Abstract
Acacetin, a natural product, has a wide spectrum of biological activities such as antioxidant properties. In the present study, we examined whether Acacetin has any beneficial role on lipopolysaccharide (LPS)-induced acute lung injury (ALI) and, if so, whether its effect is mediated via heme oxygenase-1 (HO-1), an antioxidant enzyme playing an important role in ALI. Male BALB/c mice were stimulated with LPS intratracheal instillation to induce ALI. Acacetin was administrated 2 h after LPS challenge. Samples were harvested 10 h after LPS administration. We demonstrated that LPS challenge significantly induced lung histological alterations such as inflammation and edema. Acacetin administration notably attenuated these changes and reduced tumor necrosis factor-α and interleukin-1β in lung tissues. The LPS-induced reactive oxygen species generation was markedly suppressed by Acacetin. Furthermore, Acacetin treatment significantly elevated pulmonary HO-1 and nuclear factor erythroid-2-related factor 2 (Nrf2) activities. However, the beneficial action of Acacetin was markedly abolished when pretreated with zinc protoporphyrin, an inhibitor of HO-1. In in vitro studies, Acacetin notably increased the HO-1 expression in pulmonary microvascular endothelial cells. During knockdown of Nrf2 by siRNA, the effect of Acacetin on HO-1 expression was significantly reversed. Acacetin attenuates LPS-induced ALI in mice. This protective effect of Acacetin may be mediated, in part, through an HO-1-dependent pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory distress syndrome (ARDS), a common complication of sepsis, is a severe health problem with an extremely high morbidity and mortality (Sweeney and McAuley 2016). Similar to sepsis (Gotts and Matthay 2016; Tao et al. 2016a; van der Poll et al. 2017), although great progress has been made in understanding the mechanism of ARDS, effective treatments for it are desirable (Sweeney and McAuley 2016). Animal models of acute lung injury (ALI) are widely used to investigate pathogenesis and treatment of ARDS (Tao et al. 2012a, b; Wu et al. 2015).
Anti-oxidant enzymes are critical in protecting the lung against oxidative stress (Maines 1988; Tao et al. 2012b). Heme oxygenase (HO) is a ubiquitous heme-degrading enzyme which catalyzes the rate-limiting step in the oxidative degradation of heme to biliverdin (Tenhunen et al. 1969). HO-1, an isoform of HO, is highly inducible (Maines 1988). Under oxidative stress, the expression of HO-1 is upregulated (Maines 1988). HO-1 knockout mice are associated with severe lung injury compared with wild-type (Constantin et al. 2012). Induction of HO-1 by pharmacology agents is protection from ALI in a number of animal studies (Chi et al. 2016; Takashima et al. 2014).
Acacetin (5,7-dihydroxy-4′-methoxyflavone), a flavonoid isolated from Agastache rugosa, is known to exert a wide spectrum of biological activities such as anti-inflammatory and antioxidant properties (Cho et al. 2014; Ha et al. 2012; Tanigawa et al. 2013). Acacetin suppressed tumor necrosis factor (TNF)-α-induced phosphorylation of p38 mitogen-activated protein kinases and activation of nuclear factor (NF)-κB in human umbilical vein endothelial cells (Tanigawa et al. 2013). In a mouse study, d-galactosamine/LPS-induced liver injury was reduced by Acacetin via a mechanism involving suppression of toll-like receptor 4 signaling (Cho et al. 2014). The anti-inflammatory properties of Acacetin may play a beneficial effect on ALI. Recent study reported that Acacetin prevented rat cardiomyocyte ischemia/reperfusion-induced reduction of anti-oxidative proteins superoxide dismutas-2 and thioredoxin (Liu et al. 2016). Hypoxia/reoxygenation-induced neonatal cardiomyocyte injury was protected by Acacetin via reduction of lipid peroxidation and enhancement of antioxidant activity (Yang et al. 2014). These results suggest the potential antioxidant features of Acacetin. However, the underlying mechanisms are yet to be elucidated. In the current study, we hypothesized that Acacetin dampens LPS-induced ALI via an HO-1 dependent pathway.
