Abstract
Treatment with inhaled carbon monoxide (CO) has been shown to ameliorate intestinal injury induced by lipopolysaccharide (LPS) or ischemia-reperfusion in experimental animals. We hypothesized that CO intraperitoneal administration (i.p) might provide similar protection against inhaled gas. In the present study, 1 h after intravenously receiving 5 mg/kg LPS, rats were exposed to either room air or 2 ml/kg of 250 ppm CO i.p for 1, 3, and 6 h. Intestinal tissues were collected to determine the levels of platelet activator factor (PAF), intercellular adhesion molecule-1 (ICAM-1), interlukin-10 (IL-10), maleic dialdehyde (MDA), cell apoptotic rate and the phosphorylated p38 mitogen activated protein kinase (MAPK), as well as myeloperoxidase (MPO) and superoxide dismutase (SOD) activity. After CO i.p, the increase of PAF, ICAM-1, MDA, MPO, and cell apoptosis rate induced by LPS was markedly reduced (P < 0.05 or 0.01), while the decrease of IL-10 and SOD was significantly increased (P < 0.05). Western blotting showed that the effects of CO i.p were mediated by p38 MAPK pathway. Thus, the results of our study show that CO i.p exerts potent protection against LPS induced injury to the intestine via anti-oxidant, anti-inflammation and anti-apoptosis, which may involve the p38 MAPK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The intestine is still considered to be the critical organ in the development of multiple organ dysfunction (Moore 1998) that is a major cause of morbidity and mortality in intensive care units (Nieuwenhuijzen and Goris 1999). Although the pathophysiological basis of intestine dysfunction induced by lipopolysaccharide (LPS) is not completely understood, there is increasing evidence that peroxidize damage, deregulated inflammatory response, and cell apoptosis play a primary role (Nieuwenhuijzen and Goris 1999; Gibbon et al. 2004). How to protect the intestine from LPS-induced injury remains of considerable interest, and numerous pharmacological strategies to ameliorate this problem have been investigated (Delaney et al. 2005; Wolff et al. 2004).
Compelling evidence during the last few years indicates that endogenous carbon monoxide (CO), a major byproduct of heme catalysis by heme oxygenase (HO)-1, not only serves as a signal molecule for mediating the integrity of the physiological function of organs, but also exerts anti-oxidative, anti-inflammatory, and anti-apoptotic effects (Wagener et al. 2003; Bauer et al. 2008). Recent data also showed that inhalation of low concentrations of CO has been shown to ameliorate intestinal injury induced by LPS or ischemia-reperfusion (IR) in experimental animals (Liu et al. 2007; Ryter and Choi 2007; Nakao et al. 2003; Zuckerbraun et al. 2005). Conversely, the injury can be exacerbated by blockading the effects of inhaled CO (Nakao et al. 2006a, b).
The cytoprotective effects of CO have generated considerable interest in using CO as a therapeutic agent to prevent intestinal injury induced by a number of diseases (Liu et al. 2007; Ryter and Choi 2007; Nakao et al. 2003; Zuckerbraun et al. 2005). However, these results were acquired by inhaled CO and have some inherent problems due to CO inhalation. Thus, other approaches for using CO as a therapeutic agent are warranted. To develop a more practical and safe means of delivering the gas, we performed this present study in which 2 ml/kg of 250 ppm CO was infused into peritoneal lavage for testing whether there exists a similar protection to inhaled gas against intestinal injury induced by LPS. One hour after receiving LPS, rats were treated with either room air or 250 ppm CO at a single dose of 2 ml/kg intrapertioneal administration (i.p), the effects of CO were observed and its physiologic mechanisms were investigated. Since the mitogen-activated protein kinase (MAPK) pathways play an important role in mediating the expression of a number of inflammatory cytokines and cell apoptosis (Arbabi and Maier 2002), we monitored the potential signal pathway by which CO administration might confer protection against LPS induced injury.
2 Materials and methods
2.1 Animal care
Seventy-two adult (6 months old) healthy male Sprague Dawley rats were used in this study. They were obtained from a licensed laboratory animal vendor (Animals Experimental Center of Nanjing Medical University, Grade II, Certificate No. NMXK2004-0018), and weighed 200-250 g. The research protocol complied with the regulations regarding animal care as published by the National Institutes of Health and was approved by the Institutional Animal Use and Care Committee of the Nanjing Medical University. Rats were acclimatized in the animal room for a week, were fed standard rat chow and water and maintained on a 12:12 h light-dark photoperiod in an environment-controlled room (22.0 ± 0.5°C, 55.0 ± 5.0% relative humidity).
2.2 Experimental design
Rats were randomly divided into control, CO i.p, LPS, and LPS + CO i.p four groups (n = 18), and each group was subdivided into three subgroups according to the time after administration of room air or 250 ppm CO (1, 3, and 6 h). All animals were anesthetized with pentobarbital (30 mg/kg i.p). One hour after intravenously receiving 5 mg/kg LPS (Escherichia coli, Serotype O111: B4, Sigma), the rats in the LPS group and LPS + CO i.p group were treated with room air and 2 ml/kg of 250 ppm CO i.p, respectively, and the rats in the control group and CO i.p group intravenously received an equal volume of 0.9% NaCl and 1 h later, were exposed to room air and 2 ml/kg of 250 ppm CO i.p, respectively. The CO gas was at a concentration of 250 ppm with balanced air compressed in a stainless steel mixing cylinder (Nanjing Special Gas Co., China). The concentration of CO in the cylinder was determined using a CO analyzer (Taiyo Instruments Inc., Osaka, Japan).
