93.5 °C has tremendous effects on cellular survival. Upon comparing with the actual temperature profiles (Fig. 2), we learn that shifting the temperature from 75 to 87.5 °C in 5 min does not impair viability (92.5 °C HS-experiment, Fig. 3b, d), while a more rapid heating from 75 to 90.5 °C in 5 min (93.5 °C HS-experiment, Fig. 3c, d) leads to a large decrease in survival with approximately 80% of the cells being killed off. Upon this rapid heating, S. acidocaldarius’ defense mechanisms immediately fail. Furthermore, the increase in temperature to Tmax 93.5 °C is lethal with only about 0.1% of cells surviving at timepoint 15 min and no cells surviving after 30 min (Fig. 3c, d). Possibly, important HSPs are denatured at these high temperatures. Indeed, both the Thα and Thβ subunits of S. solfataricus have been shown to start unfolding at around 94 °C in vitro (Chaston et al. 2016).
These results demonstrate that the response to HS is a dynamic process in S. acidocaldarius, with not only the HS-temperature being important but also the rate of heating of the culture and the duration of the high-temperature stress. This is nicely illustrated by comparing the experiment in which S. acidocaldarius is exposed to a gradual heating to 90.5 °C over the course of 15 min in the 90.5 °C HS-experiment (Fig. 2), in which survival is only decreased to 85% (Fig. 3a, d), with the more rapid heating to 90.5 °C over a course of 5 min in the 93.5 °C HS-experiment (Fig. 2), in which only 20% of the cells survive (Fig. 3c, d). Secondly, maintaining a higher plateau temperature for 45 min, such as in the 92.5 °C temperature profile (Fig. 2), completely kills off the remaining 33% of cells (Fig. 3b, d). The observation that half of the population of S. shibitae cells survives a severe 95 °C-HS during 90 min (Trent et al. 1994) might be explained by the fact that these experiments employed a less robust HS medium, possibly a water or oil bath, and other volumes of culture.
In conclusion, the cell viability tests confirm the results of the growth rate measurements by demonstrating that the tipping point of cell survival of S. acidocaldarius is situated around a temperature of 90 °C, which is between 10 and 15 °C above the optimal growth temperature. Such growth dynamics are comparable to those observed for the mesophilic bacterium Escherichia coli, growing optimally at 37 °C and for which strongly impaired growth was observed upon an increase in cultivation temperature of 7–8 °C and for which cultivation at 47 °C results in no growth (Nguyen 2006; Mordukhova et al. 2008; Van Derlinden et al. 2008). Furthermore, the tipping point of cell survival for E. coli also seems to be situated around 15 °C above the optimal growth temperature, since rapidly heat shocking cells from 37 to 50 °C does not impair survival for at least 20 min, but switching to 54 °C is lethal after 15 min, with approximately only 0.3% of cells surviving (Marcén et al. 2017).
Transcriptional expression of important heat shock proteins upon heat shock
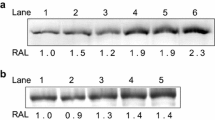
To obtain insights into the transcriptional regulation of the HS response of S. acidocaldarius, we examined the relative transcriptional expression of some of the most important HSPs and of (putative) TFs upon high-temperature stress by qRT-PCR (Figs. 4 and 5; Supplementary Tables S2 and S3; Supplementary Files 1 and 2). We decided to subject the cells to a rather mild high-temperature stress, in order not to incorporate variations in gene expression due to cell death. This strategy has previously been used for HS-response gene expression studies in S. solfataricus and P. furiosus (Tachdjian & Kelly, 2006; Esteves et al. 2019). According to the above-described growth and cell survival experiments, we considered that a 10 °C-HS of 84 °C (corresponding to the “85 °C//84 °C”-temperature profile in Fig. 2) was of interest as cells experience high-temperature stress at this cultivation temperature, reflected by an increased td (Fig. 1; Table 1), but not resulting in a loss in cell viability as demonstrated by the spot tests (Fig. 3; Supplementary Figure S8). Due to the complex nature of HS response, a large biological variation was observed in the differential gene expression levels of both HSPs and TFs. Therefore, fold change (FC) values for each gene of each separate biological replicate (BR) are given in Supplementary Tables S2 and S3. Nevertheless, substantial trends can be observed for genes that are differentially expressed in all BRs (FC ≥ 2 or ≤ 0.5) (annotated by a star in Figs. 4 and 5).
