Abstract
We retrospectively evaluated the outcome of administering low-dose rabbit anti-thymocyte globulin (thymoglobulin: ATG-T) to 219 patients (ATG-T group, n = 30; no-ATG-T group, n = 189) who received an initial unrelated hematopoietic stem cell transplantation (uHSCT). The median total dose of ATG-T was 1.5 mg/kg. There was no significant difference in the cumulative incidences of grade II–IV (42 vs. 38 %, P = 0.87) and grade III–IV (5 vs. 7 %, P = 0.52) acute GVHD. In patients who received uHSCT from a donor with at least one HLA allele mismatch, the cumulative incidence of extensive chronic GVHD was significantly lower in the ATG-T group than that in the no-ATG-T group (13 vs. 44 %, P = 0.02). No patient in the ATG-T group developed chronic lung dysfunction. The probabilities of 1-year, GVHD-free/relapse-free survival (GRFS) were 61 % in the ATG-T group and 35 % in the no-ATG-T group (P = 0.02). Patients in the ATG-T group discontinued immunosuppressive drugs significantly earlier than those in the no-ATG-T group (P < 0.01). The use of low-dose ATG-T did not increase the incidence of severe infectious disease. The use of low-dose ATG-T in patients who received uHSCT was associated with a superior GRFS, reflecting the reduced incidence of severe/persistent GVHD without compromising overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has become an integral part of treatment for various hematological disorders [1–3]. Although acute GVHD is still a major obstacle to success in allogeneic HSCT, chronic GVHD is also an important morbidity that is a major cause of treatment-related mortality in long-term survivors [4–6].
Although there is no established GVHD prophylaxis that can reduce the incidence of acute/chronic GVHD without increasing the risk of relapse, one promising agent is anti-thymocyte globulin (ATG). Finke et al. conducted a large, randomized controlled trial in patients who received an unrelated HSCT and found that ATG-Fresenius (ATG-F) as GVHD prophylaxis reduced the incidences of acute and chronic GVHD without compromising survival [7, 8]. A randomized trial by Bacigalupo et al. also showed that ATG-Thymoglobulin (ATG-T) reduced the incidence of chronic GVHD, especially chronic lung dysfunction, in patients who received an unrelated HSCT [9]. These studies suggested that ATG as GVHD prophylaxis reduced the incidence of GVHD without compromising survival. However, there is still controversy regarding the optimal dose of ATG.
The risk of GVHD might differ among different ethnicities and races [10–12]. Asian populations were reported to have a lower risk of GVHD compared to Caucasian populations [13, 14]. As excessive doses of ATG will attenuate the graft-versus-leukemia effect and increase viral infections, it is desirable to choose the minimum dose of ATG that is sufficient to control GVHD. In our previous single-institute retrospective study of patients who received an unrelated bone marrow transplantation (BMT) using a reduced-intensity conditioning (RIC) regimen, the incidences of acute and chronic GVHD were promisingly low in patients who received low-dose ATG-F (5 or 10 mg/kg in total) [15]. In another retrospective study of Japanese nationwide transplant outcomes after unrelated BMT, the incidences of acute and chronic GVHD were again promisingly low considering the dose of ATG-F (median dose 10 mg/kg) [16]. Such low-dose ATG-F might be sufficient in Asian populations who receive unrelated BMT. However, no published data are available regarding the doses of different formulations of ATG-T.
Here, we retrospectively analyzed clinical outcomes in patients who received an unrelated HSCT at our institute to assess the impact of low-dose ATG-T.
Patients and methods
Study design
This was a single-center retrospective study that assessed the impact of ATG-T on clinical outcomes in consecutive patients who received an unrelated HSCT from 2009 to 2013 (ATG-T group, n = 30; no-ATG-T group, n = 189). In Japan, until September 2010 only BMT was approved for unrelated HSCT. This study was approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan.
Clinical outcomes
Endpoints included neutrophil recovery, overall survival (OS), relapse, non-relapse mortality (NRM), acute GVHD, chronic GVHD, infection, discontinuation of immunosuppressive drugs and GVHD-free relapse-free survival (GRFS). Neutrophil recovery was defined as an absolute neutrophil count (ANC) of 0.5 × 109/L for 3 consecutive days. OS was defined as time from HSCT to death from any cause or the time of the last follow-up. The incidences of grade II–IV or grade III–IV acute GVHD were based on standard criteria [17]. Chronic GVHD was defined according to previously published criteria [18]. Chronic lung dysfunction was defined according to Bacigalupo’s report [9]. Discontinuation of immunosuppressive drugs was defined accordingly to previously studies [15, 19–21]. GRFS events were defined as grade III–IV acute GVHD, chronic GVHD requiring systemic immunosuppressive treatment, disease relapse, or death from any cause after HSCT [22].