Methods
Reagents
Acacetin (molecular weight: 284.26 g/mol, Fig. 1a), LPS (Escherichia coli serotype O111:B4), zinc protoporphyrin (ZnPP), 2-hydroxypropyl-β-cyclodextrin (HBC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), and Trizol reagent were products of Sigma (St. Louis, MO, USA). TNF-α and interleukin (IL)-1β enzyme-linked immunosorbent assay (ELISA) kit, dimethyl sulfoxide (DMSO), and phosphate-buffered saline (PBS) were purchased from R&D Systems Inc (Minneapolis, MN, USA). TransAM™ Nuclear factor erythroid-2-related factor 2 (Nrf2) kit and Lipofectamine 2000 were purchased from Active Motif (Carlsbad, CA, USA). Horseradish peroxidase-linked anti-rabbit IgG and anti-β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-HO-1 and anti-Nrf2 antibody were products of Abcam (Cambridge, MA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin, and streptomycin (10,000 U/ml) were purchased from GIBCO (Grand Island, NY, USA). Lipid hydroperoxide assay kit was product of Cayman Chemical (Ann Arbor, USA). Nuclear/Cytosol Extraction kit was product of BioVision (Mountain View, CA, USA). Small interfering RNA (siRNA)-Nrf2, siRNA-HO-1, and siRNA-negative control were products of Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-TATA box binding protein (TBP) antibodies were products of Abcam (Cambridge, UK). Fluorescent probe dihydroethidium (DHE) and carboxy-dichlorodihydrofluorescein (H2DCFDA) were products of Thermo Fisher Scientific (Waltham, MA, USA).
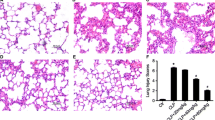
Male BALB/c mice were administrated with Acacetin 2 h after lipopolysaccharide (LPS) or equivalent sterilized phosphate-buffered saline (PBS) challenged. Samples were collected 10 h after LPS or PBS challenge. a Chemical structures of Acacetin. b Pulmonary histological images: control (PBS + vehicle-treated group) (1); LPS + vehicle-treated group (2); zinc protoporphyrin (ZnPP) + LPS + Acacetin-treated group (3); LPS + Acacetin-treated group (4). Original magnification: ×400. c Lung injury scores. d The lung wet to dry weight (W/D) ratio, and e myeloperoxidase (MPO) activity in lung tissues. Results are shown as the mean ± SD (n = 6–10 per group). Scale bars, 10 μm. *P < 0.05 when compared with control group; † P < 0.05 when compared with LPS + vehicle group; # P < 0.05 when compared with LPS + Acacetin group
Animals and experimental protocol
The Institutional Animal Care and Use Committee of General Hospital of Chinese People’s Liberation Army approved all mouse protocols at April 20, 2016 (no. 2016-X1-56). Healthy male BALB/c mice (8–12 weeks old) were purchased from General Hospital of Chinese People’s Liberation Army. The animals were housed in a temperature-controlled (23 ± 0.5 °C) room. Mice were maintained on a 12 h light/12 h dark cycle. All animals had access to food and water ad libitum.
The mice were randomly assigned to five groups (n = 10): control (PBS + vehicle-treated group), PBS + Acacetin-treated group, LPS + vehicle-treated group, LPS + Acacetin-treated group, and ZnPP + LPS + Acacetin-treated group. Mice were anesthetized by an intraperitoneal injection of ketamine/xylazine mixture. 50 μl of LPS intratracheal instillation was performed to induce ALI as described previously (Lin et al. 2011). Before use, LPS was dissolved in PBS at 10 mg/ml. For control and PBS + Acacetin group, equivalent PBS was administrated. Two hours after LPS or PBS administration, Acacetin (50 mg/kg) intraperitoneal injection was performed. The dose of Acacetin selected in the present study was based on our preliminary study (data not shown). Acacetin was dissolved in 5% DMSO to the concentration of 20 mg/ml before use. Equivalent DMSO was used as vehicle. ZnPP is an effective inhibitor of HO-1. ZnPP (5 mg/kg) was administered intraperitoneally 30 min before Acacetin treatment. The dose of ZnPP was determined according to a previous study (Xiong et al. 2017). Mice were killed by cervical dislocation and exsanguinated by cutting the vena cava inferior at 10 h after LPS or PBS treatment.
