Abstract
Anti-T lymphocyte globulin (ATG) is commonly used as prophylaxis for graft-versus-host disease (GVHD), especially in patients who are at high risk of GVHD. The appropriate dosage of ATG in Japan has not yet been assessed. We therefore conducted a nationwide survey of patients who received ATG-Fresenius as GVHD prophylaxis for unrelated bone marrow transplantation (uBMT). A total of 86 patients were identified (median age 31 years, range 1–68). The median total dose of ATG was 10 mg/kg. The cumulative incidence of neutrophil engraftment was 90 %. The probability of 2-year overall survival (OS) was 67 %. The cumulative incidence of 2-year non-relapse mortality was 25 %. The incidences of grade II–IV and grade III–IV acute GVHD were 20 and 8 %, respectively. The incidences of chronic and extensive chronic GVHD were 19 and 8 %, respectively. In adult patients, there was a reduction of acute GVHD with high-dose ATG (>10 mg/kg), which did not reach statistical significance. In conclusion, the addition of low-dose ATG to GVHD prophylaxis in Japanese patients who received uBMT resulted in decreased incidences of both acute and chronic GVHD without compromising OS. The effects of low-dose ATG should be assessed in a prospective clinical trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past several decades, significant advances have been made in the field of allogeneic hematopoietic stem cell transplantation (HSCT), and allogeneic HSCT has become an integral part of treatment for a variety of hematological malignancies and some non-malignant diseases [1, 2]. However, acute and chronic graft-versus-host diseases (GVHD) are still major morbidities after allogeneic HSCT, especially in patients who receive stem cells from an unrelated donor. Finke et al. [3] conducted a large randomized-control trial which showed that anti-T lymphocyte globulin Fresenius (ATG-F) has a beneficial effect as GVHD prophylaxis in patients who have undergone unrelated HSCT. Furthermore, a long-term follow-up of this study demonstrated that the use of ATG-F significantly reduced the incidence of chronic GVHD, which should reduce late non-relapse mortality (NRM) and improve the quality of life (QOL) after unrelated HSCT [4].
Different preparations of ATG including Thymoglobulin and ATG-F have been tested as part of conditioning regimens to achieve in vivo T cell depletion. These two ATG preparations can differ substantially in their potency due to differences in production methods, i.e., in the cells used for immunization [5]. In addition, the intensity of GVHD prophylaxis may depend on the patient’s ethnicity, since the risk of GVHD might differ among races [6, 7]. For example, regarding Thymoglobulin, 6.0–7.5 mg/kg is the usual dose for preventing GVHD, and 4 mg/kg was shown to be insufficient in Caucasian patients [5]. In contrast, in Korea, Kim et al. reported that the use of a very low dose of Thymoglobulin (total 2.5 mg/kg) was still associated with lower incidences of acute GVHD and NRM [8, 9]. Furthermore, ATG has multiple immunomodulatory effects, including the expansion of regulatory T cells [10]. Therefore, the effectiveness of ATG as GVHD prophylaxis cannot be assessed simply in terms of the degree of lymphodepletion. Profound immunosuppression could lead to an inferior outcome due to a high rate of infections or possibly to an increase in relapse caused by a loss of graft-versus-leukemia (GVL) effects.
Therefore, we assessed the clinical outcomes of patients who underwent unrelated HSCT using ATG-F as GVHD prophylaxis to identify a candidate dose of ATG-F for testing in a prospective clinical trial.
Patients and methods
Study design
This was a retrospective study that surveyed the use of ATG-F as GVHD prophylaxis in Japan. Patients who received ATG-F as GVHD prophylaxis for bone marrow transplant from an unrelated donor (uBMT) from April 2001 to December 2006 were included. In the era of this study, only BMT could be collected as a stem cell source from an unrelated volunteer donor. We performed a nationwide questionnaire survey at institutions in Japan that performed allogeneic HSCT. For patients who received ATG-F as GVHD prophylaxis, we also collected data regarding the dose and duration of ATG-F along with data from the national registry system of the Japan Society of Hematopoietic Cell Transplantation [11]. We identified 581 cases who received ATG for related or unrelated HSCT, and questionnaires were obtained for 509 (87.6 %). Among these 509 cases, 182 (35.8 %) received ATG-F. We only included patients who received ATG-F for an unrelated BMT, because most of the patients who received ATG for a related HSCT underwent a haploidentical HSCT. In terms of HLA typing, allele typing of 6 loci including HLA A, B and DRB1 was available, while information regarding HLA-C was unavailable. This study was approved by the Institutional Review Board of the Japan Society of Hematopoietic Cell Transplantation and Rinku General Medical Center, Osaka, Japan.