Statistical analysis
Fisher’s exact test was used to compare differences in the distribution of clinical features. The probability of OS was calculated by the Kaplan–Meier method. Comparison of survival curves was performed using the log-rank test. The cumulative incidences of engraftment, NRM, relapse, GVHD, cytomegalovirus (CMV) infection, and discontinuation of immunosuppressive drugs were evaluated using Gray’s method. The cumulative incidence of extensive chronic GVHD was evaluated using the model by Fine and Gray for univariate and multivariate analyses. In the competing risk models for engraftment, GVHD and discontinuation of immunosuppressive drugs, relapse, and death before these events were defined as competing risks. In the competing risk models for NRM, relapse was defined as a competing risk. In the competing risk models for CMV infection, relapse and death without this event was defined as competing risks. The variables that were evaluated in these analyses were follows: patient’s sex (male vs. female), patient’s age at transplant (Age ≥ 60 vs. Age < 60), disease risk (low vs. intermediate vs. high risk), stem cell source (bone marrow vs. peripheral blood stem cells), intensity of the conditioning regimen (MAC vs. RIC), using of ATG-T (yes vs. no), HLA mismatch (none vs. 1 allele vs. more than 1 allele) and disease status (CR vs. non CR). Disease risk was based on the Center for International Blood and Marrow Transplant (CIBMTR) classifications [23]. For all analyses, P < 0.05 was considered as statistically significant. The statistical analyses were carried out using the EZR software package (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0) [24].
Results
Patient characteristics
Patient characteristics are summarized in Table 1. The median age was 48 years (range 17–69). There was no statistically significant difference in the disease risk between the two groups. In the ATG-T group, most patients received a RIC regimen (n = 23, 77 %). The ATG-T group included more HSCT from an HLA mismatched donor than the no-ATG-T group (83 vs. 48 %, P < 0.01). As GVHD prophylaxis, tacrolimus and a short course of methotrexate was mainly used in both groups. The median total dose of ATG-T was 1.5 mg/kg (range 1.0–4.0 mg/kg). ATG-T was administered at a median of day −2 (range day −4 to −1; day-4, n = 6; day-3, n = 1; day-2, n = 22; day-1, n = 1). We used 2 mg/kg/day of methylprednisolone in total on the day of ATG-T administration to prevent the infusion reaction. The median follow-up period among survivors was 567 days (range 16–1562 days) after HSCT.
Engraftment
The cumulative incidences of neutrophil engraftment at day 28 were 100 and 93 % in the ATG-T and no-ATG-T groups (P < 0.01; Fig. 1a), respectively. The median times to neutrophil engraftment were 17 days (range 11–25 days) in the ATG-T group and 18 days (range 7–40 days) in the no-ATG-T group, respectively. In the ATG-T group, no graft failure was observed.
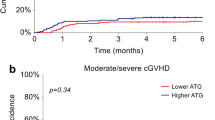
a Engraftment, b grade II–IV acute GVHD, c grade III–IV acute GVHD, d extensive chronic GVHD, e extensive chronic GVHD in patients who received HSCT from a donor with at least one HLA allele mismatch, f chronic lung dysfunction, g discontinuation of immunosuppressive drugs shown by the lower curve. The competing risks of relapse and death are shown by the upper curve. Differences between upper curves and lower curves show the proportion of surviving patients who continued to receive immunosuppressive drugs
Acute and chronic GVHD
There was no statistically significant difference in the cumulative incidences of grade II–IV (42 vs. 38 %, P = 0.87; Fig. 1b) and grade III–IV (5 vs. 7 %, P = 0.52; Fig. 1c) acute GVHD. The cumulative incidence of extensive chronic GVHD in the ATG-T group tended to be lower than that in the no-ATG-T group (19 vs. 38 %, P = 0.13; Fig. 1d). In patients who received HSCT from a donor with at least one HLA allele mismatch, the cumulative incidence of extensive chronic GVHD was significantly lower in the ATG-T group than that in the no-ATG-T group (13 vs. 44 %, P = 0.02; Fig. 1e). In multivariate analysis of extensive chronic GVHD, The administration of ATG-T trended to be associated with a lower incidence of extensive chronic GVHD (Table 2). In all patients, there was a trend towards a lower incidence of chronic lung dysfunction in the ATG-T group than in the no-ATG-T group (0 vs. 24 %, P = 0.07; Fig. 1f).
