Abstract
The diagnosis of primary immune thrombocytopenia (ITP) is based on differential diagnosis. Although the measurement of percentages of reticulated platelets (RP%) by flow cytometry is useful as a supportive diagnostic test, this method is nonetheless a time-consuming, laboratory-based assay. To identify alternative assays that are useful in daily practice, we compared three methods in parallel, IPF% measured by XE-2100 [IPF% (XE), Sysmex Corp.], IPF% measured by new XN-1000 [IPF% (XN)], and RP%. We examined 47 patients with primary ITP, 28 patients with aplastic thrombocytopenia (18 aplastic anemia and 10 chemotherapy-induced thrombocytopenia) and 80 healthy controls. In a selected experiment, we examined 16 patients with paroxysmal nocturnal hemoglobinuria (PNH) to examine the effect of hemolysis. As compared with IPF% (XE), IPF% (XN) showed better within-run reproducibility. The sensitivity and specificity for the diagnosis of ITP were 83.0 and 75.0 % for IPF% (XE), 85.1 and 89.3 % for IPF% (XN), and 93.6 and 89.3 % for RP%, respectively. Examination of PNH patients revealed that hemolysis and/or red blood cell fragments interfered with IPF% (XE) values, but not with IFP % (XN) values. Our results suggest that IPF% measured by XN-1000 may be of comparable value with RP% as a supportive diagnostic test for ITP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disease characterized by early platelet destruction due to anti-platelet autoantibodies and slightly impaired platelet production [1–3]. Despite recent advances in understanding of its pathophysiology, the diagnosis of ITP is still mainly based on differential diagnosis [4]. However, it is sometimes very difficult to distinguish ITP from isolated thrombocytopenia due to aplastic thrombocytopenic disorders such as aplastic anemia (AA) and amegakaryocytic thrombocytopenia. To resolve this issue, several laboratory-based assays have been developed: detection of anti-platelet autoantibodies, measurement of percentage of reticulated platelets (RP%) and plasma thrombopoietin (TPO) concentrations [3]. Regarding detection of platelet-associated autoantibodies, it has been shown that its specificity for the diagnosis of ITP is very high (80–90 %) in prospective studies. However, the drawback in this assay is its relatively low sensitivity as well as being time-consuming, laboratory-based assay: platelet-associated anti-GPIIb/IIIa and/or anti-GPIb/IX antibodies are detected in only 51–55 % of ITP [5–7]. Alternatively, measurement of RP% and plasma TPO concentrations is useful to distinguish between ITP and aplastic thrombocytopenic disorders [8–10]. RPs are reported to be younger platelets (i.e., immature platelets) that have been released recently into the circulation and are probably analogous to reticulocytes reflecting erythropoiesis. RPs can be distinguished from mature platelets by their RNA contents using flow cytometry with an RNA-binding fluorochrome, such as thiazole orange, and RP% and absolute number of RPs are reflecting platelet production and hence platelet turnover [11, 12]. In ITP patients RP% was markedly increased compared with healthy controls, whereas RP% in patients with AA or chemotherapy-induced thrombocytopenia (CIT) was within normal range [10–12]. In contrast, plasma TPO levels in ITP patients are within normal range or only slightly increased, whereas those in patients with aplastic thrombocytopenic disorders are markedly increased [8–10]. Accordingly, Japanese ITP working group including us proposed preliminary diagnostic criteria for ITP by incorporating anti-platelet autoantibody detection, RP%, and plasma TPO level. In a multi-center prospective study, the criteria showed high sensitivity and specificity for the diagnosis of ITP [13]. However, the method for the measurement of RP% is nonetheless a time-consuming, laboratory-based assay and has not been standardized yet.
We have been seeking alternative assays to measure RP% that are useful in daily practice, although measurement of RP% by flow cytometry is the gold standard method. One candidate is measurement of percentage of immature platelet fraction (IPF%) using Sysmex XE-2100 (or XE-5000) automated hematology analyzer (Sysmex Corp., Kobe, Japan). This IPF% method becomes very popular because of its convenience [14]. However, we previously demonstrated that IPF% measured by XE-2100 showed less sensitivity and specificity as compared to RP% method to distinguish between ITP and AA patients [3, 15]. Thus, IPF% measured by XE-2100 was neither so accurate nor satisfactory in daily practice. To improve the accuracy of IPF% method, new generation analyzer, XN-1000 has been developed and become commercially available. In this study, we compared these three methods in parallel, IPF% measured by XE-2100 [IPF% (XE)], IPF% measured by XN-1000 [IPF% (XN)], and RP%, for their utility in differential diagnosis between ITP and aplastic thrombocytopenia (AA and CIT). In addition, effects of hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH) on IPF% (XE), IPF% (XN), and RP% were examined.