Histological examination
For immediate fixation, formalin was instilled through the right primary bronchi, and then the lung tissue specimen was submerged in formalin. After being washed in fresh PBS, the fixed tissue specimens were dehydrated in alcohol, cleared and embedded in paraffin and then cut into 5 μm thick sections, floated on warm water, and transferred to glass slides and mounted. The sections were stained with hematoxylin and eosin (H&E) after deparaffinization by routine methods. Histological examinations were performed by light microscopy and graded by two blinded examiners based on the following histological features: edema, neutrophil infiltration, intraalveolar hemorrhage, and congestion, each with a score of 0–3 (0, absent; 1, mild; 2, moderate; 3, severe). We calculated a total score for each animal (Bachofen and Weibel 1982).
Lung wet to dry (W/D) weight ratio
The lung was harvested and weighed. Then, the lung was placed in an oven at 80 °C for 48 to obtain the dry weight. The W/D ratio was measured to evaluate pulmonary edema.
Myeloperoxidase (MPO) activity and proinflammatory cytokine measurements
MPO activity in lung tissues was measured as described previously (Zhu et al. 2013). The concentrations of TNF-α and IL-1β in the lung tissues and pulmonary microvascular endothelial cells (PMVECs) were measured by ELISA according to the manufacturer’s instructions.
Cell culture and cell viability
We isolated PMVECs using a method described previously (Cheng et al. 2007). Briefly, the lung from male BALB/c mice was used to isolate PMVECs. The lung was extracted, minced, and digested. The PMVECs were harvested by immunoselection using an anti-CD31 antibody (Boster, Wuhan, China). PMVECs were cultured in Dulbecco’s modified Eagle’s medium (containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37 °C in a humidified incubator with 5% CO2.
Cell viability was determined using the MTT assay after 10 h of treatment with various concentrations of Acacetin. Briefly, PMVECs were seeded at a density of 5 × 103 cells/well into 96-well plates and treated with or without Acacetin (0–100 μM) for 10 h. Subsequently, 10 μl of 5 mg/ml MTT solution was added and further incubated for 4 h to form formazan crystals. Then, 100 μl of DMSO was transferred into each well to dissolve the formazan crystals, and results were measured using a microplate reader (Biotek, Winooski, VT, USA) at an absorbance of 570 nm. The IC50 values were obtained from the MTT viability growth curve.
Cell siRNA transfection
PMVECs were transfected with siRNA-Nrf2, siRNA-HO-1, or siRNA-negative control with Lipofectamine 2000 according to the manufacturer’s instructions. The effects of siRNA on Nrf2 expression was detected by western blot analysis.
Cell stimulation experiments
PMVECs were stimulated with 10 μg/ml LPS or equivalent sterilized PBS. LPS was dissolved in PBS before use. Thirty min. after LPS or PBS stimulation, the cells were treated with 50 μM Acacetin.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
The RT-PCR analysis was used to investigate the effect of Acacetin on HO-1 expression. Briefly, total RNA was extracted by using Trizol reagent according to the manufacturer’s instructions, and the RNA concentration was detected by using a spectrophotometer (Thermo Fisher, Boston, MA, USA). 0.5 μg sample of total RNA was converted to cDNA and Real-time PCR was performed with PrimeScript™ RT reagent kit. The forward (F) and the reverse (R) primers for HO-1 were (F) 5′-AAGCCGAGAATGCTGAGTTCA and (R) 5′-GCCGTGTAGATATGGTACAAGGA, respectively, and for β-actin were (F) 5′-AGCCATGTACGTAGCCATCC and (R) 5′-CTCTCAGCTGTGGTGGTGAA, respectively. β-Actin was used as internal control to evaluate relative expressions of HO-1.