Discontinuation of immunosuppressive drugs
At 2 years after HSCT, immunosuppressive drugs were discontinued in 60 and 25 % of the patients in the ATG-T and no-ATG groups, respectively. Patients in the ATG-T group discontinued immunosuppressive drugs significantly earlier than those in the no-ATG-T group (P < 0.01; Fig. 1g).
Infection
The incidence of CMV antigenemia in the ATG-T group was significantly higher compared to that in the no-ATG-T group (83 vs. 67 %, P < 0.01; Fig. 2a). However, there was no statistically significant difference in the cumulative incidence of CMV disease between the ATG-T and no-ATG-T groups (0 vs. 7 %, P = 0.14; Fig. 2b). There was no statistically significant difference in the incidence of Epstein-Barr virus disease (0 vs. 0.5 %), adenovirus disease (3 vs. 4 %), or BK virus disease (0 vs. 3 %) between the ATG-T and no-ATG-T groups. No patients developed post-transplant lymphoproliferative disorder (PTLD) in the ATG-T group.
NRM, OS, and GRFS
The cumulative incidences of 1-year NRM were 7 and 9 % in the ATG-T and no-ATG-T groups, respectively (P = 0.65; Fig. 3a). The use of low-dose ATG-T was not associated with an increased risk of relapse (P = 0.20; 18 vs. 29 % at 1 year, Fig. 3b). The probabilities of 1-year OS were 85 and 67 % in the ATG-T and no-ATG-T groups, respectively. There was no statistically significant difference between the 2 groups (P = 0.14; Fig. 3c). Grouped according to the pretransplant disease risk, the probabilities of OS at 1-year did not differ statistically between ATG-T and no-ATG-T groups in the low-, intermediate- and high-risk groups, respectively (P = 0.40; 88 vs. 82 % Fig. 3d, P = 0.65; 89 vs. 80 % Fig. 3e, P = 0.22; 84 vs. 62 % Fig. 3f). The probabilities of 1-year GRFS were 61 and 35 % in the ATG-T and no-ATG-T groups, respectively (P = 0.02; Fig. 3g).
Discussion
In this retrospective study of 219 patients who received unrelated HSCT, we evaluated the impact of low-dose ATG-T on clinical outcomes, focusing on the incidence of GVHD. The most important finding in this study was the significant impact of ATG-T on chronic GVHD as assessed by several clinical outcomes. Compared to the no-ATG-T group, the incidence of extensive chronic GVHD was significantly lower in the ATG-T group in patients who received an HSCT from a donor with at least one HLA allele mismatch. Patients in the ATG-T group did not suffer from chronic lung dysfunction during the study period, and discontinued immunosuppressive drugs significantly earlier than those in the no-ATG-T group. GRFS, a new clinical outcome proposed by Shernan et al. was significantly better in the ATG-T group than that in the no-ATG-T group [22]. Therefore, our study suggests the possibility that even low-dose ATG-T could reduce the incidence of severe/refractory chronic GVHD in the setting of unrelated HSCT, thereby lowering the rate of chronic lung dysfunction and allowing for the discontinuation of immunosuppressive drugs.
Previous studies conducted in Western countries showed that the risks of acute and chronic GVHD were significantly reduced by the use of ATG [9, 25]. In practice, in Asian countries such as Korea and Japan we use a smaller dose of ATG than in Western countries, since the incidence of GVHD in Asian populations was reported to be lower than that in Caucasian populations [10–12]. Kim et al. reported that the use of low-dose ATG-T (2.5 mg/kg) was associated with a low incidence of acute GVHD in patients who received an HLA-mismatched unrelated HSCT [14]. In our previous single-institute retrospective study of patients who received an unrelated BMT using a RIC regimen, the incidences of acute and chronic GVHD were low in patients who received low-dose ATG-F (5 or 10 mg/kg in total) [15]. In the current study of low-dose ATG-T (median 1.5 mg/kg), the incidence of extensive chronic GVHD was low in patients who received an HLA-mismatched unrelated HSCT. However, there were some differences in patient characteristics between the ATG group and the no-ATG group. In multivariate analysis, there was a trend toward a lower incidence of extensive chronic GVHD. Although it was not a statistically significant difference, it could be due to the limited number of cases who received ATG-T. Although there have been no well-designed randomized controlled trials in Asia, low-dose ATG-T would contribute to the reduced incidence of GVHD. The reason why ATG reduces the risk of chronic GVHD independent of the impact on acute GVHD is still unclear as reviewed previously [26]. ATG might have immunomodulatory activity to induce immune tolerance, which should be further clarified in the future.