2.3 Sample collection
Animals were sacrificed by rapid abdominal aorta exsanguination under pentobarbital (20 mg/kg i.p) anesthesia at the indicated time points (six rats, once). The first 3-cm long segment of ileum and the first 3-cm long segment of the ileocecal junction were removed, the surplus ileum was cleaned with 4°C 0.9% NaCl and divided into nine segments of equal length. Segments were snap frozen in liquid nitrogen and stored at −70°C until assay. Arterial blood was taken for gas analysis and carboxyhemoglobin (COHb) measurement.
2.4 Arterial blood gas analysis
Abdominal arterial blood samples (1.5 ml) were taken at the indicated time points after 0.9% NaCl, LPS and 250 ppm CO challenge to analyze blood gas. COHb, serum lactate, partial pressure of arterial oxygen (PaO2), and saturation of arterial oxygen (SaO2) were measured using blood gas analyzer (Roche OMNI S6, USA).
2.5 MDA content determination
Maleic dialdehyde (MDA), a reliable marker of lipid peroxidation, was determined with thiobarbituric acid (TBA) according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, China). Stored specimens were weighed and immersed into 0.02 mmol/L Tris-HCl (pH 7.4) at the ratio of 1:10 (mg/mL). Tissue homogenate was centrifuged at 13,000 r/min for 15 min and precipitates were discarded. Supernatant (0.15 ml) was transferred to a testing tube. A standard solution (0.15 ml) was added to each standard testing tube and 0.15 ml of distilled water was added to another blank testing tube, 2.5 ml TBA was added into each of these two tubes, which were agitated several times, and then incubated at 100°C for 1 h. After that, each specimen was cooled to room temperature and centrifuged at 3,000 r/min for 15 min. Finally, supernatant in each tube underwent colorimetric assay at 532 nm. Total protein content in samples was analyzed using a bicinchoninic acid protein assay kit (BioSource, Camarillo, CA, USA). MDA content (nanomole per gram protein) was calculated according to the following formula:
2.6 MPO activity determination
Myeloperoxidase (MPO) is a constituent enzyme found principally in neutrophils that results in the formation of hypochlorous acid. Its presence in neutrophils allows it to be extracted from tissues and represents a direct correlation to neutrophils content. Intestinal MPO activity was determined using MPO Detection Kit (Nanjing Jiancheng Bioengineering Institute, China). Briefly, tissue was homogenized in 1 ml of 50 mmol/L potassium phosphate buffered saline (PBS, pH 6.0) containing 0.5% hexadecyltrimethylammonium hydroxide and centrifuged at 12,000 r/min at 4°C for 20 min. 10 μl of the supernatant was transferred into PBS (pH 6.0) containing 0.17 mg/mL 3,3′-dimethoxybenzidine and 0.0005% H2O2. MPO activity of the supernatant was determined by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine and expressed as units per gram of total protein (u/g). Total protein content in samples was analyzed using a bicinchoninic acid protein assay kit.
2.7 SOD activity determination
Superoxide dismutase (SOD) is a key enzyme in the dismutation of superoxide radicals resulting from cellular oxidative metabolism into hydrogen peroxide, and prevents LPS induced penetration. Because CO is an anti-oxidant, we speculated that SOD activity would be increased after CO exposure. Stored samples were homogenized in 100 mmol/L Tris-HCl buffer and centrifuged at 10,000 r/min for 20 min, and then the SOD activity was determined using assay kits (Nanjing Jiancheng Bioengineering Institute, China) and expressed as units per microgram of total protein (u/mg). Total protein content in samples was analyzed using a bicinchoninic acid protein assay kit.
2.8 PAF measurement
Stored segments were suspended in 1 ml PBS (pH 7.4) and sonicated at 30 cycles, twice, for 30 s. Homogenates were centrifuged at 3,000 r/min at 4°C for 10 min. The supernatants were collected for the platelet activator factor (PAF) measurement. PAF was assayed by an enzyme immunoassay kit according to the manufacturer’s instructions (BioSource International, USA). The mobile phase was required according to chromatography. Conditions of chromatography: the velocity of flow was 1.0 ml/min, the column temperature was at 25°C, and the wave length for determinating was 208 nm. The PAF values of 20 randomly selected samples were determined by the biological technique. The protein content in samples was analyzed using a bicinchoninic acid protein assay kit. The results were expressed as nanograms per gram of protein (ng/g).
2.9 ICAM-1 measurement
The enzyme-linked immunosorbent assay (ELISA) method was adapted to measure the intercellular adhesion molecule-1 (ICAM-1). Stored segments were weighed and homogenized in 5 ml of 0.1 mol/L PBS (pH 7.4) containing 0.5 g/L of sodium azide at 4°C. The homogenates were centrifuged at 2,000 r/min for 10 min to remove solid tissue debris. The supernatant was assayed using commercially available kits according to the manufacturer’s instructions (R&D Systems Inc, USA). The concentration of antigens in intestinal tissue homogenates was standardized to the total protein content in each specimen as measured by a bicinchoninic acid protein assay kit. The results were expressed as pictograms per microgram of protein (pg/mg).
2.10 IL-10 measurement
Stored segments were homogenized in 100 mg tissue/mL cold lysis buffer (20 mM Tris, 0.25 M sucrose, 2 mM EDTA, 10 mM EGTA, 1% Triton × 100) and one tablet of complete mini protease inhibitor cocktail tablets/10 ml (Roche Diagnostics, Indianapolis, IN, USA). Homogenates were centrifuged at 10,000 r/min for 40 min, the supernatant was collected, and the levels of interlukin-10 (IL-10) in the supernatants from the intestinal tissues from the different treatments were analyzed by ELISA following the manufacturer’s protocol (BioSource International, USA). OD values were measured at 450 nm using a microtiter ELISA reader. Protein content in samples was analyzed using a bicinchoninic acid protein assay kit. The results were expressed as pictograms per microgram of protein.