Western blotting analysis
Western immunoblotting was used to assess protein levels of HO-1 and Nrf2. Briefly, cytoplasmic and nuclear proteins were extracted by a nuclear/cytosol extraction kit according to the manufacturer’s instructions. The extracted proteins (50 μg) were subjected to SDS–polyacrylamide gels (PAGE) and transferred to polyvinylidene fluoride membranes. The membranes were blocked by incubation in TBST [0.1% Tween 20, 10 mM Tris–HCl (pH 7.5), 150 mM NaCl] containing 5% nonfat milk for 1 h at room temperature. Then, blots in the membranes were incubated with primary antibodies specific to HO-1 (diluted 1:5000) or anti-Nrf2 antibody (diluted 1:5000) at 4 °C overnight. An anti-β-actin antibody (diluted 1:10,000) was used as control. The blots were washed in TBST five times for 10 min. Blots were then incubated with horseradish peroxidase-linked anti-rabbit IgG for 1 h at room temperature. Bands were developed using Super Signal West Pico Chemiluminescent Substrate (Pierce, Woburn, MA, USA) according to the manufacturer’s instructions. Then, the bands were analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
HO-1 and Nrf2 activity analysis
The HO-1 activity in lung tissues was measured as previously described (Ryter et al. 2000). The absorbance of the sample was measured by spectrophotometer at 530 nm.
The nuclear extractions from lung tissues were used for measuring Nrf2 binding activity to immobilized antioxidant response elements (ARE) using a TransAM™ Nrf2 kit according to the manufacturer’s instructions.
Reactive oxygen species (ROS) measurement
For animal studies, the ROS was determined by measuring lipid hydroperoxide in lung tissues using a lipid hydroperoxide assay kit according to the manufacturers’ manual.
For cell studies, ROS was measured by using DHE and H2DCFDA probe according to the manufacturer’s instructions. Fluorescence intensity was analyzed by flow cytometry (ACEA NovoCyte, Hangzhou, China).
Statistical analysis
Statistical calculations were performed by using SPSS 17.0 for Windows software (IBM, Armonk, NY, USA). All data are expressed as mean ± SD and analyzed with one-way analysis of variance (ANOVA) followed by Bonferroni t test. Differences between groups in histopathological scores were tested using the Kruskal–Wallis one-way analysis of variance on ranks and the Student–Newman–Keuls method. The survival rate was estimated by the Kaplan–Meier method and compared by log-rank test. Statistically significance was accepted at P < 0.05.
Results
Acacetin attenuates LPS-induced lung injury
Histological assessment revealed that LPS stimulated a marked influx of inflammatory cells to the alveolar space, hemorrhage, and pulmonary edema (Fig. 1b). Administration of Acacetin attenuated these changes (Fig. 1b). Lung injury score demonstrated that LPS-induced lung injury was attenuated by Acacetin treatment (Fig. 1c). Moreover, W/D, an indicator of lung edema, was markedly improved by Acacetin treatment (Fig. 1d). MPO, an indicator of neutrophil activation, was significantly inhibited by Acacetin treatment (Fig. 1e). However, these protective effects of Acacetin were significantly abolished when pretreated with ZnPP (Fig. 1). Ten hours after LPS challenge, animals in vehicle-treated group were weak, less eating and drinking compared with Acacetin treated group. Four mice died in vehicle-treated group, and no animal died in Acacetin treated group (P < 0.05). Four mice died in ZnPP + Acacetin-treated group. These results suggest that Acacetin significantly reduced LPS-induced ALI and mortality.
Acacetin inhibits LPS-induced ROS production
In animal studies, the LPS-induced ROS generation in lung tissues was significantly attenuated by Acacetin treatment (Fig. 2a). However, the antioxidant effect of Acacetin was significantly abolished when pretreated with ZnPP (Fig. 2a).