Relative gene expression of HSP-encoding genes after 15, 30 and 60 min of subjecting cells to a 84 °C-HS, according to the temperature profile given in Fig. 2. Error bars represent the standard deviation. Stars indicate the genes which are considerably differentially expressed (≥ 2 or ≤ 0.5) in all BRs (Supplementary Table S2)
Relative gene expression of putative TF encoding genes after 15, 30 and 60 min of 84 °C HS, according to the temperature profile given in Fig. 2. Error bars represent the standard deviation. Stars indicate the genes which are considerably differentially expressed (≥ 2 or ≤ 0.5) in all BRs (Supplementary Table S3)
The effect of a mild HS on transcriptional expression was investigated for a set of eight important HSP genes: the three thermosome subunits (Thα, Thβ and Thγ), the two small HSPs (sHSP-1 and sHSP-2), an HtpX protease/chaperone (HtpX) and the two PFD subunits (Pfdα and Pfdβ) (Fig. 4; Supplementary Table S2). After 15 min of HS treatment, during which the cultures were exposed to rapid heating (Fig. 2), a trend of increased mRNA levels was observed for some HSP-encoding genes, with Thα being upregulated in all BRs. At 30 min, when a constant temperature of 84 °C was maintained for 10–15 min (Fig. 2), an extensive upregulation was observed for all HSP-encoding genes except Thγ, with notably high FC ratios in all BRs for Thα, Thβ and sHSP-1 (FC of 72.9, 29.7 and 47.3, respectively) (Fig. 4; Supplementary Table S2). After 60 min, when the HS-temperature persisted for approximately 45 min (Fig. 2), most HSPs are still transcriptionally upregulated with the exception of sHSP-2 and Pfdβ, however, with a less pronounced differential expression as compared to the 30-minutes timepoint. These results suggest that cells need to be exposed for at least 15 min to a steady, constant high-temperature stress of 84 °C (Fig. 2) to have a sufficient transcriptional upregulation of their HSPs, which can last up to at least 45 min, depending on the HSP.
The thermosome of S. solfataricus is characterized by a differential subunit composition in response to temperature, which is assumed to be crucial for its functioning upon temperature shocks (Chaston et al. 2016). While an octameric structure composed of alternating Thα and Thβ subunits is present at the optimal growth temperature, a translational upregulation of the β-subunit (Trent et al. 1990) leads to an all-β nonameric thermosome at HS temperatures of 85 °C (Chaston et al. 2016). The observed increase in transcripts encoding both the Thα and Thβ subunits upon a 10 °C-HS in S. acidocaldarius (Fig. 4; Supplementary Table S2) is remarkable, as transcriptional regulation of Thα and Thβ was not observed in a similar HS experiment of S. solfataricus (Tachdjian & Kelly, 2006). On the other hand, in S. shibatae, Thα and Thβ were shown to be upregulated upon HS on both the transcriptional and translational level (Kagawa et al. 2003). In contrast to Thα and Thβ, transcriptional expression of Thγ is not influenced by HS (Fig. 4; Supplementary Table S2). In S. solfataricus, the homologous subunit is heat-labile and a prominent part of the “cold-shock thermosome” (Chaston et al. 2016) and was shown to be transcriptionally downregulated immediately after HS (Tachdjian and Kelly 2006). Whether the individual subunits of the S. acidocaldarius thermosome are also regulated post-transcriptionally, translationally or post-translationally and whether the exact composition of the thermosome complex resembles that of S. solfataricus in these conditions (Chaston et al. 2016), remains to be studied.
The observed transcriptional upregulation of the Pfdα and Pfdβ genes after 30 min of HS treatment (Fig. 4; Supplementary Table S2) was not found for the homologs in S. solfataricus (Tachdjian & Kelly, 2006; D’Amaro et al. 2008). Although their upregulation was less pronounced than that of Thα and Thβ, it might be indirectly linked, keeping in mind that the function of PFD is intertwined with the chaperone functioning of the thermosome complex (Vainberg et al. 1998; Okochi et al. 2004; Ohtaki et al. 2010; Lemmens et al. 2018).