Materials and methods
Subjects
For a period of 6 months (October 2013 through March 2014) we examined 47 patients with primary ITP [9 males and 38 females, age 59 ± 17 years, platelet count 57 ± 34 × 103/μl (mean ± SD)], 28 patients with aplastic (or hypoplastic) thrombocytopenia [18 AA and 10 chemotherapy-induced thrombocytopenia (CIT)] [11 males and 17 females, age 50 ± 15 years, platelet count 43 ± 28 × 103/µl (mean ± SD)], and 80 healthy controls [35 males and 45 females, age 34 ± 12 years, platelet count 269 ± 58 × 103/µl (mean ± SD)]. Diagnosis of primary ITP and aplastic anemia was based on reports from an international working group and International Agranulocytosis and Aplastic Anemia Study group, respectively [4, 16]. With regard to management of 47 patients with ITP, 19 patients managed with observation alone, 14 patients mainly with prednisolone, 9 patients with TPO receptor agonist (TPORA) and prednisolone, and 5 patients with TPORA only. Thus, 14 ITP patients treated with TPO receptor agonist such as eltrombopag and romiplostim were included, and 12 of these patients still showed thrombocytopenia less than 100 × 103/µl. Patients with CIT include 6 patients with AML, 2 patients with ALL, and 2 patients with MDS, and samples were obtained on day 1 or day 2 for myeloablative allogeneic hematopoietic stem cell transplantation. We obtained informed consent from all subjects, in accordance with the declaration of Helsinki. This study was approved by Osaka University Institutional Review Board.
In a selected experiment, we examined 16 patients with PNH to investigate effects of hemolysis and/or fragmentation of red blood cells (RBC) on the measurement of IPF% and RP%.
Measurement of RP%
RP% was measured as previously described with a slight modification [15]. In brief, 15-µl aliquots of whole blood anti-coagulated with ethylenediamineteraacetic acid (EDTA) were incubated with 5 µl of phycoerythrin-conjugated anti-CD42b monoclonal antibody (BD Pharmingen, Tokyo, Japan) and 20 µl of 2 % paraformaldehyde for 15 min at room temperature. After adding 1 ml of thiazole orange (Retic-COUNT; Becton–Dickinson, San Jose, CA, USA) diluted to 8 times by phosphate-buffered saline, the whole blood samples were centrifuged at 350 g for 30 s to remove red blood cells, and then the platelet-rich suspensions were incubated at room temperature for 90 min. RP% was analyzed on a flow cytometer (FACScan, Becton–Dickinson) by measuring 10,000 events in the CD42b-positive fraction. To exclude cell autofluorescence and instrument background, platelet-rich suspension without thiazole orange was prepared as a negative control for each sample.
Measurement of IPF% by Sysmex automated hematology analyzer XE-2100 and XN-1000
EDTA-anti-coagulated whole blood samples were also used to measure IPF% employing automated hematology analyzer XE-2100 (XE, Sysmex) and the newer generation analyzer, XN-1000 (XN, Sysmex). XE used polymethine and oxazine to stain nucleic acid and RET-channel for the measurement of IPF, whereas XN used only oxazine and PLT-F channel to more accurately detect platelets and IPF [17]. All samples were measured within 7 h of venesection.
Measurement of plasma TPO concentrations
Plasma TPO concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit as previously described (R & D Systems, Minneapolis, MN, USA) [10].
Statistical analysis
The differences between mean values were evaluated using Student t test and a p value less than 0.05 was considered statistically significant. We analyzed sensitivity and specificity of IPF% (XE), IPF% (XN), and RP% for the diagnosis of ITP as previously described [10]. Constructed receiver operating characteristics (ROC) curves were analyzed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA).