Clinical outcomes
Endpoints included neutrophil recovery, overall survival (OS), NRM, acute GVHD and chronic GVHD. Neutrophil recovery was defined as an absolute neutrophil count of ≥0.5 × 109/L for 3 consecutive days. The incidences of grade II–IV or III–IV acute and chronic or extensive chronic GVHD were based on standard criteria [12, 13].
Statistical analysis
The probability of OS was calculated by the Kaplan–Meier method. The cumulative incidences of engraftment, NRM and GVHD were evaluated using Gray’s method. In the competing risk models for engraftment and GVHD, relapse and death before these events were defined as competing risks. In the competing risk models for NRM, relapse was defined as a competing risk. A two-sided P value of <0.05 was considered statistically significant. Standard risk was defined as the first complete remission of acute leukemia, the first chronic phase of chronic myeloid leukemia, or non-malignant diseases. High risk was defined as other hematological malignancies. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0) [14]. More precisely, it is a modified version of R commander (version 1.6-3) that was designed to add statistical functions that are frequently used in biostatistics.
Results
Patients’ characteristics
The details of the patients’ characteristics are shown in Table 1. The median age was 31 years (range 1–68). The underlying disease was non-malignant and hematologically malignant in 51 and 35 patients, respectively. Among hematological malignancies, 27 patients (84 %) had a high-risk disease. In pediatric patients (age < 18), 31 of 34 patients had non-malignant disease. Among the 85 patients for whom data on HLA typing were available, 41 received bone marrow from a donor with an HLA mismatch (one antigen mismatch n = 13, one allele mismatch n = 24, 2–3 allele mismatch n = 4). As GVHD prophylaxis, tacrolimus was used in 67 patients (78 %). Most patients received a reduced-intensity conditioning regimen (RIC n = 33) or a non-myeloablative conditioning regimen (NMA n = 46) [15, 16]. NMA was only used in patients with a non-malignant disease.
Regarding the total dose of ATG-F, 10 and 20 mg/kg were used in 35 and 20 patients, respectively. ATG-F was administered on 2 and 4 days in 28 patients and 44 patients, respectively.
Clinical outcomes
The median follow-up of surviving patients was 888 days after uBMT (range 118–2122 days). The cumulative incidence of neutrophil engraftment was 90 % (Fig. 1a). The probability of 2-year OS was 67 % (Fig. 1b). The cumulative incidence of 2-year NRM was 25 % (Fig. 1c).
Patients with a non-malignant disease had a significantly better 2-year OS than those with a malignant disease (81 vs. 44 %, P = 0.0004; Fig. 2a), but there was no significant difference in the incidence of NRM between the 2 groups (19 vs. 35 %, P = 0.16, Fig. 2b). UBMT from an HLA antigen-mismatched donor had a significantly inferior OS compared to uBMT from an HLA-matched donor (37 vs. 69 %, P = 0.008), but there was no significant difference between uBMT from an HLA allele-mismatched donor and that from an HLA-matched donor (78 vs. 69 %, P = 0.50). Patients who received an uBMT from an HLA antigen-mismatched donor tended to have a higher incidence of NRM than those who received uBMT from an HLA-matched donor (46 vs. 21 %, P = 0.051). However, there was no significant difference between uBMT from an HLA allele-mismatched donor and that from an HLA-matched donor (22 vs. 21 %, P = 0.91).