Transcriptional expression of putative transcription factors upon heat shock
Since it is currently unknown how HSP gene expression is regulated, genes encoding interesting putative transcriptional regulators were selected based on an extensive differential expression after a 10 °C-HS of their S. solfataricus homologs in a microarray transcriptomic analysis (Tachdjian and Kelly 2006). Eight genes encoding putative regulators, belonging to a variety of TF-families, were subsequently tested for differential expression in S. acidocaldarius at 15, 30 and 60 min post 84 °C HS (Figs. 2, 5; Supplementary Table S3). Of these, four genes (Saci_1851, Saci_0446, Saci_0102 and Saci_1242) are significantly upregulated and one gene (Saci_1012) is significantly downregulated after HS. The dynamic response of these TF-encoding genes is similar to that of HSP-encoding genes, with the highest upregulation occurring at 30 min after HS, or even slower, with the maximal differential expression only occurring at 60 min post-HS, as observed for Saci_0102, Saci_1242 and Saci_1012. Such a slow dynamic behavior would be unexpected for TFs directly responsible for the regulation of structural genes involved in HS response, such as HSP-encoding genes, for which a fast regulation in response to HS is required.
A late transcriptional response to HS is most explicit for Saci_1012, which shows a strong transcriptional downregulation at 60 min after HS (FC of 0.02) (Fig. 5; Supplementary Table S3), in agreement with the transcriptional response of the homologous gene in S. solfataricus (Tachdjian and Kelly 2006). Saci_1012 encodes an uncharacterized DNA-binding protein with a putative helix-turn-helix motif, annotated as a transcriptional regulator of an unknown family and function. Given that the expression levels of Saci_1012 were only responsive to HS after 60 min, it is plausible to hypothesize that this protein is not directly involved in the regulation of HSPs, which need to protect protein integrity and function immediately after HS exposure. Nevertheless, the effect of HS on the transcriptional expression level of this putative DNA-binding protein is remarkable, possibly indicative of an indirect relationship between the protein’s function and HS response.
Several Lrs14-family members showed a differential transcriptional level upon HS: Saci_0446, Saci_0102 and Saci_1242 (Fig. 5; Supplementary Table S3). Expression of Saci_0446, encoding the Lrs14-family biofilm regulator AbfR1 (Orell et al. 2013), displayed the highest upregulation after HS (FC of 31.5 after 30 min) (Fig. 5; Supplementary Table S3). This DNA-binding protein binds in a non-sequence specific to the DNA (Napoli et al. 2001; Orell et al. 2013; Li et al. 2017). Based on the observations that Lrs14 members have been retrieved from independently performed pulldown assays using Sulfolobus crude cell extracts and seem to be involved in a variety of cellular processes (Napoli et al. 2001; Fiorentino et al. 2003; Kessler et al. 2006; Abella et al. 2007), it was cautiously postulated that Lrs14 regulators might rather be chromatin-organizing proteins than transcriptional regulators and regulate transcription indirectly (Karr et al. 2017). Previously, it was demonstrated that heat and cold shock influences the overall topology of the DNA in hyperthermophilic archaea leading to, respectively, positively supercoiled and negative supercoiled DNA (López-García and Forterre 2000). Interestingly, the AbfR1 orthologue in S. solfataricus, Smj12, is able to introduce positive supercoiling in DNA and is capable of stabilizing double-strand DNA against thermodenaturation (Napoli et al. 2001). Since it is known that DNA-binding proteins provide a helper role in the control of DNA topology during thermal stress (López-García et al. 1998; López-García and Forterre 2000), we hypothesize that Lrs14-like proteins might play a role in controlling DNA topology upon heat stress and in the stabilization of double-stranded DNA against thermodenaturation, rather than specifically regulating the expression of genes involved in HS response such as those encoding molecular chaperones.
Saci_1851 is the only gene which displayed an upregulation already after 15 min of HS treatment in all BRs and that showed a high upregulation upon thermal stress, peaking at 30 min (FC of 12.1 and 28.3, respectively) (Fig. 5; Supplementary Table S3). This gene encodes YtrASa, a GntR-like regulator belonging to the YtrA subfamily with a highly restricted regulon, consisting of two (putative) membrane protein-encoding genes (Lemmens et al. 2019). Since it does not regulate a HSP, not directly nor indirectly (Lemmens et al. 2019), the reason for this strong upregulation after HS remains unclear. Although the function of these membrane proteins is not unraveled, there is no direct link with thermal stress apparent.