Results
Within-run reproducibility
We first examined within-run reproducibility for IPF% measurement in 4 control subjects and 2 ITP patients employing XE and XN in parallel. Each sample was measured 10 times, and mean intra-assay coefficients of variation [CV(%)] for these samples were 14.2 ± 2.8 and 5.6 ± 2.0 % for XE and XN, respectively (p < 0.001) (Table 1).
IPF%, RP%, and plasma TPO levels in thrombocytopenic disorders due to either accelerated platelet destruction or deficient platelet production.
We examined 47 patients with ITP as a thrombocytopenic disorder due to early platelet destruction and 18 patients with aplastic anemia and 10 patient with CIT as aplastic (or hypoplastic) thrombocytopenic disorders. IPF% values obtained from 80 control subjects were 2.5 ± 1.3 and 2.2 ± 1.2 % (mean ± SD) for XE and XN, respectively. As we defined an upper limit for healthy control subjects as mean + 3SD in this study, the upper limits were 6.4 and 5.8 % for XE and XN, respectively. RP% value obtained from 80 control subjects measured by flow cytometry was 4.8 ± 1.1 % (mean ± SD) and its upper limit of reference range was defined as 8.1 % (mean + 3SD).
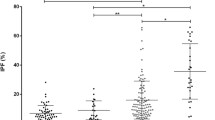
Figure 1 shows correlations between IPF% (XE), IPF% (XN), and RP%. Good linear correlation between IPF% (XE) and IPF% (XN) was obtained (r = 0.94), whereas only moderate correlation between RP% and IPF% (XE) (r = 0.72) or IPF% (XN) (r = 0.71) was obtained. IPF and RPs were measured with oxazine and thiazole orange, respectively, and they were thought to be equivalent. However, our data suggested that IPF and RPs were similar, but not quantitatively identical. Next, we examined the sensitivity and specificity of IPF% and RP% to distinguish ITP from AA/CIT. There was no significant difference in platelets count between two groups (57 ± 34 × 103/µl for ITP, 43 ± 28 × 103/µl for AA/CIT). Figure 2 shows IPF% (XE), IPF% (XN) and RP% in ITP patients and AA/CIT patients. As expected, IPF% (XE), IPF% (XN) and RP% showed clear difference between ITP and AA/CIT. However, elevated values were detected in 83.0, 85.1, and 93.6 % of ITP measured by IPF% (XE), IPF% (XN) and RP%, respectively. In sharp contrast, the elevated values were detected in 25.0, 10.7, and 10.7 % of AA/CIT by IPF% (XE), IPF% (XN) and RP%, respectively (Fig. 2). As compared with IPF% (XN) and RP%, IPF% measured by XE-2100 appeared to be less frequently elevated in ITP and much frequently elevated in AA/CIT. Thus, the sensitivity and specificity of the elevation of IPF% or RP% for the diagnosis of ITP were 83.0 and 75.0 % for IPF% (XE), 85.1 and 89.3 % for IPF% (XN), and 93.6 and 89.3 % for RP%, respectively (Table 2).
In parallel we measured plasma TPO concentration in each sample, which further confirmed the diagnosis (Fig. 3). TPO levels obtained from 80 control subjects were 16.3 ± 21.8 pg/ml (mean ± SD), and the upper limit of reference range for TPO was 81.7 pg/ml (mean + 3SD). Patients with AA/CIT showed markedly increased plasma TPO levels, whereas patients with ITP showed normal or modestly increased TPO levels. In addition, we measured mean platelet volume (MPV) for ITP and AA/CIT. However, MPV could be measured by XN-1000 in only 28 out of 47 ITP patients, probably because of abnormal size distribution of platelet volume in ITP. Nonetheless, MPVs for ITP (12.5 ± 1.0 fl, n = 28) were significantly larger than 80 controls (10.5 ± 1.0 fl, p < 0.001) and 23 AA/CIT (10.7 ± 0.9 fl, p < 0.001).
Receiver operating characteristic (ROC) analysis
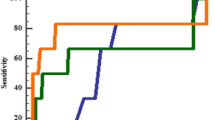
ROC curves were constructed for the sensitivity and specificity of the differential diagnosis of ITP from AA/CIT patients, and IPF% (XE), IPF% (XN), and RP% data were analyzed separately. ROC curve for IPF% (XE), IPF% (XN), and RP% showed area under curve (AUC) of 0.863, 0.956, and 0.959, respectively (Fig. 4).