Male BALB/c mice were administrated with Acacetin 2 h after lipopolysaccharide (LPS) or equivalent sterilized phosphate-buffered saline (PBS) challenge. Samples were collected 10 h after LPS or PBS challenge. Alterations of lipid hydroperoxide (a), tumor necrosis factor (TNF)-α (b), interleukin-1β (c), nuclear factor erythroid-2-related factor 2 (Nrf2) (d), and heme oxygenase (HO)-1 activity (e) in lung tissues. Results are shown as the mean ± SD (n = 6–10 per group). ZnPP zinc protoporphyrin. *P < 0.05 when compared with control group; † P < 0.05 when compared with LPS + vehicle group; # P < 0.05 when compared with LPS + Acacetin group
In cell studies, Acacetin markedly inhibited LPS-induced ROS generation (Table 1). However, this protective effect of Acacetin was dampened in siRNA-HO-1 transfected cells (Table 1).
Acacetin attenuates inflammatory mediators
We measured inflammatory mediators to evaluate the anti-inflammatory property of Acacetin. Ten hours after LPS stimulation, TNF-α and IL-1β were significantly increased (Fig. 2b, c). Acacetin treatment notably decreased the LPS-induced TNF-α and IL-1β elevation in pulmonary tissues (Fig. 2b, c). However, the anti-inflammatory action of Acacetin was markedly dampened when pretreated with ZnPP (Fig. 2b, c). In cell studies, the LPS-induced IL-1β and TNF-α elevation were significantly reduced by Acacetin (Table 1). However, the effect of Acacetin on inhibition of IL-1β and TNF-α was significantly reversed in siRNA-HO-1 transfected cells (Table 1).
Acacetin enhances HO-1 and Nrf2 activity
HO-1 and Nrf2 are critical in protecting the lung against oxidative stress. Our animal studies’ results have shown that the activity of Nrf2 and HO-1 was significantly increased in Acacetin-treated animals compared with vehicle-treated group (Fig. 2d, e). This result suggests the potential antioxidant feature of Acacetin in in vivo.
Acacetin enhances HO-1 expression in PMVECs
As shown in Fig. 3a, Acacetin did not show any significant cellular toxicity up to 100 μM (25, 50, 100 μM). We used 50 μM Acacetin in our in vitro study. Acacetin significantly induced HO-1 expression both in mRNA and protein levels in PMVECs (Fig. 3b). This effect was markedly enhanced when the cells were stimulated with LPS (Fig. 3b).
a Cell viability. b Pulmonary microvascular endothelial cells were stimulated with lipopolysaccharide (LPS) or equivalent sterilized phosphate-buffered saline (PBS). Thirty min after LPS or PBS stimulation, 50 μM Acacetin was supplemented and treated for 10 h. The mRNA expression of heme oxygenase (HO)-1 was analyzed by real-time reverse transcriptase-polymerase chain reaction analysis. HO-1 protein was analyzed by western blotting analysis. β-Actin was used as internal controls. c Expression of nuclear factor erythroid-2-related factor 2 (Nrf2) protein in cytoplasmic or nuclear in PMVECs treated with small interfering RNA (siRNA)-Nrf2 or siRNA-negative control. β-actin and TATA box binding protein (TBP) were used as controls for cytoplasmic and nuclear proteins, respectively. d Effect of Acacetin on Nrf2 expression was analyzed by western blotting analysis. TBP was used as internal controls. Acacetin was dissolved in dimethyl sulfoxide (DMSO) before use. Results are shown as the mean ± SD of six independent experiments. *P < 0.05 when compared with control (PBS + DMSO); † P < 0.05 when compared with LPS + DMSO group; †† P < 0.05 when compared with LPS + Acacetin group
Acacetin augments Nrf2-regulated HO-1 expression
Cell studies were performed to investigate whether the Acacetin-triggered upregulation of HO-1 was through Nrf2 signaling pathway. Nrf2 was knockdown by siRNA in PMVECs (Fig. 3c). Acacetin significantly increased Nrf2 expression in PMVECs simulated with LPS (Fig. 3d). As shown in Fig. 3b, Acacetin treatment failed to induce an upregulation of HO-1 in siRNA-Nrf2 transfected cells. This result indicated the Acacetin induced HO-1 was Nrf2 dependent.