Finally, the effect of HS on the other investigated TF-encoding genes Saci_0800 and Saci_1107 is not that pronounced (Fig. 5; Supplementary Table S3). Saci_0800, encoding an uncharacterized ArsR-family member, is not downregulated like its homolog in S. solfataricus (Tachdjian & Kelly, 2006) and seems not to be differentially expressed in S. acidocaldarius. Likewise, Saci_1107, encoding the TetR-family regulator FadRSa (Wang et al. 2019), seems not to be differentially expressed upon HS in S. acidocaldarius, while a strong upregulation upon HS was observed for its homolog in S. solfataricus (Tachdjian & Kelly, 2006). FadRSa represses a 30-kb gene cluster encoding enzymes involved in fatty acid metabolism (Wang et al. 2019; Takemata et al. 2019). In bacteria, there is a clear link between fatty acid metabolism and heat stress with the latter affecting the fluidity of the fatty-acid composed cell membrane through modification of the membrane lipid composition (Juneja et al. 1998; Mejía et al. 1999; Haddaji et al. 2015). In contrast to bacteria, the archaeal cell membrane is not composed of fatty acids but of isoprenoid-based hydrocarbon chains (Koga 2012). The function of the extensive fatty acid metabolism genes in the archaeal cell remains unclear, but since FadRSa seems not differentially expressed upon HS, we expect that fatty acids do not play an important role in the HS response of Sulfolobus cells.
Taken together, the results of the qRT-PCR suggest that a HS of 85 °C is sensed by the cells, reflected by an increase in mRNA levels of all relevant HSPs and some TFs, mainly after 15 min of steady and constant high-temperature stress (30 min post HS in our set-up). Since none of the investigated TFs seem to specifically regulate HSP gene expression, the question still remains whether there is indeed a direct HS-regulating TF present in S. acidocaldarius or whether the increase in mRNA levels of the HSPs is due to other causes, such as effects of HS on RNA stability. Nevertheless, due to the complexity of the HS response, it seems very plausible that other regulatory processes are at least equally important as transcriptional regulation in finetuning the expression levels of HSPs, whether it is post-transcriptional, translational or post-translational.
Conclusions
With this work, we were able to define the consequences of high-temperature stress for the thermophilic crenarchaeon S. acidocaldarius. By performing growth experiments at high cultivation temperatures and HS-experiments using a well-validated HSset-up, it was found that temperature stress results in a molecular response by the cells at temperatures of 85 °C and higher. When cultures are rapidly heated, this is characterized by increased transcript levels of all important HSPs and a subset of transcriptional regulators, whose link to the HS response is not apparent or possibly indirect. Using spot tests, we showed that when exponentially growing cultures at 75 °C are exposed to HS, the tipping point is situated around 90 °C, which is between 10 and 15 °C above the optimal growth temperature. When cultures are gradually heated to 90.5 °C over the course of 10–20 min, cells are able to cope with this stress. In contrast, the defense mechanism immediately fails when cultures are more rapidly heated to 90.5 °C, resulting in the death of approximately 75% of cells. Therefore, S. acidocaldarius does not seem to be better equipped to handle sudden high-temperature stress than mesophilic organisms. Since in most previous studies of HS response in thermophilic archaea, a clear description of the technical set-up for exposing cells to temperature stress is lacking, possibly complicating the reproducibility of results, we would like to emphasize the importance of using a well-defined HSset-up. This is underlined by the exceptional dynamic nature of the response to HS on both the physiological and transcriptional level as described in this work.