2.11 Cell apoptosis observation
Apoptosis of intestinal cells was observed with flow cytometry in Nanjing Medical University Cell Detection Center. Apoptotic cells were detected by both propidium iodide (PI) (BD PharmMingen Co., USA) nuclei staining and Annexin V (BD PharmMingen Co., USA) binding described in Brouard et al. (Brouard et al. 2000). Intestinal tissues were collected and ground to prepare a single cell suspension by density gradient centrifugation. A 0.5 ml single cell suspension was fixed for 30 min with 70% alcohol at 4°C, then centrifuged at 1,500 r/min for 4 min. The supernatant was removed and the sediment was digested with RNase 0.2 ml (50 pg/mL) and cultured for 30 min at 37°C. Cells were washed with PBS (pH 7.4) and labeled with 1 ml PI (50 mg/L) for staining. Cells stained with PI were marked by 4 ml Annexin V. 10,000 events were counted on the flow cytometer to determine the number of apoptotic intestine cells. Data from the flow cytometry were quantified and analyzed by Mod Fit 2.3 program.
2.12 Western blot analysis
Assay kits were purchased from Cell Signaling (Beverly, MA, USA) and used per manufacturer’s instructions. At the set time points, intestinal tissue protein was extracted as described previously (Kohmoto et al. 2007). Briefly, 100 µg of protein in sodium dodecyl sulfate (SDS) sample buffer was electrophoresed, transferred, and probed as described below. Cells were scraped and sonicated for 5 s. 20 µl of each sample was boiled for 5 min and then loaded into 12% polyacrylamide gel and electrophoresed at 100 V for 4 h. The gel was transferred overnight at 40 V onto nitrocellulose membrane. Membranes were then incubated for 3 h with blocking buffer (5% nonfat dry milk in TTBS (10% Tween in Tris-buffered saline)), washed with TTBS, and then incubated overnight in the appropriate rabbit polyclonal primary antibody against phosphorylated p38. The following day the membranes were washed in TTBS and proteins were visualized using horseradish-peroxidase-conjugated antibody against rabbit IgG and the enhanced chemiluminesence assay (Amersham Life Science, Arlington Heights, IL, USA) according to the manufacturer’s instructions. All membranes were stripped using a standard stripping solution (100 mmol/L β-mercaptoethanol, 2% SDS and 62.5 mmol/L Tris-HCl, pH 6.8) at 50°C, and then were re-probed with rabbit polyclonal antibody targeting total non-phosphorylated p38, to confirm equal loading of samples.
2.13 Histopathological study
The mid-ileum specimens were fixed in 10% paraformaldehyde for 2 h and then in 30% sucrose for 12 h. Specimens were then slowly frozen in cold 2-methylbutane, and sections of 6 μm were stained with hematoxylin eosin (H&E) staining for light microscopy evaluation. Degrees of injury were blindly assessed by two experienced independent pathologists (Department of Pathology, Jiangsu Province People Hospital, China), based on the extent of mucosal erosion in at least 12 sections per animal. Mucosal loss (degree of mucosal erosion) and the percentage of mucosal erosion area were defined as a percent of surface area with mucosal loss exceeding approximately one half of the villous length. The depth of mucosal loss was graded as: grade 0, involvement in less than 25% of the overall sample; grade 1, involvement in 25–50% of the villous length; and grade 2, more than 50% of the villous height involved (Nakao et al. 2003). A scoring system was used for each item using 0 up to 2 points for the different grades of the depth of mucosal loss. Afterwards, the mean ± SEM of each item was calculated.
2.14 Statistical methods
All assays were performed in duplicate, and the mean values were used for statistical analysis. The data are expressed as means ± SD and analyzed with SPSS 11.0 statistical software package. Differences were assessed with one-way analysis of variance followed by Fisher’s least significant difference test. The P < 0.05 values were considered significant.
3 Results
3.1 CO does not affect oxygenation, but increases COHb
CO can be highly toxic in vivo and has primarily been viewed as such. This toxicity is related directly to the formation of COHb and the resulting anoxia given the higher binding-affinity of CO for heme compared with oxygen, so we speculated that CO i.p should impair oxygenation. PaO2 and SaO2 are important evaluations of oxygenation, and serum lactate is thought to primarily reflect hypoxia. Therefore, we monitored PaO2, SaO2 and serum lactate for the duration of the experiment as described in “Materials and methods” section. There were no significant differences in PaO2 and SaO2 among these four groups. The level of serum lactate in the control group was (1.8 ± 0.5) mmol/L. At 1, 3, and 6 h after LPS injection, serum lactate levels in the LPS group were (2.4 ± 0.8) mmol/L, (2.6 ± 0.6) mmol/L and (2.8 ± 1.0) mmol/L, respectively. There were no significant differences between these two groups. In the LPS + CO i.p group, the levels of serum lactate were (2.2 ± 0.8) mmol/L, (2.4 ± 0.9) mmol/L and (2.5 ± 0.7) mmol/L, respectively. Compared to the LPS group, there were no significant differences. However, COHb in CO i.p, LPS and LPS + CO i.p groups was increased, significantly higher than that of the control group (P < 0.05; Table 1).