Discussion
ARDS is characterized by diffuse alveolar infiltration with inflammatory cells, hemorrhage, pulmonary edema, and hyaline membrane formation (Sweeney and McAuley 2016; Wheeler and Bernard 2007). These histological changes lead to impaired gas exchange and acute respiratory failure (Bachofen and Weibel 1982; Sweeney and McAuley 2016). In the current study, our results demonstrate that Acacetin reduces LPS-induced pulmonary inflammation and edema. Furthermore, our results also suggest that the beneficial effect of Acacetin may, in part, mediate via HO-1-dependent pathway.
Multiple reports confirmed that HO-1 was considered as one of the most important regulators in oxidative stress (Agarwal and Bolisetty 2013; Gozzelino et al. 2010). The protective effects of HO-1 are considered correlated with its ability to decrease harmful heme and to produce the cytoprotective carbon monoxide (CO) and bilirubin (Baranano et al. 2002; Otterbein et al. 2000). CO is a by-product produced by HO during the procedure that catalyzes the degradation of heme to biliverdin (Tenhunen et al. 1969). CO can act as an anti-inflammatory effector in mouse models of lung injury through the downregulation of pro-inflammatory cytokine production (Joe et al. 2015). Kim et al. (2017) reported that IL-10-dependent upregulation of pyrin expression is involved in the anti-inflammatory effects of CO. HO-1/CO system ameliorated endotoxin-induced lung injury by modulating the imbalance of the dynamic mitochondrial fusion/fission process (Yu et al. 2016a). Consistent with previous studies (Yu et al. 2016b), HO-1 was upregulated as a response to relieve LPS stimulation in the current study. Acacetin significantly further elevated HO-1 expression and activity both in vivo and in vitro. And the ROS generation was markedly reduced in Acacetin-treated group. When pretreated with ZnPP, a HO-1 inhibitor, the antioxidant feature of Acacetin was markedly abolished. These results suggest that Acacetin has antioxidant feature and its antioxidant effect may mainly depend on a HO-1-dependent manner. Nrf2 has been shown to regulate expression of HO-1 (Na and Surh 2014). As a redox-sensitive transcription factor, Nrf2 mediates basal and induced transcription of phase II antioxidant proteins and is critical in protecting the lung against oxidative stress (Kobayashi et al. 2004; Zhao et al. 2017). Acacetin has antioxidant feature (Yang et al. 2014). However, its mechanism is not well defined. Our results showed that Acacetin activated Nrf2 directly. Nrf2 could be activated by downregulating Keap1 or phosphorylation of Nrf2 at Ser40 (Huang et al. 2002; Zhao et al. 2017). Confirmatory studies of potential mechanisms that involved in Acacetin’s effects on activation of Nrf2 are required. In the present study, we investigate whether the Acacetin-triggered upregulation of HO-1 was Nrf2 dependent. Our in vitro study suggests Nrf2 signaling pathway playing a key role in Acacetin induced HO-1 expression.
Acacetin shows anti-inflammatory pharmacological values (Carballo-Villalobos et al. 2014; Cho et al. 2014; Ha et al. 2012; Liu et al. 2016; Tanigawa et al. 2013). Huang et al. found that Acacetin reduced IL-6 and TNF-α in OVA-sensitized asthmatic mice (Huang and Liou 2012). Proinflammatory mediators are known to play an important effect in the pathogenesis of inflammatory disorders. IL-1β has a key role in the development of ALI (Ganter et al. 2008). Qiu et al. (2013) reported that anti-TNF therapy is associated with modest but significant decrease in mortality in patients with sepsis, based on the results of their meta-analysis. These results suggest that inhibition of proinflammatory mediators may contribute to a beneficial effect on inflammatory disorders. In the current study, we observed that Acacetin treatment markedly reduced LPS-induced up-regulation of TNF-α and IL-1β both in vivo and in vitro studies. Previous study has shown that induction of HO-1 is protective against IL-1β induced inflammatory responses (Clerigues et al. 2012). Induction of HO-1 decreases pro-inflammatory cytokines IL-1β and TNF-α (Ma et al. 2007). Our in vitro studies show that the effect of Acacetin on reduction of proinflammatory mediators TNF-α and IL-1β was markedly dampened in siRNA-HO-1 transfected PMVECs. These results suggest a potential anti-inflammatory action of Acacetin may be mediated through an HO-1-dependent pathway.