Abbreviations
- BR:
-
Biological replicate
- HS:
-
Heat shock
- HSP:
-
Heat shock protein
- Pfdα:
-
Prefoldin subunit α
- Pfdβ:
-
Prefoldin subunit β
- sHSP:
-
Small heat shock protein
- t d :
-
Doubling time
- TF:
-
Transcription factor
- Thα:
-
Thermosome subunit α
- Thβ:
-
Thermosome subunit β
- Thγ:
-
Thermosome subunit γ
- µ :
-
Specific growth rate
References
Abella M, Rodriguez S, Paytubi S, Campoy S, White MF, Barbe J (2007) The Sulfolobus solfataricus radA paralogue sso0777 is DNA damage inducible and positively regulated by the Sta1 protein. Nucleic Acids Res 35:6788–6797. https://doi.org/10.1093/nar/gkm782
Brock TD, Brock KM, Belly RT, Weiss RL (1972) Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol 84:54–68. https://doi.org/10.1007/BF00408082
Chaston JJ, Smits C, Aragão D, Wong ASW, Ahsan B, Sandin S, Molugu SK, Molugu SK, Bernal RA, Stock D, Stewart AG (2016) Structural and functional insights into the evolution and stress adaptation of type II Chaperonins. Structure 24:364–374. https://doi.org/10.1016/j.str.2015.12.016
D’Amaro A, Valenti A, Napoli A, Rossi M, Ciaramella M (2008) The Prefoldin of the Crenarchaeon Sulfolobus solfataricus. Protein Pept Lett 15:1055–1062. https://doi.org/10.2174/092986608786071094
Esteves AM, Graça G, Peyriga L, Torcato IM, Borges N, Portais JC, Santos H (2019) Combined transcriptomics–metabolomics profiling of the heat shock response in the hyperthermophilic archaeon Pyrococcus furiosus. Extremophiles 23:101–118. https://doi.org/10.1007/s00792-018-1065-0
Fiorentino G, Cannio R, Rossi M, Bartolucci S (2003) Transcriptional regulation of the gene encoding an alcohol dehydrogenase in the archaeon Sulfolobus solfataricus involves multiple factors and control elements. J Bacteriol 185:3926–3934. https://doi.org/10.1128/JB.185.13.3926-3934.2003
Haddaji N, Mahdhi AK, Krifi B, Ben Ismail M, Bakhrouf A (2015) Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol Lett 362:1–7. https://doi.org/10.1093/femsle/fnv047
Juneja VK, Foglia TA, Marmer BS (1998) Heat resistance and fatty acid composition of Listeria monocytogenes: effect of pH, acidulant, and growth temperature. J Food Prot 61:683–687. https://doi.org/10.4315/0362-028X-61.6.683
Kagawa HK, Yaoi T, Brocchieri L, McMillan RA, Alton T, Trent JD (2003) The composition, structure and stability of a group II chaperonin are temperature regulated in a hyperthermophilic archaeon. Mol Microbiol 48:143–156. https://doi.org/10.1046/j.1365-2958.2003.03418.x
Kanai T, Takedomi S, Fujiwara S, Atomi H, Imanaka T (2009) Identification of the Phr-dependent heat shock regulon in the hyperthermophilic archaeon, Thermococcus kodakaraensis. J Biochem 147:361–370. https://doi.org/10.1093/jb/mvp177
Karr EA, Isom CE, Trinh V, Peeters E (2017) Chapter 2: Transcription factor-mediated gene regulation in Archaea. In: Clouet-d’Orval B (ed) RNA metabolism and gene expression in Archaea. Springer International Publishing, Switzerland, pp 27–69
Kessler A, Sezonov G, Inaki Guijarro J, Desnoues N, Rose T, Delepierre M, Bell SD, Prangishvili D (2006) A novel archaeal regulatory protein, Sta1, activates transcription from viral promoters. Nucleic Acids Res 34:4837–4845. https://doi.org/10.1093/nar/gkl502
Kocabiyik S (2009) Essential structural and functional features of small heat shock proteins in molecular chaperoning process. Protein Pept Lett 16:613–622. https://doi.org/10.2174/092986609788490249
Koga Y (2012) Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea. https://doi.org/10.1155/2012/789652
Laksanalamai P, Robb FT (2004) Small heat shock proteins from extremophiles: a review. Extremophiles 8:1–11. https://doi.org/10.1007/s00792-003-0362-3
Lemmens L, Baes R, Peeters E (2018) Heat shock response in archaea. Emerg Top Life Sci 2:581–593. https://doi.org/10.1042/ETLS20180024