3.2 CO reduces MDA level, inhibits MPO and SOD activity
Peroxidize damage can lead to severe cellular injury that can be prevented by anti-oxidative agents. It has been previously demonstrated that inhaled CO has been shown to ameliorate intestinal injury induced by LPS via anti-oxidant. However, the mechanisms responsible for the anti-oxidant are poorly understood. Accordingly, we sought to determine whether CO i.p would affect peroxidative products and/or anti-oxidative agents by measuring intestinal MDA level, MPO, and SOD activity. The intestinal tissues from sham-treated animals contained low MDA, MPO, and SOD regardless of whether the rats were treated with room air or CO. LPS exposure resulted in a significant increase in MDA, as well as markedly promoted MPO activity. After LPS challenge followed by CO i.p at 1 and 3 h, MDA concentration and MPO activity were decreased, and there were significant differences as compared with that of LPS group (P < 0.05 or 0.01), but no significant differences at 6 h (Figs. 1 and 2). These results suggested that the inhibition of CO on peroxidize damage reached the maximum effect at 3 h and disappeared at 6 h because CO is quickly scavenged in the body, and its inhibiting action is not maintained so long. SOD is capable of scavenging intracellular oxygen free radical (OFR) via dismutation of superoxide radicals. In LPS-induced intestine injury, SOD was reduced, which contributed to injury and led to a significant increase in intestinal superoxide radicals, accompanied by inflammatory cell infiltration and peroxidize damage. However, treatment with CO prevented the histological injury, and suppressed the increase of OFR generation after receiving LPS (Fig. 3). Here, data imply that CO delivery seems to be crucial for the maintenance of a renewed balance of oxidant-antioxidant after the intestine was challenged by LPS. Strategies either to prevent SOD inactivation or to augment its levels might provide useful in lessening the injury.
3.3 CO targets pro-and anti-inflammatory cytokines
It has been known that intestinal injury induced by LPS is associated with an increase in the expression of a number of pro-inflammatory gene products within intestinal tissues, including TNF-α, IL-6, PAF, and ICAM-1, that are contribute to intestinal injury and trigger a deregulation inflammatory reaction. Therefore, we sought to determine whether CO i.p is sufficient to modulate the gut inflammatory response to LPS injection. We used ELISA to measure the expression of several cytokines such as PAF, ICAM-1, and IL-10 in the samples of isolated intestinal tissues obtained from all the experimental rats. PAF and ICAM-1 in the LPS group were increased, and were significantly higher than that of the control and CO i.p groups at all observed time points (P < 0.01). In rats with intestinal injury treated with CO, the levels of PAF and ICAM-1 were decreased. Statistical analysis showed that the values of the LPS + CO i.p group were lower than those of LPS group at 1 and 3 h (P < 0.05 or 0.01), while no significant difference was observed at 6 h. We presume that that is due to CO being quickly absorbed and rapidly exhausted in the body so that its decreasing action disappeared swiftly and is not maintained up to 6 h (Figs. 4 and 5). IL-10 is an inhibitor of pro-inflammatory cytokine synthesis and can limit the inflammatory process. Consequently, we examined the changes in the expression of IL-10. Transcripts for the IL-10 genes were significantly down-regulated after LPS challenge, while the down-regulation of IL-10 expression was partially reversed by CO i.p. Fig. 6 showed that IL-10 of the LPS + CO i.p group were higher than that of the LPS group (P < 0.05), but lower than the corresponding values of the control group and the CO i.p group at each time point (P < 0.05).
3.4 CO suppresses cell apoptosis
In the control group, the apoptotic cell rate was (8.02 ± 2.10)%. At 1, 3, and 6 h after the induction of intestinal injury, the percentages of apoptotic cells in the LPS group were (38.13 ± 8.59)%, (41.52 ± 3.36)%, and (47.12 ± 3.58)%, respectively, which were significantly higher than that of the control group (P < 0.01). In contrast, the rats with intestinal injury that were exposed to CO exhibited a marked decrease in the number of apoptotic cells. At 1, 3, and 6 h after CO i.p, the percentages of apoptotic cells in the LPS + CO i.p group were (29.78 ± 4.24)%, (34.45 ± 5.77)%, and (31.46 ± 6.67)%, respectively, which were significantly lower than that of the LPS group (P < 0.01). CO i.p alone did not affect intestinal cells apoptosis. The cell apoptotic rate in the CO i.p group relative to that of the control group at all observed time points showed no statistical differences (Fig. 7).
3.5 CO activates p38 MAPK
The p38 MAPK pathway plays a primary role in regulating the expression of inflammatory cytokines and the induction of cell apoptosis caused by multiple stress or injury, and CO inhalation confers anti-inflammatory and anti-apoptotic effects associated with the CO-dependent activation of the p38 MAPK (Zhang et al. 2003a, b). Thus, we tested the hypothesis that the protective effects of CO i.p were also associated with the CO-dependent activation of the p38 MAPK. As can be seen by studying Fig. 8a, LPS markedly increased the expression of the phosphorylated p38 MAPK, and was further up-regulated by CO i.p. The expression of the phosphorylated p38 MAPK in the control group and the CO i.p group was (1,421 ± 237) density unit (DU) and (1,742 ± 344) DU, respectively. There was no significant difference between these two groups. In the LPS group, the expression of the phosphorylated p38 MAPK was (1,742 ± 344) DU and it was significantly higher than that of control group (P < 0.01). After treatment with CO, the expression of the phosphorylated p38 MAPK in the LPS + CO i.p group was (9,774 ± 986) DU, and was significantly increased compared to the LPS group (P < 0.01; Fig. 8b). To further confirm whether the p38 MAPK pathway mediate cytoprotection is involved, we used the p38 MAPK inhibitor SB203580 that selectively inhibits the p38 pathway. SB203580 (20 mg/kg i.p) mainly prevented the protective effects of CO challenge (data not shown).