In the present study, although a high dosage of HO-1 inhibitor was used, the protective effect of Acacetin was partially inhibited. This result suggests that other mechanisms such as inhibition of NF-κB and toll-like receptor 4 signaling may be involved in the effect of Acacetin on endotoxin-induced ALI (Cho et al. 2014; Tanigawa et al. 2013). Further study is still required to unveil the potential mechanisms.
Conclusion
Acacetin attenuates LPS-induced ALI in mice. This protective effect of Acacetin may be mediated, in part, through an HO-1-dependent pathway.
References
Agarwal A, Bolisetty S (2013) Adaptive responses to tissue injury: role of heme oxygenase-1. Trans Am Clin Climatol Assoc 124:111–122
Bachofen M, Weibel ER (1982) Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 3:35–56
Baranano DE, Rao M, Ferris CD, Snyder SH (2002) Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA 99:16093–16098
Carballo-Villalobos AI, Gonzalez-Trujano ME, Lopez-Munoz FJ (2014) Evidence of mechanism of action of anti-inflammatory/antinociceptive activities of acacetin. Eur J Pain 18:396–405
Cheng C et al (2007) Lipopolysaccharide induces expression of SSeCKS in rat lung microvascular endothelial cell. Mol Cell Biochem 305:1–8. doi:10.1007/s11010-007-9521-7
Chi X, Guo N, Yao W, Jin Y, Gao W, Cai J, Hei Z (2016) Induction of heme oxygenase-1 by hemin protects lung against orthotopic autologous liver transplantation-induced acute lung injury in rats. J Transl Med 14:35
Cho HI, Park JH, Choi HS, Kwak JH, Lee DU, Lee SK, Lee SM (2014) Protective mechanisms of acacetin against d-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. J Nat Prod 77:2497–2503
Clerigues V, Guillen MI, Castejon MA, Gomar F, Mirabet V, Alcaraz MJ (2012) Heme oxygenase-1 mediates protective effects on inflammatory, catabolic and senescence responses induced by interleukin-1beta in osteoarthritic osteoblasts. Biochem Pharmacol 83:395–405. doi:10.1016/j.bcp.2011.11.024
Constantin M, Choi AJ, Cloonan SM, Ryter SW (2012) Therapeutic potential of heme oxygenase-1/carbon monoxide in lung disease Int. J Hypertens 2012:859235
Ganter MT et al (2008) Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res 102:804–812. doi:10.1161/circresaha.107.161067
Gotts JE, Matthay MA (2016) Sepsis: pathophysiology and clinical management. BMJ (Clin Res Ed) 353:i1585. doi:10.1136/bmj.i1585
Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354
Ha SK, Moon E, Lee P, Ryu JH, Oh MS, Kim SY (2012) Acacetin attenuates neuroinflammation via regulation the response to LPS stimuli in vitro and in vivo. Neurochem Res 37:1560–1567
Huang WC, Liou CJ (2012) Dietary acacetin reduces airway hyperresponsiveness and eosinophil infiltration by modulating eotaxin-1 and th2 cytokines in a mouse model of asthma. Evid Based Complement Altern Med 2012:910520
Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem 277:42769–42774. doi:10.1074/jbc.M206911200
Joe Y et al (2015) Tristetraprolin mediates anti-inflammatory effects of carbon monoxide on lipopolysaccharide-induced acute lung injury. Am J Pathol 185:2867–2874
Kim SK et al (2017) Carbon monoxide decreases interleukin-1beta levels in the lung through the induction of pyrin. Cell Mol Immunol 14:349–359
Kobayashi A, Ohta T, Yamamoto M (2004) Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol 378:273–286
Lin WC, Lin CF, Chen CL, Chen CW, Lin YS (2011) Inhibition of neutrophil apoptosis via sphingolipid signaling in acute lung injury. J Pharmacol Exp Ther 339:45–53
Liu H et al (2016) Water-soluble acacetin prodrug confers significant cardioprotection against ischemia/reperfusion injury. Sci Rep 6:36435
Ma JL, Yang PY, Rui YC, Lu L, Kang H, Zhang J (2007) Hemin modulates cytokine expressions in macrophage-derived foam cells via heme oxygenase-1 induction. J Pharmacol Sci 103:261–266