IPF% and RP% in patients with PNH
To examine effects of hemolysis on the measurement of IPF% and RP% we examined patients with PNH. Ten patients out of 16 PNH patients were treated with eculizumab, a humanized monoclonal antibody against terminal complement protein C5 that inhibits terminal complement activation. As shown in Fig. 5, 6 PNH patients showed elevated IPF% (XE), whereas none and only two patients showed elevated IPF% (XN) and RP%, respectively. Five out of 6 PNH patients with elevated IPF% (XE) were treated with eculizumab, suggesting active hemolysis may interfere with the measurement of IPF% by XE-2100.
Discussion
Recent in vivo vital imaging as well as biochemical and genetic approaches have revealed the mechanism of platelet production (thrombopoiesis) from mature megakaryocytes. Mature megakaryocytes localized in bone marrow sinusoids extend proplatelets into the lumen of the sinusoids, and then new platelets are shed as fragments from the tips of intravascular proplatelets [18, 19], and newly produced platelets can be distinguished from mature platelets by their content of RNA as RPs by flow cytometry [20]. Despite our progress in understanding of pathophysiology of ITP as well as mechanism of thrombopoiesis, diagnosis of ITP has been still based on differential diagnosis [1–3].
Several laboratory-based tests, detection of platelet-associated autoantibodies, measurement of RP% and plasma TPO level, could be useful for the diagnosis of ITP [10]. However, the measurement of RP% is laboratory-based assay, and not used in daily practice yet. In addition, methods for RP% measurement have not been standardized. High concentrations of thiazole orange as well as longer incubation time, more than 2.5 h, induced dramatically higher fluorescence intensities, probably due to penetration of the dye into the dense granules [20]. Accordingly, we used 8-times-diluted thiazole orange and shorter incubation time (90 min) to measure RP%. To examine the reliability of automated hematology analyzer-based IPF% method for the differential diagnosis of ITP, we compared IPF% (XE), IPF% (XN), and RP% in parallel. Platelets were precisely monitored by anti-CD42b antibody and 10,000 events were analyzed even under thrombocytopenic conditions in the RP% method, but not in IPF% (XE) or IPF% (XN). However, in the new XN series a novel PLT-F channel was introduced to more specifically gate platelets than in XE series [17, 20]. Actually, we confirmed that data obtained by XN-1000 were much more accurate than XE-2100 regarding within-run reproducibility.
We examined the correlation between IPF% (XE), IPF% (XN), and RP%. Excellent correlation between IPF% (XE) and IPF% (XN) was obtained, while only moderate correlation even between RP% and IPF% (XN) existed. The difference between IPF% and RP% is probably caused by the difference in the fluorescent dyes: oxazine and thiazole orange. Our data first revealed that IFP % (XN) values and RP% values were moderately related, but not quantitatively identical.
In a selected experiment we examined samples obtained from PNH patients, since in XE-2100 RBC fraction and platelet fraction were relatively close each other and both polymethine and oxazine were used as fluorescent dyes. As expected, hemolysis and/or RBC fragments interfered with IPF% (XE) values and 6 out of 16 PNH samples showed elevated IPF% (XE) values. However, none and two samples showed elevated IFP % (XN) and RP%, respectively. In contrast to XE series employing both polymethine and oxazine as fluorescent dyes to measure reticulocytes and immature platelets, XN-1000 employs only oxazine to measure immature platelets more specifically. In addition, the novel PLT-F channel enables us to more accurately differentiate platelets from other cells and interfering particles such as RBC fragments than XE series [21]. The false positive results in IPF% (XE) may be partially caused by nonspecific binding of polymethine to RBC fragments. These data suggested that influence of hemolysis and/or RBC fragmentation was only minimal on measurement of IPF% by XN-1000 as well as RP% by flow cytometry.