3.6 CO ameliorates intestinal damage
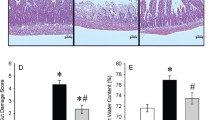
To determine the magnitude of intestinal injury caused by LPS, we performed ileum histologic studies. Morphological study clearly revealed that the rat’s ileum from the control and CO i.p groups had a normal structure of the intestinal epithelium and wall, whereas after LPS injection, there was severe structural damage associated with a massive loss of the intestinal villi, congestion of the villi, and inflammatory cells sequestration. In the rats given CO i.p, these histopathological injuries were significantly improved and the rats showed nearly intact intestinal histopathology (Liu et al. 2007). Scoring analysis showed that the degrees of injury in LPS group were significantly increased compared to those of the control and CO i.p groups (P < 0.01). CO i.p significantly decreased the depth of mucosal loss, with lower scores than that of the LPS group (P < 0.05; Fig. 9).
4 Discussion
Previously, a series of articles on the cytoprotective effects of CO generated considerable interest in the idea of using CO as a therapeutic agent to prevent intestinal IR or LPS-induced injury (Liu et al. 2007; Nakao et al. 2003, 2006a, b). However, CO is a toxic gas that interferes with the oxygen carrying capacity of the blood. Hence, using inhaled CO as a therapeutic agent for preventing organ injury would require the use of a closed circuit ventilation system that would minimize or remove the risk of contaminating the operating theater with the gas, as well as monitor the concentration of rendered CO and the level of circulatory COHb. In addition, studies reported so far have shown that CO inhaled for the prevention of intestinal injury has inherent problems. In order to overcome these flaws, other approaches for using CO have been described recently (Nakao et al. 2006a, 2006b). Rather than giving CO by inhalation, these investigators dissolved CO into UW solution (University of Wisconsin solution, an organ preservation solution; Nakao et al. 2006a) or ringer’s lactate solution (Nakao et al. 2006b), and a similar protection to inhaled gas was shown. Prompted by these intriguing results, we tried to develop a more safe and practical method of delivering the gas. Therefore, 2 ml/kg of 250 ppm CO was directly infused into the peritoneal cavity to investigate the effects of CO on LPS-induced intestinal injury.
As our results show, CO i.p is quite effective in preventing the development of intestinal injury after LPS injection. A similar protection is confirmed by measuring oxygen stress products, inflammatory cytokine levels, and the apoptotic cell rate. The precise mechanisms underlying this protection remain vague and require further investigation. The following mechanisms may explain its potential protection.
Several studies have shown that CO has the ability to provide protection against oxidative stress. CO exposure protected rats from LPS-induced intestinal injury by decreasing MDA content (Liu et al. 2007, 2008), inhibiting MPO activity (Liu et al. 2007; De Backer et al. 2009), and increasing SOD levels (Liu et al. 2008). The injury to the intestine caused by LPS leads to the peroxidation of lipids, to multivalence unsaturated fatty acids in the biomembrane and to the formation of lipids peroxidation including MDA. SOD is the scavenger of peroxide anon, which can turn peroxide anon to O2 and H2O2. Active H2O2 is harmful to the intestine and can be turned to H2O by SOD and eliminated. MPO is an enzyme that is found predominantly in the azurophilic granules of PMNs. Tissue MPO activity is frequently used to estimate tissue PMN accumulation in inflamed tissues, and correlates significantly with the number of PMNs in tissues (Sun et al. 2007). In the present study, we found that intestinal MPO activity was markedly elevated after LPS injection, and significantly decreased after CO i.p. This indicates that CO effectively prevents PMN chemotaxis and infiltration in the intestine after LPS challenge, and consequently decreases the production of oxidants and reduces tissue oxidative damage.
CO exerts a potent anti-inflammatory action (Dolinay et al. 2004; Otterbein et al. 2000; Song et al. 2003), is a component of the pathogenesis of the intestine after LPS injection. The biochemical basis for the anti-inflammatory effects of CO remains very poorly understood. In several models of cellular injury with LPS, the effect of CO on the down-regulation of the pro-inflammatory proteins has been observed (Zuckerbraun et al. 2005; Dolinay et al. 2004). In this context, it is noteworthy that treatment with CO at the conclusion of LPS limited the development of inflammation in the intestine, as evidenced by the reduction of the pro-inflammatory mediators PAF and ICAM-1 (Liu et al. 2007; Sun et al. 2007; Moore et al. 2003; Moore et al. 2005; Morisaki et al. 2001, 2002), as well as by the increase in the expression of a key anti-inflammatory cytokine IL-10 (Otterbein et al. 2000; Zuckerbraun et al. 2005; Dolinay et al. 2004; Goebel et al. 2008).
Cell apoptosis is responsible for injury of the intestine in response to a variety of microbial products, oxidative stress, and pro-inflammatory cytokines. The suppression of apoptosis by CO may represent an additional mechanism by which CO provides protection from LPS-induced injury (Song et al. 2003; Goebel et al. 2008; Wang et al. 2007). The mechanistic details underlining the anti-apoptotic effects are presently unclear. It is possible that the effect of CO may be connected to its powerful anti-oxidative and anti-inflammatory effects (Song et al. 2003; Wang et al. 2007). CO might limit the generation of oxygen free radicals by lowering the presence of free metal irons (Wang et al. 2007). CO may also down-regulate pro-inflammatory cytokines (Song et al. 2003; Otterbein et al. 2003). More recently, evidence suggests that CO can trigger up-regulation of the expression of anti-apoptotic genes such as the Bcl-2 family members, and down-regulate the pro-apoptotic genes Fax and Bax (Zhang et al. 2003a, b). These anti-apoptotic genes prevail over the pro-apoptotic signals thus preventing apoptosis in LPS-mediated cells. Our study shows that the rate of apoptosis in the intestinal cells of injured rats was decreased after CO i.p. The rate of apoptosis in cells can serve as a useful marker of organ injury in response to oxidative stress and inflammation.