GVHD and post-transplant lymphoproliferative disease (PTLD)
The cumulative incidences of grade II–IV and grade III–IV acute GVHD were 20 and 8 %, respectively (Fig. 3a). The cumulative incidences of chronic GVHD and extensive chronic GVHD were 19 and 8 %, respectively (Fig. 3b). There was no significant difference in the incidence of acute GVHD between patients with a non-malignant disease and those with a malignant disease (19 vs. 22 %, P = 0.79). In terms of the incidences of grade II–IV and grade III–IV acute GVHD, there was no significant difference regardless of the presence of an HLA mismatch (grade II–IV 31, 18, 16 % and grade III–IV 23, 4, 7 % in patients with an HLA antigen-mismatched donor, HLA allele-mismatched donor, and HLA-matched donor, respectively). There were no reported cases of PTLD.
Dose of ATG-F
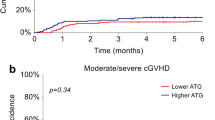
For a comparison of the effect of the ATG-F dose, we included only adult patients (≥18 years old). The median dose of ATG-F was 10 mg/kg. We divided patients into 2 groups: those who received more than 10 mg/kg of ATG-F (n = 21, high ATG group) and those who received 10 mg/kg or less of ATG-F (n = 31, low ATG group). The characteristics of the 2 groups are shown in Supplementary Table 1. The high-ATG group included significantly more patients with a non-malignant disease (76 vs. 23 %, P < 0.001) and more younger patients compared to the low-ATG group. The high-ATG group included more patients with an HLA mismatch, but this difference was not statistically significant. The cumulative incidences of grade II–IV acute GVHD in the low- and high-ATG groups were 23 and 10 %, respectively (P = 0.23; Fig. 4a). The incidence of grade II–IV acute GVHD in the low-ATG group tended to be higher than that in the high-ATG group, but this difference was not statistically significant. The cumulative incidences of grade III–IV acute GVHD in the low- and high-ATG groups were 10 and 5 %, respectively (P = 0.50; Fig. 4b). The cumulative incidences of chronic GVHD in the low- and high-ATG groups were 19 and 10 %, respectively (P = 0.40; Fig. 4c). The cumulative incidences of extensive chronic GVHD in the low- and high-ATG groups were 10 and 5 %, respectively (P = 0.51; Fig. 4d). The probabilities of 2-year OS in the low- and high-ATG groups were 39 and 57 %, respectively (P = 0.74; Fig. 4e). The cumulative incidences of 2-year NRM in the low- and high-ATG groups were 34 and 38 %, respectively (P = 0.62; Fig. 4f).
Discussion
We determined the clinical outcomes of Japanese patients who received ATG-F as GVHD prophylaxis for an uBMT. We found low incidences of both acute and chronic GVHD with the use of low-dose ATG-F, considering that all patients received BMT from an unrelated donor and about half received BMT from a donor with an HLA mismatch.
A previous large Japanese retrospective study reported that the incidences of grade II–IV and grade III–IV acute GVHD in patients who received uBMT from an HLA-matched donor were 34.5 and 11.8 %, respectively [17]. In addition, that study reported that the incidence of grade III–IV acute GVHD in patients who received an unrelated BMT from an HLA one allele-mismatched donor was 16.1–27.8 %, depending on the locus of mismatch [17]. In that study, only 176 of 1282 patients (14 %) received ATG. Therefore, it seems that the incidence of acute GVHD in patients who received low-dose ATG-F (grade II–IV 20 %, grade III–IV 8 %) was lower than that for all of the registered patients in Japan.
Our study also showed low incidences of chronic GVHD and extensive chronic GVHD (19 and 8 %, respectively). A previous report that focused on chronic GVHD showed that the incidences of chronic GVHD and extensive chronic GVHD after uBMT in Japan were 45.8 and 28.2 %, respectively [18]. In that study, only 203 of 2937 patients (7 %) received ATG [18]. In patients with an HLA-mismatched donor, the incidence of chronic GVHD was significantly higher than that in patients with an HLA-matched donor in previous studies [12, 13]. Compared to a previous report from Japan, the incidence of chronic GVHD in patients with ATG-F in our study seems to be promising, and the reduction of chronic GVHD was consistent with previous reports [4, 18].