Previous studies found that RIC regimens, including those with ATG-T (5 mg/kg), were associated with low mortality and high long-term disease-free survival without GVHD [27], while the use of higher-dose ATG-T (8 mg/kg) increased the risk of relapse after unrelated HSCT [28]. Previous studies demonstrated no impact of ATG on OS [7, 9, 25, 29]. However the use of ATG improved survival free of immunosuppressive therapy for chronic GVHD [8]. Similarly in our study, while the use of low-dose ATG-T did not improve OS, patients in the ATG-T group discontinued immunosuppressive drugs significantly earlier than in the no-ATG-T group. Focusing on chronic lung dysfunction, recent studies demonstrated that the use of ATG-T in HSCT reduced the incidence of this condition. Bacigalupo et al. reported that ATG-T reduced the incidence of chronic lung dysfunction in patients who received an unrelated HSCT [9]. Dirou et al. also reported a low rate of pulmonary complications and lung function impairment in patients who received RIC regimens that included low-dose ATG-T (5 mg/kg), [30] although Milano et al. reported that the use of ATG-T (range 4.5–6 mg/kg) did not decrease the incidence of pulmonary complications at 1 year after HSCT [31]. In our study, the incidence of chronic lung dysfunction in the ATG-T group tended to be lower than that in the no-ATG-T group. However, the follow-up period might be insufficient to evaluate the incidence of chronic lung dysfunction. Further study incorporating routine serial monitoring of lung function is needed to confirm the beneficial impact of ATG on the incidence of chronic lung dysfunction. A recent study proposed the use of GRFS as a novel valuable composite endpoint comprising grade III–IV acute GVHD, chronic GVHD requiring systemic therapy, relapse, and death after HSCT [22]. GRFS is considered to measure the probability of OS with a good QoL after allogeneic HSCT. In our study, the probability of GRFS in the ATG-T group was significantly higher than that in the no-ATG-T group. Our results are consistent with results from previous studies, and suggest that the use of low-dose ATG-T might lead to a better QoL after unrelated HSCT.
A concern associated with the use of ATG is the increased risk of infectious diseases. Several studies suggested that ATG did not increase the risk of infection-related mortality [7, 32–34]. Bacigalupo et al. reported higher infection-related mortality using high-dose ATG-T (15 mg/kg), but not using low-dose ATG-T (7.5 mg/kg) [9]. In our study, the use of low-dose ATG-T did not increase the incidence of severe infectious disease. Furthermore, there were no cases of PTLD in the ATG-T group, suggesting that low-dose ATG-T did not cause marked immunosuppression. As the incidence of PTLD was previously reported to be rather low even in patients who received high-dose ATG [35], the incidence of PTLD should be confirmed in larger studies.
In previous randomized studies, ATG-F was shown to have a negative effect on both neutrophil and platelet engraftment [7, 29]. Other reports, however, found that ATG-T had no negative effect on neutrophil engraftment [36]. In our previous study, the use of low-dose ATG-F (5 or 10 mg/kg in total) increased the incidence of graft failure [15]. In contrast, our current study showed that low-dose ATG-T did not increase graft failure. The reason for this discrepancy is unclear, but 2 factors in this study might have contributed to the avoidance of graft failure: the formulation of ATG, and our practice of using melphalan or adding low-dose total body irradiation in patients at high-risk of graft failure, such as those with untreated myelodysplastic syndrome.
The limitations of this study should be clarified. We retrospectively analyzed the data at our center. The major limitation of the study was the limited number of cases who received ATG-T and significant difference in patient characteristics between the ATG-T and no-ATG-T groups. We consider that the benefit of low-dose ATG-T should be re-evaluated in prospective studies and larger retrospective clinical studies like a study using a registry data.
In conclusion, the use of low-dose ATG-T in patients who received unrelated HSCT was associated with a superior GRFS, reflecting the reduced incidence of severe/persistent GVHD without compromising OS. The results of this study support further investigation of ATG as prophylaxis for GVHD. The clinical role of low-dose ATG-T as prophylaxis for GVHD should be assessed in prospective clinical trials.
References
Thomas E, Storb R, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975;292:832–43.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–13.
Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21.
Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–84.
Jamil MO, Mineishi S. State-of-the-art acute and chronic GVHD treatment. Int J Hematol. 2015;101:452–66.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–5.
Oh H, Loberiza FR Jr, Zhang MJ, Ringden O, Akiyama H, Asai T, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105:1408–16.