In our study, treatment with CO revealed that its anti-oxidative, anti-inflammatory, and anti-apoptotic effects clearly involve the MAPK signal cascade, in particular the p38. The phosphorylated p38 MAPK level is assessed by Western blotting at 3 h after LPS challenge in the absence or presence CO. At the indicated time points, the increase of the phosphorylated p38 MAPK expression induced by LPS was further up-regulated by CO i.p. This finding is consistent with some prior studies of the effects of CO on MAPKs-dependent signaling (Kohmoto et al. 2007; De Backer et al. 2009; Otterbein et al. 2000, 2003; Zhang et al. 2003a, 2003b; Zhang et al. 2005). In support of this, we describe that activation and/or inhibition p38 MAPK not only modulated the expression of the inflammatory cytokines and the production of peroxidize radicals, but also abrogated the CO suppressed apoptosis of intestinal cells induced by LPS. Additionally, CO has the effect of increasing anti-inflammatory cytokines IL-10 simultaneously via the activation of the p38 MAPK pathway (Otterbein et al. 2000). The precise biochemical mechanism by which CO modulates the p38 MAPK pathway remains unclear at this time. Given that none of the upstream kinases in the MAPK pathway contains a heme moiety, a common target for CO, it could be that CO modulates the upstream kinases through an unknown or unidentified intermediate molecule, which is supported by several studies which observed that the protective effects of CO require new protein synthesis in a model of apoptosis induced by TNF-α (Kohmoto et al. 2007; Otterbein et al. 2003; Zhang et al. 2003a, 2003b; Zhang et al. 2005).
The induction of most pro-inflammatory cytokines requires the activation of NF-κB. Since CO has an anti-inflammatory effect via a reduction of pro-inflammatory cytokines products, it seemed logical to speculate that CO might suppress the activation of NF-κB. However, there are two opposing observations that CO modulates NF-κB activity. Brouard et al. have demonstrated that HO-1-derived CO requires the activation of NF-κB to protect endothelial cells from TNF-α mediated apoptosis (Brouard et al. 2000). CO liberated from CO-releasing molecule attenuates IR-induced inflammation in the intestine, which is associated with the activity of NF-κB (Katada et al. 2009). In cardiac myocytes, the stimulators of p38 MAPK can augment the activity of NF-κB via cross-talk (Craig et al. 2000). We have found that in intestinal cells CO is a potent activator of p38, so it is tempting to speculate that CO also activates NF-κB, or that p38 contributes to the activation of NF-κB after CO i.p. In contrast, based on the study of Zabalgoitia et al., HO-1 inducers and CO donors significantly inhibit IL-18-mediated human cardiac endothelial cell death and limit tissue inflammation by attenuating NF-κB activation (Zabalgoitia et al. 2007). Studies of the interactions between STAT1 and NF-κB signal pathway have demonstrated that STAT1 acts as a TNF-α receptor 1 signal molecule to suppress NF-κB activation. TNF-α mediated IκB degradation and NF-κB activation were markedly enhanced in STAT1-deficient Hela cells, whereas overexpression of STAT1 in 293 T cells blocked NF-κB activity induced by TNF-α (Wang et al. 2000). It has been previously reported that CO modulates STAT1 and inhibits apoptosis during anoxid-reoxygenation injury (Zhang et al. 2005), so it is conceivable that CO may inhibit NF-κB activity via the up-regulation of STAT1. The increase in p38 MAPK activity induced by CO may be associated with the inhibition of NF-κB activity. It has been demonstrated that indomethacin-induced activation of p38 MAPK results in the inhibition of NF-κB in response to the cytotoxicity of IR (Bradbury et al. 2001). These conflicting observations suggest that the role of NF-κB in CO-mediated cytoprotection is cell- and stimulus-specific, and is complex. Much remains incompletely understood and it is necessary to investigate further the connection between CO and the NF-κB pathway.
Despite its tantalizing potential as a therapeutic agent, translating the use of CO from the laboratory to the clinical arena has been a formidable challenge. Being a well-known toxic agent, administration of CO to patients by inhalation will require careful measurement to minimize the risk of environmental contamination, and careful monitoring of the inhaled CO concentration and COHb levels. In our studies, a single dose of CO i.p is sufficient to provide a therapeutic effect without any observed adverse effects. Although circulating COHb level increase, the peak level (5.5 ± 0.8%) is comparable with the level observed in healthy volunteers who smoke (6.0 ± 1.0%; Zevin et al. 2001). Moreover, the concentration used for the current study is less than one tenth of the CO dose administered to patients during measurement of diffusion of the lung for carbon monoxide in pulmonary function testing (Song et al. 2003). To test the safeness of 2 ml/kg of 250 ppm CO i.p, we measured PaO2 and SaO2. Surprisingly, no significant differences were found among the rats exposed to room air, to LPS and to CO. This suggests that CO administration at the current dose and at low concentrations is safe and does not cause hypoxia. Based on the results presented here, we believe that a careful clinical evaluation of CO for the prevention of intestinal injury induced by LPS may be warranted.
In summary, a single dose of CO i.p acts as a powerful protection against intestinal injury induced by LPS, at least in part, via anti-oxidant, anti-inflammation, and anti-apoptosis mechanisms, which may involve the p38 MAPK pathway. A single dose of low concentrations of CO i.p may be a more practical and a more safe approach for using CO to study cytoprotection from gut injury.
MDA measurement reveals an increase after LPS injection, while the increase is inhibited by CO i.p. P < 0.01 compared with control group and CO i.p group at the same time point. Comparison of LPS + CO i.p group with LPS group at 1 h, P < 0.05; at 3 h, P < 0.01. Comparison of 3 h with 1 h, and 6 h with 3 h in LPS group, all P < 0.01. Comparison of 3 h with 1 h in LPS + CO i.p group, P < 0.05; 6 h with 3 h, P < 0.05. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05.