In Western countries, the total dose of ATG-F for GVHD prophylaxis is usually 30–60 mg/kg [3, 4]. In Asian countries, as shown in the current study, a smaller dose of ATG is commonly used, since the incidence of GVHD itself is lower in Asian patients than in Caucasian patients [6, 7]. Kim et al. [8] reported that the use of low-dose ATG (Thymoglobulin 2.5 mg/kg) was associated with a low incidence of acute GVHD in patients who received an HLA-mismatched unrelated HSCT. In our study with a low dose of ATG-F (median 10 mg/kg), the incidences of both acute and chronic GVHD were relatively low. In a subset analysis, the use of a lower dose of ATG-F (≤10 mg/kg) did not significantly increase the incidence of GVHD compared to ATG-F at a higher dose, albeit the size of the study was limited. A reduction of chronic GVHD should lead to not only a reduction of morbidity and mortality associated with chronic GVHD but also an improvement of QOL which is an important clinical outcome in long-term survivors [4, 19]. Such low dosages of ATG-F should be good candidates for testing in a prospective clinical trial.
Another concern with the use of ATG is a possible increase in infectious diseases [20]. The CIBMTR study showed that the incidence of EBV-related PTLD in patients with ATG was significantly higher than that in those without T cell depletion (2 vs. 0.1 %, P = 0.005) [20]. Although the number of patients was limited, there were no cases of PTLD in this study, which suggested that the immunosuppression with low-dose ATG-F was not very intense. However, the incidence of PTLD should be confirmed in a larger study.
This study has several limitations. Even though we included all patients in the registry who received ATG-F as GVHD prophylaxis for uBMT, the number of patients was still quite small. This must be because of the use of ATG-F as GVHD prophylaxis was not covered by insurance in Japan in the era of this study. Furthermore, the patients had heterogeneous characteristics. Especially, the underlying disease and the conditioning regimen varied significantly. As recently reported by Soiffer et al. [20], the benefit of ATG could differ depending on the intensity of the conditioning regimen. Therefore, based on the results of the current study, the impact of low-dose ATG-F with a uniform conditioning regimen and GVHD prophylaxis should be assessed.
In conclusion, the use of low-dose ATG-F in Japanese patients who underwent an uBMT was associated with promisingly low incidences of both acute and chronic GVHD, with a low incidence of late NRM. The role of low-dose ATG-F as prophylaxis for GVHD should be further assessed in a prospective clinical trial.
References
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–13.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Socié G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Kumar A, Mhaskar AR, Reljic T, Mhaskar RS, Kharfan-Dabaja MA, Anasetti C, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia. 2012;26:582–8.
Oh H, Loberiza FR Jr, Zhang MJ, Ringdén O, Akiyama H, Asai T, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105:1408–16.
Hahn T, McCarthy PL Jr, Zhang MJ, Wang D, Arora M, Frangoul H, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–34.
Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ, Min CK, et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15:704–17.
Remberger M, Svahn BM, Mattsson J, Ringdén O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–7.
Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–94.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Kanda Y. Free statistical software: EZR (Easy R) on R commander. http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html. Accessed 1 Apr 2012.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Morishima Y, Sasazuki T, Inoko H, Juji T, Akaza T, Yamamoto K, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99:4200–6.
Ozawa S, Nakaseko C, Nishimura M, Maruta A, Cho R, Ohwada C, et al. Chronic graft-versus-host disease after allogeneic bone marrow transplantation from an unrelated donor: incidence, risk factors and association with relapse: a report from the Japan Marrow Donor Program. Br J Haematol. 2007;137:142–51.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–5.
Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–70.
Acknowledgments
We thank the medical, nursing, data-processing, laboratory, and clinical staff at the participating centers for their important contributions to this study and their dedicated care of the patients. This work was supported by grants from the Japanese Ministry of Health, Labour and Welfare and the National Cancer Research and Development Fund (23-A-28).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Hatanaka and S. Fuji contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hatanaka, K., Fuji, S., Ikegame, K. et al. Low incidences of acute and chronic graft-versus-host disease after unrelated bone marrow transplantation with low-dose anti-T lymphocyte globulin. Int J Hematol 96, 773–780 (2012). https://doi.org/10.1007/s12185-012-1209-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1209-4