Hahn T, McCarthy PL Jr, Zhang MJ, Wang D, Arora M, Frangoul H, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–34.
Murata M. Prophylactic and therapeutic treatment of graft-versus-host disease in Japan. Int J Hematol. 2015;101:467–86.
Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–7.
Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ, Min CK, et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15:704–17.
Fuji S, Ueno N, Hiramoto N, Asakura Y, Yakushijin K, Kamiyama Y, et al. Reduced-intensity conditioning regimen with low-dose ATG-F for unrelated bone marrow transplant is associated with lower non-relapse mortality than a regimen with low-dose TBI: a single-center retrospective analysis of 103 cases. Int J Hematol. 2013;98:608–14.
Hatanaka K, Fuji S, Ikegame K, Kato R, Wake A, Hidaka M, et al. Low incidences of acute and chronic graft-versus-host disease after unrelated bone marrow transplantation with low-dose anti-T lymphocyte globulin. Int J Hematol. 2012;96:773–80.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51.
Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–6.
Fuji S, Kim SW, Yano S, Hagiwara S, Nakamae H, Hidaka M et al. A prospective multicenter study of unrelated bone marrow transplants using a reduced-intensity conditioning regimen with low-dose ATG-F. Bone Marrow Transplant. 2015. doi:10.1038/bmt.2015.268 [Epub ahead of print].
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Walker I, Schultz KR, Toze CL, Kerr HM, Moore J, Szwajcer D et al. Thymoglobulin decreases the need for immunosuppression at 12 months after myeloablative unrelated donor transplantation: CBMTG0801, a controlled trial. American Society of Hematology Annual Meeting. Blood. 2014;124. Abstract 38.
Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–94.
Devillier R, Furst S, El-Cheikh J, Castagna L, Harbi S, Granata A, et al. Antithymocyte globulin in reduced-intensity conditioning regimen allows a high disease-free survival exempt of long-term chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:370–4.
Remberger M, Ringden O, Hagglund H, Svahn BM, Ljungman P, Uhlin M, et al. A high antithymocyte globulin dose increases the risk of relapse after reduced intensity conditioning HSCT with unrelated donors. Clin Transplant. 2013;27:E368–74.
Bonifazi F, Solano C, Wolschke C, et al. Prevention of chronic GvHD after HLA-indentical sibling peripheral hematopoietic stem cell transplantation with or without anti-lymphocyte globulin (ATG). Results from a prospective, multicenter randomized phase III trial (ATG family study). American Society of Hematology Annual Meeting. Blood. 2014;124. Abstract 37.
Dirou S, Malard F, Chambellan A, Chevallier P, Germaud P, Guillaume T, et al. Stable long-term pulmonary function after fludarabine, antithymocyte globulin and i.v. BU for reduced-intensity conditioning allogeneic SCT. Bone Marrow Transplant. 2014;49:622–7.
Milano F, Au MA, Boeckh MJ, Deeg HJ, Chien JW. Evaluating the impact of antithymocyte globulin on lung function at 1 year after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:703–9.
Zander AR, Kroger N, Schleuning M, Finke J, Zabelina T, Beelen D, et al. ATG as part of the conditioning regimen reduces transplant-related mortality (TRM) and improves overall survival after unrelated stem cell transplantation in patients with chronic myelogenous leukemia (CML). Bone Marrow Transplant. 2003;32:355–61.
Schattenberg A, van der Meer A, Preijers F, Schaap N, Rinkes M, van der Maazen R, et al. Addition of ATG to the conditioning regimen is a major determinant for outcome after transplantation with partially lymphocyte-depleted grafts from voluntary unrelated donors. Bone Marrow Transplant. 2004;33:1115–21.
Crocchiolo R, Esterni B, Castagna L, Furst S, El-Cheikh J, Devillier R, et al. Two days of antithymocyte globulin are associated with a reduced incidence of acute and chronic graft-versus-host disease in reduced-intensity conditioning transplantation for hematologic diseases. Cancer. 2013;119:986–92.
Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–70.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Acknowledgments
This work was supported by grants from the Japanese Ministry of Health, Labour and Welfare and the National Cancer Research and Development Fund. We thank the medical, nursing, data-processing, laboratory, and clinical staffs at the participating centers for their important contributions to this study and their dedicated patient care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Kuriyama, K., Fuji, S., Inamoto, Y. et al. Impact of low-dose rabbit anti-thymocyte globulin in unrelated hematopoietic stem cell transplantation. Int J Hematol 103, 453–460 (2016). https://doi.org/10.1007/s12185-016-1947-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-1947-9