Intestinal MPO activity of injured rat is increased, and is reduced by CO i.p. P < 0.01 compared with control group and CO i.p group at the same time point. Comparison of LPS + CO i.p group with LPS group at 1 h, P < 0.05; at 3 h, P < 0.01. Comparison of 3 h with 1 h, and 6 h with 3 h in LPS group, all P < 0.01. Comparison of 3 h with 1 h in LPS + CO i.p group, P < 0.05; 6 h with 3 h, P < 0.05. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05.
As analyzed, the activity of SOD shows a decrease after LPS injection, but the decrease is prevented by CO i.p. P < 0.01 compared with control group and CO i.p group at the same time point. Comparison of LPS + CO i.p with LPS group at 1 h, P < 0.01; at 3 h, P < 0.05. At the different time points of LPS group and LPS + CO i.p group, there are no significant differences. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05.
Treatment with CO significantly prevents the increase of PAF induced by LPS. P < 0.01 compared with control group and CO i.p group at the same time point. Comparison of LPS + CO i.p group with LPS group at 1 h, P < 0.05; at 3 h, P < 0.01. Comparison of 3 h with 1 h, and 6 h with 3 h in LPS group, all P < 0.01. Comparison of 3 h with 1 h in LPS + CO i.p group, P < 0.05. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05.
After CO i.p, the increase of ICAM-1 induced by LPS is markedly reduced. P < 0.01 compared with control group and CO i.p group at the same time point. Comparison of LPS + CO i.p group with LPS group at 1 h, P < 0.05; at 3 h, P < 0.01. Comparison of 3 h with 1 h, and 6 h with 3 h in LPS group, all P < 0.01. Comparison of 3 h with 1 h in LPS + CO i.p group, P < 0.05. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05.
The decrease of IL-10 caused by LPS is reversed by CO i.p. At all observed time points, IL-10 in LPS group decreased, significantly lower than that of control group (P < 0.05). However, after treatment with CO, IL-10 in LPS + CO i.p group increased, significantly higher than that of LPS group at the same time point (P < 0.05). At the different time points of LPS group and LPS + CO i.p group, there are no statistical differences. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05.
Apoptotic cells are determined by flow-cytometry that can detect the apoptotic cells stained with both Annexin V and PI, then to determine the percentage of apoptotic cells. CO i.p significantly reduces the increase of apoptotic cells rate induced by LPS. P < 0.01 compared with control group and CO i.p group at the same time point. Comparison of LPS + CO i.p group with LPS group at 1 h, P < 0.05; at 3 h, P < 0.01. Comparison of 3 h with 1 h, and 6 h with 3 h in LPS group, all P < 0.01. Compared 3 h with 1 h in LPS + CO i.p group, P < 0.05. Analysis of variance results between control group and CO i.p group for 1, 3, and 6 h are all >0.05
Western blotting shows that the expression of the phosphorylated p38 MAPK significantly increased after LPS challenge, and increased more after CO i.p (a). The levels of the phosphorylated p38 MAPK in the LPS group are higher than that of the control group and the CO i.p group (P < 0.01). CO i.p increases the levels of the phosphorylated p38 MAPK in the LPS + CO i.p group, with significant differences compared with those of the LPS group (P < 0.01; b).
H&E stained intestinal tissues sections were scored under light microscopy base on 0 up to 2 points for the different grades of the depth of mucosal loss after receiving 0.9% NaCl, LPS, and CO i.p, then compared the mean value of each item. The injured scores of the LPS group are higher than those of the control group and CO i.p group (P < 0.01), CO i.p reduces the rates of injury, with significant differences compared with that of the LPS group (P < 0.05)
References
Arbabi S, Maier RV (2002) Mitogen-activated protein kinases. Crit Care Med 30:s74–s79
Bauer M, Huse K, Settmacher U, Claus RA (2008) The heme oxygenase-carbon monoxide system: regulation and role in stress response and organ failure. Intensive Care Med 34:640–648
Bradbury CM, Markovina S, Wei SJ, Rene LM, Zoberi I, Horikoshi N, Gius D (2001) Indomethacin-induced radiosensitization and inhibition of ionizing radiation-induced NF-kappaB activation in HeLa cells occur via a mechanism involving p38 MAP kinase. Cancer Res 61:7689–7696
Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP (2000) Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J Exp Med 192:1015–1026
Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, Glembotski CC (2000) p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release. J Biol Chem 275:23814–23824
De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA (2009) Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut 58:347–356
Delaney CP, Weese JL, Hyman NH, Bauer J, Techner L, Gabriel K, Du W, Schmidt WK, Wallin BA (2005) Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileum after major abdominal surgery. Dis Colon Rectum 48:1114–1127
Dolinay T, Szilasi M, Liu M, Choi AM (2004) Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med 170:613–620
Gibbon SJ, Farrugia G, Haga M (2004) The role of carbon monoxide in the gastrointestinal tract. J Physiol 556(Pt 2):325–336
Goebel U, Siepe M, Mecklenburg A, Stein P, Roesslein M, Schwer CI, Cchmidt R, Doenst T, Geiger KK, Pahl HL, Schlensak C, Loop T (2008) Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology 108:1025–1036
Katada K, Bihari A, Mizuguchi S, Yoshida N, Yoshikawa T, Fraser DD, Potter RF, Cepinskas G (2009) Carbon monoxide liberated from CO-releasing molecule (CORM-2) attenuates ischemia/reperfusion-induced inflammation in the small intestine. Inflammation 33(2):92–100