We then compared the sensitivity and specificity of IPF% (XE), IPF% (XN), and RP% for the differential diagnosis between ITP and AA/CIT. There were clear differences in plasma TPO levels between ITP and AA/CIT. We confirmed our previous data that IPF% (XE) showed less sensitivity and specificity (83.0 and 78.6 %, respectively) as compared with RP% (93.6 and 89.3 %, respectively) [15]. In sharp contrast, IPF% by XN-1000 showed comparable sensitivity and specificity (85.1 and 92.9 %, respectively) with RP%. The sensitivity and specificity of IPF% (XE) was relatively high as compared with our previous study (sensitivity 67 %, specificity 63 %). This difference is probably due to the inclusion of ITP patients treated with TPORA in this study, because TPORA effectively increased the absolute number of RPs (and IPF) and maintained elevated RP% (and IPF%) even after improvement of thrombocytopenia [14]. In fact, exclusion of 14 ITP patients treated with TPORA led to the decrease in the sensitivity of these assays [IPF% (XE) 78.8 %, IPF% (XN) 81.8 %, and RP% 90.9 %] (Table 2). ROC curve for IPF% (XE), IPF% (XN), and RP% showed area under curve (AUC) of 0.86, 0.96, and 0.96, respectively, indicating that IPF% measured by XN-1000 may be comparable with RP% by flow cytometry.
In summary, the data obtained from our study suggested that IPF% measured by XN-1000 may be of comparable value with RP% as a supportive diagnostic test in distinguishing between thrombocytopenic disorders due to early platelet destruction such as ITP and aplastic thrombocytopenic disorders such as aplastic anemia. In addition, automated hematology analyzer is easy to handle and suitable for daily practice. Limitation of our study is that we examined patients with definitive diagnosis from single institute. Multi-center prospective study would be necessary to further confirm our data.
References
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008.
McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44:S3–11.
Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98:24–33.
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93.
Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88:194–201.
McMillan R, Wang L, Tani P. Prospective evaluation of the immunobead assay for the diagnosis of adult chronic immune thrombocytopenic purpura (ITP). J Thromb Haemost. 2003;1:485–91.
Tomiyama Y, Kosugi S. Autoantigenic epitopes on platelet glycoproteins. Int J Hematol. 2005;81:100–5.
Kosugi S, Kurata Y, Tomiyama Y, Tahara T, Kato T, Tadokoro S, et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93:704–6.
Emmons RV, Reid DM, Cohen RL, Meng G, Young NS, Dunbar CE, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87:4068–71.
Kurata Y, Hayashi S, Kiyoi T, Kosugi S, Kashiwagi H, Honda S, et al. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol. 2001;115:656–64.
Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75:116–21.
Richards EM, Baglin TP. Quantitation of reticulated platelets: methodology and clinical application. Br J Haematol. 1995;91:445–51.
Kuwana M, Kurata Y, Fujimura K, Fujisawa K, Wada H, Nagasawa T, et al. Preliminary laboratory based diagnostic criteria for immune thrombocytopenic purpura: evaluation by multi-center prospective study. J Thromb Haemost. 2006;4:1936–43.
Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011;117:5723–32.
Hayashi S, Nishiyama M, Suehisa E, Kashiwagi H, Kurata Y, Tomiyama Y. Comparison between two methods for the measurement reticulated platelet and their clinical significance—flow cytometry (FCM) method and IPF method using automated hematology analyzer (XE-2000). Rinsho Byori. 2009;57:1039–44.
International agranulocytosis and aplastic anemia study. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718–21.
van der Linden N, Klinkenberg LJ, Meex SJ, Beckers EA, de Wit NC, Prinzen L. Immature platelet fraction measured on the Sysmex XN hemocytometer predicts thrombopoietic recovery after autologous stem cell transplantation. Eur J Haematol. 2014;93:150–6.
Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70.
Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–81.
Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75:116–21.
Tanaka Y, Tanaka Y, Gondo K, Maruki Y, Kondo T, Asai S, et al. Performance evaluation of platelet counting by novel fluorescent dye staining in the XN-series automated hematology analyzers. J Clin Lab Anal. 2014;28:341–8.
Acknowledgments
This work was supported by Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan and the Ministry of Health, Labor and Welfare in Japan.
Conflict of interest
Automated hematology analyzers, XE-2100 and XN-1000, were supplied by Sysmex Corp. During this study the authors have no other COI to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakuragi, M., Hayashi, S., Maruyama, M. et al. Clinical significance of IPF% or RP% measurement in distinguishing primary immune thrombocytopenia from aplastic thrombocytopenic disorders. Int J Hematol 101, 369–375 (2015). https://doi.org/10.1007/s12185-015-1741-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1741-0