Maines MD (1988) Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2:2557–2568
Na HK, Surh YJ (2014) Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med 67:353–365
Otterbein LE et al (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6:422–428
Qiu P, Cui X, Sun J, Welsh J, Natanson C, Eichacker PQ (2013) Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: a meta-analysis. Crit Care Med 41:2419–2429
Ryter SW, Kvam E, Tyrrell RM (2000) Heme oxygenase activity current methods and applications. Methods Mol Biol 99:369–391
Sweeney RM, McAuley DF (2016) Acute respiratory distress syndrome. Lancet 388:2416–2430
Takashima K et al (2014) Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respir Res 15:150
Tanigawa N et al (2013) Acacetin inhibits expression of E-selectin on endothelial cells through regulation of the MAP kinase signaling pathway and activation of NF-kappaB. Immunopharmacol Immunotoxicol 35:471–477
Tao W, Miao QB, Zhu YB, Shu YS (2012a) Inhaled neutrophil elastase inhibitor reduces oleic acid-induced acute lung injury in rats. Pulm Pharmacol Ther 25:99–103. doi:10.1016/j.pupt.2011.12.006
Tao W, Shu YS, Miao QB, Zhu YB (2012b) Attenuation of hyperoxia-induced lung injury in rats by adrenomedullin. Inflammation 35:150–157. doi:10.1007/s10753-011-9300-1
Tao W, Li PS, Shen Z, Shu YS, Liu S (2016a) Effects of omega-3 fatty acid nutrition on mortality in septic patients: a meta-analysis of randomized controlled trials. BMC Anesthesiol 16:39. doi:10.1186/s12871-016-0200-7
Tao W, Li PS, Yang LQ, Ma YB (2016b) Effects of a soluble epoxide hydrolase inhibitor on lipopolysaccharide-induced acute lung injury in mice. PLoS One 11:e0160359
Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244:6388–6394
van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG (2017) The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. doi:10.1038/nri.2017.36
Wheeler AP, Bernard GR (2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553–1564
Wu SY, Tang SE, Ko FC, Wu GC, Huang KL, Chu SJ (2015) Valproic acid attenuates acute lung injury induced by ischemia-reperfusion in rats. Anesthesiology 122:1327–1337
Xiong J et al (2017) Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int J Mol Med 39:113–125. doi:10.3892/ijmm.2016.2809
Yang WJ, Liu C, Gu ZY, Zhang XY, Cheng B, Mao Y, Xue GP (2014) Protective effects of acacetin isolated from Ziziphora clinopodioides Lam. (Xintahua) on neonatal rat cardiomyocytes. Chin Med 9:28
Yu J et al (2016a) Heme oxygenase-1/carbon monoxide-regulated mitochondrial dynamic equilibrium contributes to the attenuation of endotoxin-induced acute lung injury in rats and in lipopolysaccharide-activated macrophages. Anesthesiology 125:1190–1201
Yu J et al (2016b) Effect of heme oxygenase-1 on mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Sci Rep 6:36530
Zhao H, Eguchi S, Alam A, Ma D (2017) The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am J Physiol Lung Cell Mol Physiol 312:L155–L162. doi:10.1152/ajplung.00449.2016
Zhu T et al (2013) Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappaB. PLoS One 8:e56407
Acknowledgements
The authors wish to thank Dong Tangxiao and Yan Yi for their technical assistance.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: LT, WD. Performed the experiments: WD. Analyzed the data: WY, ZH, DM. Wrote the manuscript: WD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wu, D., Wang, Y., Zhang, H. et al. Acacetin attenuates mice endotoxin-induced acute lung injury via augmentation of heme oxygenase-1 activity. Inflammopharmacol 26, 635–643 (2018). https://doi.org/10.1007/s10787-017-0398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0398-0