Kohmoto J, Nakao A, Stolz DB, Kaizu T, Tsung A, Ikeda A, Shimizu H, Takahashi T, Tomigama K, Sugimoto R, Choi AM, Billian TR, Murase N, McCurry KR (2007) Carbon monoxide protects rat lung transplants from ischemia-reperfusion injury via a mechanism involving p38 MAP kinase pathway. Am J Transplant 7:2279–2290
Liu DM, Sun BW, Sun ZW, Jin Q, Sun Y, Chen X (2008) Suppression of inflammatory cytokine production and oxidative stress by CO-releasing molecules-liberated CO in the small intestine of thermally injured mice. Acta Pharmacol Sin 29:838–846
Liu SH, Xu XR, Ma K, Xu B (2007) Protection of carbon monoxide inhalation on lipopolysaccharide induced multiple organ injury in rats. Chin Med Sci J 22:169–176
Moore BA, Overhaus M, Whitcomb J, Ifedigbo E, Choi AM, Otterbein LE, Bauer AJ (2005) Brief inhalation of low-dose carbon monoxide protects rodents and swine from postoperative ileus. Crit Care Med 33:1317–1326
Moore EE (1998) Mesenteric lympth: the critical bridge between dysfunctional gut and multiple organ failure. Shock 10:407–416
Moore ET, Otterbein LE, Turler A, Choi AM, Bauer AJ (2003) Inhalation carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology 124:377–391
Morisaki H, Katayama T, Kotake Y, Ito M, Tamatani T, Sakamoto S, Ishimura Y, Takeda J, Suematsu M (2001) Roles of carbon monoxide in leukocyte and platelet dynamics in rat mesenteric during sevoflurane anesthesia. Anesthesiology 95:192–199
Morisaki H, Katayama T, Kotake Y, Ito M, Handa M, Ikeda Y, Takeda J, Suematsu M (2002) Carbon monoxide modulates endotoxin-induced microvascular leukocyte adhesion through platelet-dependent mechanisms. Anesthesiology 97:701–709
Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, Murase N (2003) Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol 163:1587–1598
Nakao A, Schmidt J, Harada T, Tsung A, Stoffels B, Cruz RJ, Kohmoto J, Peng X, Tomiyama K, Murase N, Bauer AJ, Fink MP (2006a) A single intraperitoneal dose of carbon monoxide-saturated ringer's lactate solution ameliorates postoperative ileus in mice. J Pharmacol Exp Ther 319:1265–1275
Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, Tomiyama K, Harada T, Takahashi T, Yang R, Fink MP, Morita K, Choi AM, Murase N (2006b) Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant 6:2243–2255
Nieuwenhuijzen GA, Goris RJ (1999) The gut: the ‘motor’ of multiple organ dysfunction syndrome? Curr Opin Clin Nutr Metab Care 2:399–404
Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, Wysk M, Davis RJ, Flavell RA, Choi AM (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6:422–428
Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM (2003) MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol 163:2555–2563
Ryter SW, Choi AM (2007) Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found Symp 280:165–181
Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM (2003) Carbon monoxide induced cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol 163:231–242
Sun BW, Jin Q, Sun Y, Sun ZW, Chen X, Chen ZY, Cepinskas G (2007) Carbon liberated from CO-releasing molecules attenuates leukocyte infiltration in the small intestine of thermally injury mice. World J Gastroenterol 13:6183–6190
Wagener FADTG, Volk HD, Willuis D, Abraham NG, Soares MP (2003) Different face of the heme-heme oxygenase system in inflammation. Pharmacol Res 55:551–571
Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM (2007) Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282:1718–1732
Wang Y, Wu TR, Cai S, Welte T, Chin YE (2000) Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-κB activation. Mol Cell Biol 20:4505–4512
Wolff BG, Michelassi F, Gerkin TM, Techner L, Gabriel K, Du W, Wallin BA (2004) Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg 240:728–735
Zabalgoitia M, Colston JT, Reddy SV, Holt JW, Regan RF, Stec DE, Rimoldi JM, Valente AJ, Chandrasekar B (2007) Carbon monoxide donors or heme oxygenase-1 overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death. Free Radic Biol Med 44:284–298
Zevin S, Saunders S, Gourlay SG, Jacob P, Benowitz NL (2001) Cardiovascular effects of carbon monoxide and cigarette smoking. J Am Coll Cardiol 38:1633–1638
Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ (2003a) Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38 mitogen activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem 278:22061–22070
Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ (2003b) Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem 278:1248–1258
Zhang X, Shan P, Alam J, Fu XY, Lee PJ (2005) Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxid-reoxygenation injury. J Biol Chem 280:8714–8721
Zuckerbraun BS, McCloskey CA, Gallo D, Liu F, Ifedigbo E, Otterbein LE, Billiar TR (2005) Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resusciation. Shock 23:527–532
Acknowledgments
This work is supported by the grants from the Natural Science Foundation of Jiangsu Province (No. 98029) and the Scientific Development Foundation of Jiangsu Province (No. 2000409). We thank Professor LI Chun-Sheng for assistance with the experiments, Professor XU Jian-Guo for valuable discussions, and Dr ZHANG Xiao-Liang for statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Natural Science Foundation of Jiangsu Province (No. 98029) and Scientific Development Foundation of Jiangsu Province (No. 2000409).
No conflicts of interest (including financial and other relationships) for each author
Rights and permissions
About this article
Cite this article
Liu, SH., Ma, K., Xu, XR. et al. A single dose of carbon monoxide intraperitoneal administration protects rat intestine from injury induced by lipopolysaccharide. Cell Stress and Chaperones 15, 717–727 (2010). https://doi.org/10.1007/s12192-010-0183-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-010-0183-0