Lemmens L, Tilleman L, De Koning E, Valegård K, Lindås A-C, Van Nieuwerburgh F, Maes D, Peeters E (2019) YtrASa, a GntR-family transcription factor, represses two genetic loci encoding membrane proteins in Sulfolobus acidocaldarius. Front Microbiol 10:1–15. https://doi.org/10.3389/fmicb.2019.02084
Li D, Yang F, Lu B, Chen D, Yang W-J (2012) Thermotolerance and molecular chaperone function of the small heat shock protein HSP20 from hyperthermophilic archaeon, Sulfolobus solfataricus P2. Cell Stress Chaperones 17:103–108. https://doi.org/10.1007/s12192-011-0289-z
Li L, Banerjee A, Bischof LF, Maklad HR, Hoffmann L, Henche AL, Veliz F, Bildl W, Schulte U, Orell A, Essen LO, Peeters E, Albers SV (2017) Wing phosphorylation is a major functional determinant of the Lrs14-type biofilm and motility regulator AbfR1 in Sulfolobus acidocaldarius. Mol Microbiol 105:777–793. https://doi.org/10.1111/mmi.13735
Lopez T, Dalton K, Frydman J (2015) The mechanism and function of group II chaperonins. J Mol Biol 427:2919–2930. https://doi.org/10.1016/j.jmb.2015.04.013.The
López-García P, Forterre P (2000) DNA topology and the thermal stress response, a tale from mesophiles and hyperthermophiles. BioEssays 22:738–746. https://doi.org/10.1002/1521-1878(200008)22:8%3c738:AID-BIES7%3e3.0.CO;2-5
López-García P, Knapp S, Ladenstein R, Forterre P (1998) In vitro DNA binding of the archaeal protein Sso7d induces negative supercoiling at temperatures typical for thermophilic growth. Nucleic Acids Res 26:2322–2328. https://doi.org/10.1093/nar/26.10.2322
Marcén M, Ruiz V, Serrano MJ, Condón S, Mañas P (2017) Oxidative stress in E. coli cells upon exposure to heat treatments. Int J Food Microbiol 241:198–205. https://doi.org/10.1016/j.ijfoodmicro.2016.10.023
Mejía R, Gómez-Eichelmann MC, Fernández MS (1999) Fatty acid profile of Escherichia coli during the heat-shock response. Biochem Mol Biol Int 47:835–844. https://doi.org/10.1080/15216549900201923
Mordukhova EA, Lee HS, Pan JG (2008) Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl Environ Microbiol 74:7660–7668. https://doi.org/10.1128/AEM.00654-08
Napoli A, Kvaratskelia M, White MF, Ciaramella M (2001) A novel member of the bacterial-archaeal regulator family is a nonspecific DNA-binding protein and induces positive supercoiling. J Biol Chem 276:10745–10752. https://doi.org/10.1074/jbc.M010611200
Nguyen MT (2006) The effect of temperature on the growth of the bacteria Escherichia coli DH5α. Saint Martin’s Univ Biol J 1:87–94
Ohtaki A, Noguchi K, Yohda M (2010) Structure and function of archaeal prefoldin, a co-chaperone of group II chaperonin. Front Biosci 15:708–717. https://doi.org/10.2741/3641
Okochi M, Nomura T, Zako T, Arakawa T, Iizuka R, Ueda H, Funatsu T, Leroux M, Yohda M (2004) Kinetics and binding sites for interaction of the prefoldin with a group II chaperonin. J Biol Chem 279:31788–31795. https://doi.org/10.1074/jbc.M402889200
Orell A, Peeters E, Vassen V, Jachlewski S, Schalles S, Siebers B, Albers S-V (2013) Lrs14 transcriptional regulators influence biofilm formation and cell motility of Crenarchaea. ISME J 7:1886–1898. https://doi.org/10.1038/ismej.2013.68
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:2002–2007. https://doi.org/10.1016/S0043-1354(98)00516-8
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestkeeper—excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. https://doi.org/10.1023/B:BILE.0000019559.84305.47
Quehenberger J, Albersmeier A, Glatzel H, Hackl M, Kalinowski J, Spadiut O (2019) A defined cultivation medium for Sulfolobus acidocaldarius. J Biotechnol 301:56–67. https://doi.org/10.1016/j.jbiotec.2019.04.028
Rani S, Sharma A, Goel M (2018) Insights into archaeal chaperone machinery: a network-based approach. Cell Stress Chaperones 23:1257–1274. https://doi.org/10.1007/s12192-018-0933-y
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266. https://doi.org/10.1016/j.molcel.2010.10.006
Rohlin L, Trent JD, Salmon K, Kim U, Gunsalus RP, Liao JC (2005) Heat shock response of Archaeoglobus fulgidus. J Bacteriol 187:6046–6057. https://doi.org/10.1128/JB.187.17.6046-6057.2005
Roy M, Gupta S, Patranabis S, Ghosh A (2018) The oligomeric plasticity of Hsp20 of Sulfolobus acidocaldarius protects environment-induced protein aggregation and membrane destabilization. BBA Biomembr 1860:2549–2565. https://doi.org/10.1016/j.bbamem.2018.09.005
Sahlan M, Zako T, Yohda M (2018) Prefoldin, a jellyfish-like molecular chaperone: functional cooperation with a group II chaperonin and beyond. Biophys Rev 10:339–345. https://doi.org/10.1007/s12551-018-0400-0
Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I (2000) Structure of the molecular chaperone prefoldin: unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell 103:621–632. https://doi.org/10.1016/s0092-8674(00)00165-3
Tachdjian S, Kelly RM (2006) Dynamic metabolic adjustments and genome plasticity are implicated in the heat shock response of the extremely thermoacidophilic archaeon Sulfolobus solfataricus. J Bacteriol 188:4553–4559. https://doi.org/10.1128/JB.00080-06
Takemata N, Samson RY, Bell SD (2019) Physical and functional compartmentalization of archaeal chromosomes. Cell 179:165–179.e18. https://doi.org/10.1016/j.cell.2019.08.036
Trent JD, Osipiuk J, Pinkau T (1990) Acquired thermotolerance and heat shock in the extremely thermophilic archaebacterium Sulfolobus sp. Strain B12. J Bacteriol 172:1478–1484
Trent JD, Gabrielsen M, Jensen B, Neuhard J, Olsen J (1994) Acquired thermotolerance and heat shock proteins in thermophiles from the three phylogenetic domains. J Bacteriol 176:6148–6152. https://doi.org/10.1128/jb.176.19.6148-6152.1994
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3-new capabilities and interfaces. Nucleic Acids Res 40:1–12. https://doi.org/10.1093/nar/gks596
Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J, Klein HL, Cowan NJ (1998) Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell 93:863–873. https://doi.org/10.1016/S0092-8674(00)81446-4
Van Derlinden E, Bernaerts K, Van Impe JF (2008) Dynamics of Escherichia coli at elevated temperatures: effect of temperature history and medium. J Appl Microbiol 104:438–453. https://doi.org/10.1111/j.1365-2672.2007.03592.x
Vierke G, Engelmann A, Hebbeln C, Thomm M (2003) A novel archaeal transcriptional regulator of heat shock response. J Biol Chem 278:18–26. https://doi.org/10.1074/jbc.M209250200
Wagner M, Van Wolferen M, Wagner A, Lassak K, Meyer BH, Reimann J, Albers S (2012) Versatile genetic tool box for the crenarchaeote Sulfolobus acidocaldarius. Front Microbiol 3:1–12. https://doi.org/10.3389/fmicb.2012.00214
Wang K, Sybers D, Maklad HR, Lemmens L, Lewyllie C, Zhou X, Schult F, Bräsen C, Siebers B, Valegård K, Lindås AC, Peeters E (2019) A TetR-family transcription factor regulates fatty acid metabolism in the archaeal model organism Sulfolobus acidocaldarius. Nat Commun 10:1–16. https://doi.org/10.1038/s41467-019-09479-1
Acknowledgements
We are grateful to David Sybers for insightful discussions regarding S. acidocaldarius growth curves. We thank Aquila Biolabs for their assistance in setting up a high-temperature on-line growth measurement system. This research was supported by the Research Council of the Vrije Universiteit Brussel and the Research Foundation Flanders (FWO-Vlaanderen) (PhD fellowship 1134419N to Rani Baes, research grant 1526418N and research project G021118).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Moracci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baes, R., Lemmens, L., Mignon, K. et al. Defining heat shock response for the thermoacidophilic model crenarchaeon Sulfolobus acidocaldarius. Extremophiles 24, 681–692 (2020). https://doi.org/10.1007/s00792-020-01184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-020-01184-y